Abstract
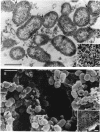
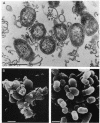
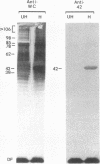
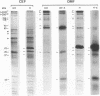
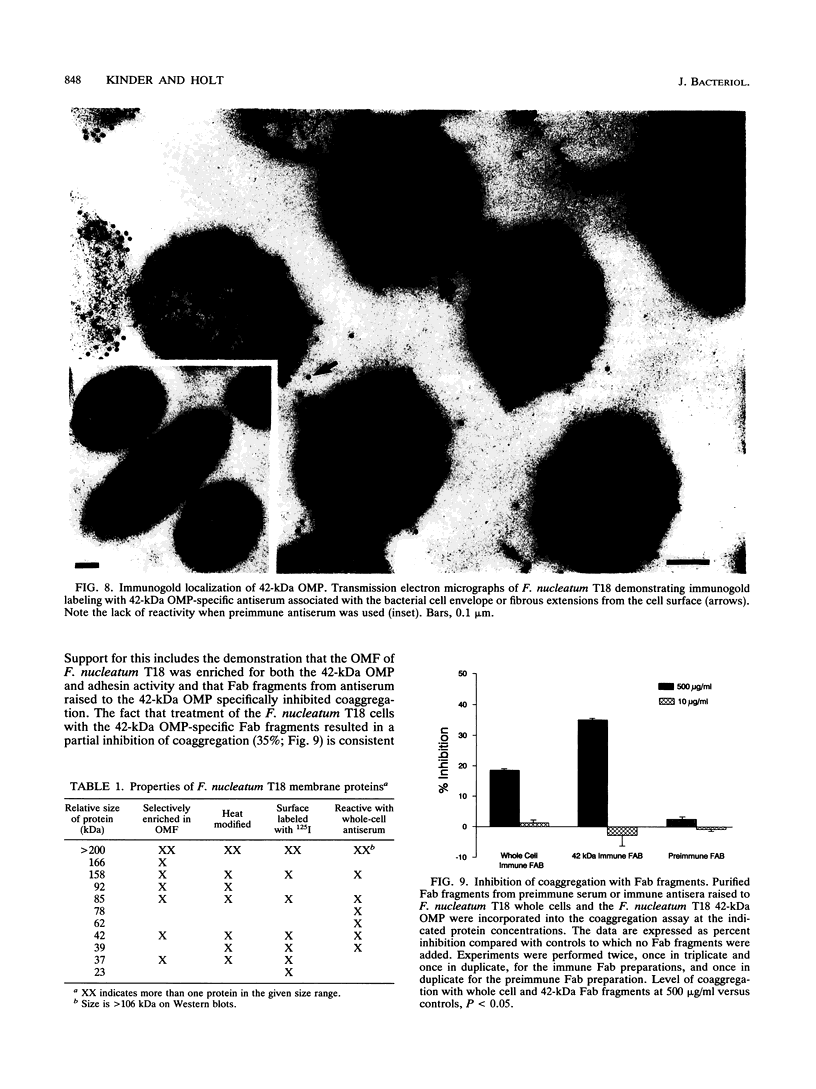
Adherence of pathogenic bacteria is often an essential first step in the infectious process. The ability of bacteria to adhere to one another, or to coaggregate, may be an important factor in their ability to colonize and function as pathogens in the periodontal pocket. Previously, a strong and specific coaggregation was demonstrated between two putative periodontal pathogens, Fusobacterium nucleatum and Porphyromonas gingivalis. The interaction appeared to be mediated by a protein adhesin on the F. nucleatum cells and a carbohydrate receptor on the P. gingivalis cells. In this investigation, we have localized the adhesin activity of F. nucleatum T18 to the outer membrane on the basis of the ability of F. nucleatum T18 vesicles to coaggregate with whole cells of P. gingivalis T22 and the ability of the outer membrane fraction of F. nucleatum T18 to inhibit coaggregation between whole cells of F. nucleatum T18 and P. gingivalis T22. Proteolytic pretreatment of the F. nucleatum T18 outer membrane fraction resulted in a loss of coaggregation inhibition, confirming the proteinaceous nature of the adhesin. The F. nucleatum T18 outer membrane fraction was found to be enriched for several proteins, including a 42-kDa major outer membrane protein which appeared to be exposed on the bacterial cell surface. Fab fragments prepared from antiserum raised to the 42-kDa outer membrane protein were found to partially but specifically block coaggregation. These data support the conclusion that the 42-kDa major outer membrane protein of F. nucleatum T18 plays a role in mediating coaggregation with P. gingivalis T22.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
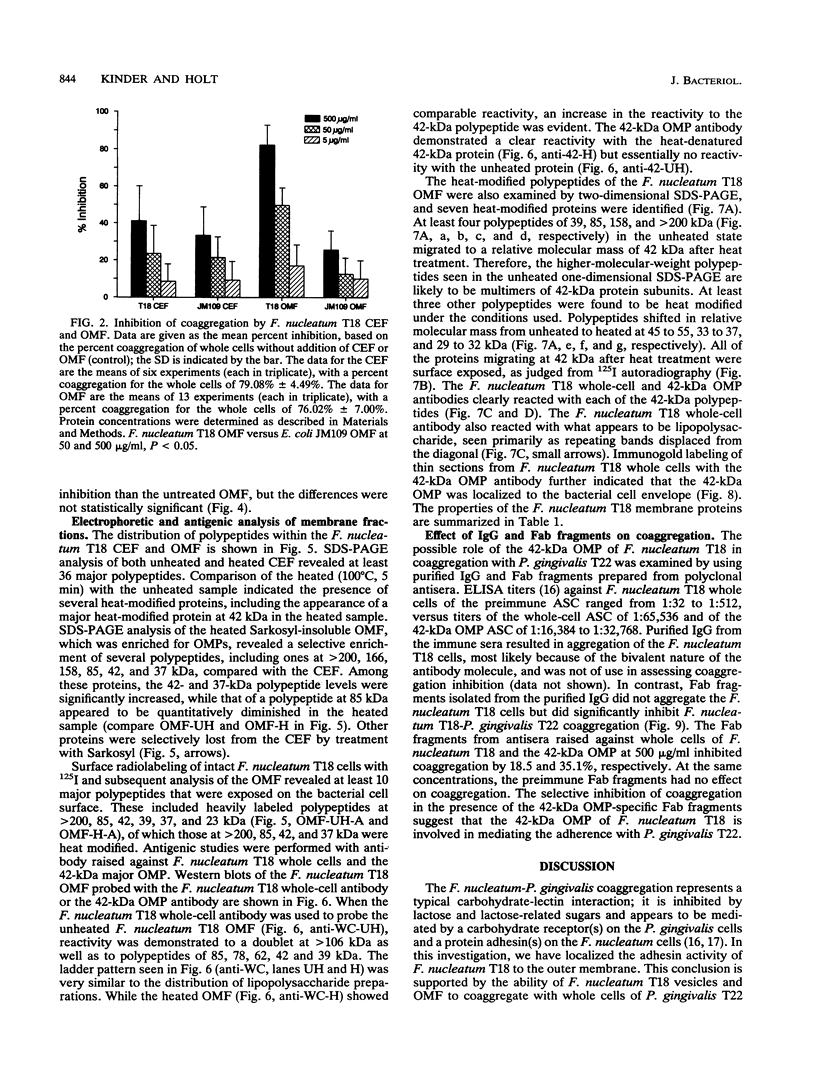
- Bakken V., Aarø S., Jensen H. B. Purification and partial characterization of a major outer-membrane protein of Fusobacterium nucleatum. J Gen Microbiol. 1989 Dec;135(12):3253–3262. doi: 10.1099/00221287-135-12-3253. [DOI] [PubMed] [Google Scholar]
- Bakken V., Jensen H. B. Outer membrane proteins of Fusobacterium nucleatum Fev1. J Gen Microbiol. 1986 Apr;132(4):1069–1078. doi: 10.1099/00221287-132-4-1069. [DOI] [PubMed] [Google Scholar]
- Beachey E. H., Giampapa C. S., Abraham S. N. Bacterial adherence. Adhesin receptor-mediated attachment of pathogenic bacteria to mucosal surfaces. Am Rev Respir Dis. 1988 Dec;138(6 Pt 2):S45–S48. doi: 10.1164/ajrccm/138.6_Pt_2.S45. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borinski R., Holt S. C. Surface characteristics of Wolinella recta ATCC 33238 and human clinical isolates: correlation of structure with function. Infect Immun. 1990 Sep;58(9):2770–2776. doi: 10.1128/iai.58.9.2770-2776.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. R., Williams P. The influence of environment on envelope properties affecting survival of bacteria in infections. Annu Rev Microbiol. 1985;39:527–556. doi: 10.1146/annurev.mi.39.100185.002523. [DOI] [PubMed] [Google Scholar]
- Datta D. B., Arden B., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane as bacteriophage receptors. J Bacteriol. 1977 Sep;131(3):821–829. doi: 10.1128/jb.131.3.821-829.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
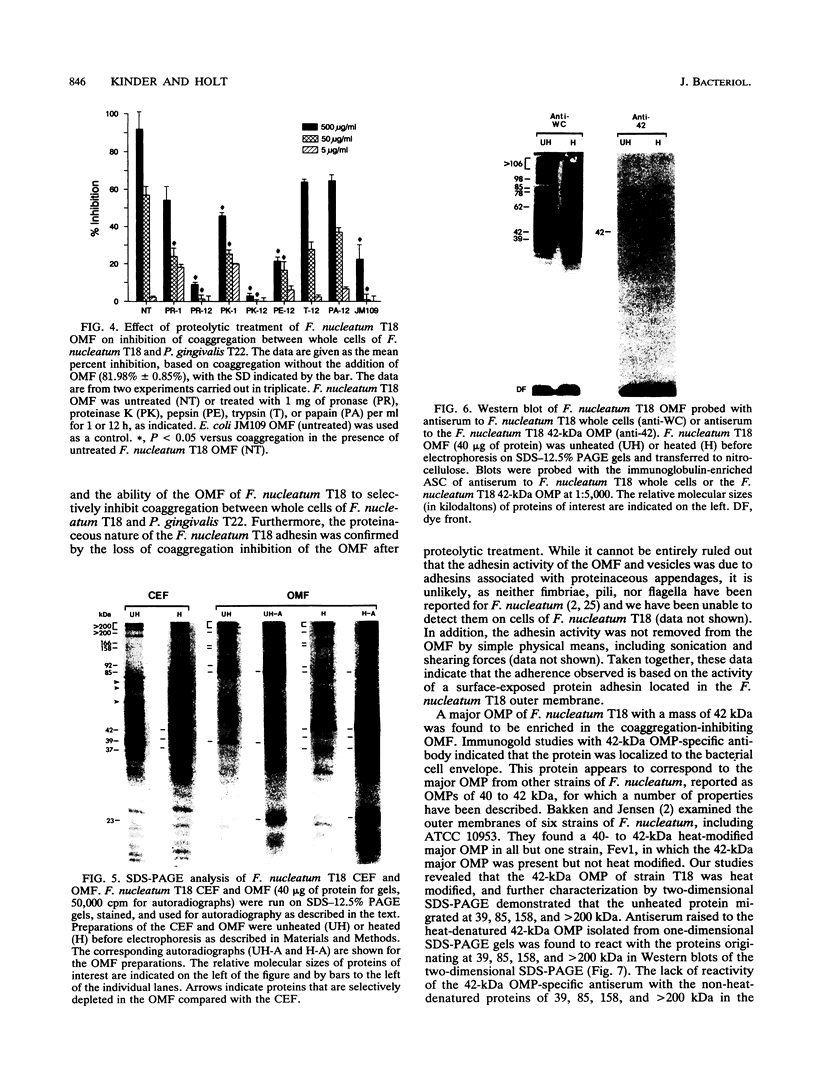
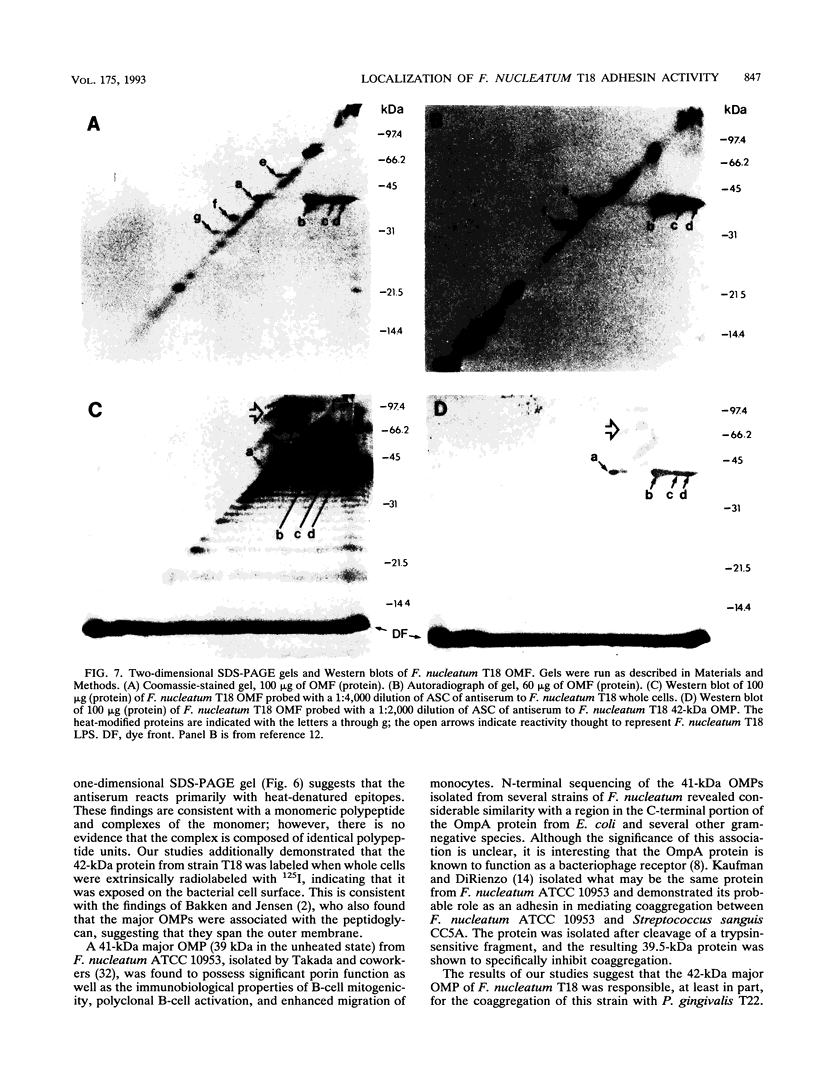
- Ebersole J. L., Frey D. E., Taubman M. A., Smith D. J. An ELISA for measuring serum antibodies to Actinobacillus actinomycetemcomitans. J Periodontal Res. 1980 Nov;15(6):621–632. doi: 10.1111/j.1600-0765.1980.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J. Bacterial adhesion to oral tissues: a model for infectious diseases. J Dent Res. 1989 May;68(5):750–760. doi: 10.1177/00220345890680050101. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Bramanti T. E. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit Rev Oral Biol Med. 1991;2(2):177–281. doi: 10.1177/10454411910020020301. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Ebersole J., Felton J., Brunsvold M., Kornman K. S. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988 Jan 1;239(4835):55–57. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- Kaufman J., DiRienzo J. M. Isolation of a corncob (coaggregation) receptor polypeptide from Fusobacterium nucleatum. Infect Immun. 1989 Feb;57(2):331–337. doi: 10.1128/iai.57.2.331-337.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell W., Holt S. C. Comparative studies of the outer membranes of Bacteroides gingivalis, strains ATCC 33277, W50, W83, 381. Oral Microbiol Immunol. 1990 Jun;5(3):121–130. doi: 10.1111/j.1399-302x.1990.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Kinder S. A., Holt S. C. Characterization of coaggregation between Bacteroides gingivalis T22 and Fusobacterium nucleatum T18. Infect Immun. 1989 Nov;57(11):3425–3433. doi: 10.1128/iai.57.11.3425-3433.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolenbrander P. E., Andersen R. N. Inhibition of coaggregation between Fusobacterium nucleatum and Porphyromonas (Bacteroides) gingivalis by lactose and related sugars. Infect Immun. 1989 Oct;57(10):3204–3209. doi: 10.1128/iai.57.10.3204-3209.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lantz M. S., Allen R. D., Bounelis P., Switalski L. M., Hook M. Bacteroides gingivalis and Bacteroides intermedius recognize different sites on human fibrinogen. J Bacteriol. 1990 Feb;172(2):716–726. doi: 10.1128/jb.172.2.716-726.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz M. S., Allen R. D., Duck L. W., Blume J. L., Switalski L. M., Hook M. Identification of Porphyromonas gingivalis components that mediate its interactions with fibronectin. J Bacteriol. 1991 Jul;173(14):4263–4270. doi: 10.1128/jb.173.14.4263-4270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Dertzbaugh M. T., Halula M. C., Krah E. R., 3rd, Jones K. R. Genetic approaches to the study of oral microflora: a review. Crit Rev Oral Biol Med. 1990;1(3):207–227. doi: 10.1177/10454411900010030401. [DOI] [PubMed] [Google Scholar]
- Mayrand D., Grenier D. Biological activities of outer membrane vesicles. Can J Microbiol. 1989 Jun;35(6):607–613. doi: 10.1139/m89-097. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Mergenhagen S. E., Sandberg A. L., Chassy B. M., Brennan M. J., Yeung M. K., Donkersloot J. A., Cisar J. O. Molecular basis of bacterial adhesion in the oral cavity. Rev Infect Dis. 1987 Sep-Oct;9 (Suppl 5):S467–S474. doi: 10.1093/clinids/9.supplement_5.s467. [DOI] [PubMed] [Google Scholar]
- Ofek I., Zafriri D., Goldhar J., Eisenstein B. I. Inability of toxin inhibitors to neutralize enhanced toxicity caused by bacteria adherent to tissue culture cells. Infect Immun. 1990 Nov;58(11):3737–3742. doi: 10.1128/iai.58.11.3737-3742.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Gibbons R. J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978 Jan;19(1):254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanborg Edén C., Hagberg L., Hanson L. A., Hull S., Hull R., Jodal U., Leffler H., Lomberg H., Straube E. Bacterial adherence--a pathogenetic mechanism in urinary tract infections caused by Escherichia coli. Prog Allergy. 1983;33:175–188. [PubMed] [Google Scholar]
- Svanborg Edén C., Hausson S., Jodal U., Lidin-Janson G., Lincoln K., Linder H., Lomberg H., de Man P., Mårild S., Martinell J. Host-parasite interaction in the urinary tract. J Infect Dis. 1988 Mar;157(3):421–426. doi: 10.1093/infdis/157.3.421. [DOI] [PubMed] [Google Scholar]
- Takada H., Ogawa T., Yoshimura F., Otsuka K., Kokeguchi S., Kato K., Umemoto T., Kotani S. Immunobiological activities of a porin fraction isolated from Fusobacterium nucleatum ATCC 10953. Infect Immun. 1988 Apr;56(4):855–863. doi: 10.1128/iai.56.4.855-863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner A. C., Haffer C., Bratthall G. T., Visconti R. A., Socransky S. S. A study of the bacteria associated with advancing periodontitis in man. J Clin Periodontol. 1979 Oct;6(5):278–307. doi: 10.1111/j.1600-051x.1979.tb01931.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A., Holt S. C. Chemical and biological activities of a 64-kilodalton outer sheath protein from Treponema denticola strains. J Bacteriol. 1991 Nov;173(21):6935–6947. doi: 10.1128/jb.173.21.6935-6947.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A., Holt S. C. Interaction of Treponema denticola TD-4, GM-1, and MS25 with human gingival fibroblasts. Infect Immun. 1990 Jun;58(6):1720–1729. doi: 10.1128/iai.58.6.1720-1729.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. I., Eli I., Shenitzki B., Smorodinsky N. Identification of the rhamnose-sensitive adhesin of Capnocytophaga ochracea ATCC 33596. Arch Oral Biol. 1990;35 (Suppl):127S–130S. doi: 10.1016/0003-9969(90)90142-w. [DOI] [PubMed] [Google Scholar]
- Weiss E. I., London J., Kolenbrander P. E., Kagermeier A. S., Andersen R. N. Characterization of lectinlike surface components on Capnocytophaga ochracea ATCC 33596 that mediate coaggregation with gram-positive oral bacteria. Infect Immun. 1987 May;55(5):1198–1202. doi: 10.1128/iai.55.5.1198-1202.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitnack E., Beachey E. H. Antiopsonic activity of fibrinogen bound to M protein on the surface of group A streptococci. J Clin Invest. 1982 Apr;69(4):1042–1045. doi: 10.1172/JCI110508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon J. J., Reynolds H. S., Slots J. Black-pigmented Bacteroides spp. in the human oral cavity. Infect Immun. 1981 Apr;32(1):198–203. doi: 10.1128/iai.32.1.198-203.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]