Abstract
An unresolved question in cystic fibrosis (CF) research is how mutations of the CF transmembrane conductance regulator, a Cl ion channel, cause airway mucus obstruction leading to fatal lung disease. Recent evidence has linked the CF transmembrane conductance regulator mutation to the onset and persistence of Pseudomonas aeruginosa infection in the airways, and here we provide evidence directly linking P. aeruginosa infection to mucus overproduction. We show that P. aeruginosa lipopolysaccharide profoundly up-regulates transcription of the mucin gene MUC 2 in epithelial cells via inducible enhancer elements and that this effect is blocked by the tyrosine kinase inhibitors genistein and tyrphostin AG 126. These findings improve our understanding of CF pathogenesis and suggest that the attenuation of mucin production by lipopolysaccharide antagonists and tyrosine kinase inhibitors could reduce morbidity and mortality in this disease.
Keywords: MUC 2 gene, CFTR, protein tyrosine phosphorylation, endotoxin
Cystic fibrosis (CF) gene mutation causes dysfunction of the CF transmembrane conductance regulator (CFTR), a Cl ion channel. Ninety-five percent of the morbidity and mortality associated with this mutation arises from lung disease characterized by chronic infection and airway mucus obstruction (1). The link between the mutation and its lethal sequelae is unknown. Recently, there has been some insight from findings indicating that the CFTR mutation is linked to three abnormalities favoring the onset and persistence of Pseudomonas aeruginosa infection in the lung: (i) undersialylated cell surface glycolipids that act as P. aeruginosa-binding sites (2), (ii) impaired capacity for bronchial epithelial cells to clear P. aeruginosa by endocytosis (3), and (iii) decreased activity of bronchial bacteriolytic substances due to abnormal airway surface liquid (4). Clinically, the onset of P. aeruginosa infection in the CF lung presages airway mucus obstruction and an overall deterioration of lung function. How this occurs is unknown. Here we show that P. aeruginosa lipopolysaccharide (LPS), a molecule commonly known to stimulate host defense responses in hematopoietic cells, is a potent stimulus of mucin transcription in epithelial cells. Thus, once airway infection has occurred, P. aeruginosa LPS is an indwelling stimulus for exaggerated airway mucin synthesis. In the underhydrated CF airway lumen (5), it is not surprising that exaggerated mucin synthesis leads to airway mucus obstruction. We hypothesize that the pathogenesis of CF lung disease proceeds in two stages: (i) the induction of P. aeruginosa infection as a direct consequence of CFTR gene mutation, and, (ii) the overproduction of, and airway plugging by, mucin as a consequence of P. aeruginosa infection.
MATERIALS AND METHODS
Reagents.
P. aeruginosa LPS from serotype 10 was purchased from Sigma. LPS from PAO1 wild-type and PAO-pmm (algC) mutants was purified as described (6). Lipid A and genistein also were purchased from Sigma. Tyrphostin AG126 was purchased from Calbiochem.
Bacterial Strains and Culture Conditions.
The P. aeruginosa strains used in these studies were grown in M9 medium with aeration at 37°C to late log phase. The broth cultures were then centrifuged at 10,000 rpm for 50 min. The supernatants containing bacterial exoproducts were sterilized by passage through a 0.22-micron polymer filter (Corning) and then were kept at −80°C until used. Bacterial culture supernatants were added to epithelial cell culture medium at a 1:4 dilution ratio.
Cell Culture.
HM3 cells were maintained in DMEM. NCIH292 cells were maintained in RPMI 1640 medium. CFTE29O cells were obtained from D. Gruenert (University of California, San Francisco) and were maintained in Eagle’s minimal essential medium with Earle’s balanced salt solution medium. 16LU cells were maintained in DMEM/Ham’s F-12 medium; 10% fetal bovine serum was added to all of the media.
In Situ Hybridization Analysis.
The experiments were carried out as described (7) and are reviewed here in brief.
Tissue preparation.
Human CF bronchial tissue was obtained at lung transplantation from the recipients, and non-CF bronchial tissue was obtained from donors. For all experiments, segmental and subsegmental bronchi were used. Slices of bronchial rings (≈0.5 mm long) were prepared within 1 h after transplantation. These human bronchial tissues were rinsed in sterile PBS to remove secretions and were incubated in serum-free medium, a 1:1 mixture of DMEM and Ham’s F-12 medium supplemented with penicillin (105 units/liter), streptomycin (100 mg/ml), gentamicin (50 mg/ml), and amphotericin B (2.5 mg/ml). The bronchial explants from CF and non-CF individuals were treated with P. aeruginosa culture supernatant or vehicle for 6 h and then were fixed in 4% paraformaldehyde/0.1 M phosphate buffer for 4 h and cryoprotected in 30% sucrose/0.1 M phosphate buffer overnight at 4°C. The next day, samples were embedded in OCT compound and quickly frozen in liquid nitrogen-cooled Freon-22. The frozen tissue was sectioned (6 mm), placed on Superfrost Plus slides (Fisher Scientific), and quickly air dried. The sections were stored at −80°C until used.
RNA probes.
The human airway mucin 1 cDNA contained a tandem repeat unit of the mucin gene MUC 2. We confirmed (by Northern blot analysis) that the human airway mucin 1 cDNA recognized mRNA transcripts in the human bronchus before using it in in situ hybridization. [35S]UTP-labeled RNA transcripts were synthesized from the cDNA in linearized pBluescript plasmids using T7 and T3 polymerases to generate antisense and sense probes at concentrations of 2–5 × 105 cpm/ml. Frozen sections of human bronchus were air dried quickly, heated at 55°C for 10 min, fixed with 4% paraformaldehyde in PBS for 10 min, washed with 2× standard saline citrate (SSC; 0.3 M NaCl/0.03 M sodium citrate, pH 7.0), immersed in 0.1 M triethanolamine HCl (pH 7.5) containing 0.25% acetic anhydride for 10 min, rinsed with 2× SSC, dehydrated with ethanol, and air dried. An RNA probe was applied in a hybridization mixture containing deionized formamide (50%), dextran sulfate (10%), tRNA (0.5 mg/ml), Ficoll 400 [0.02% (wt/vol)], salmon sperm DNA (1 mg/ml), polyvinylpyrrolidone [0.02% (wt/vol)], 10 mM DTT, 0.3 M NaCl, 0.5 mM EDTA, 10 mM Tris·HCl, and 10 mM NaPO4 (pH 6.8). The mixture was heated at 70°C for 15 min and chilled on ice. Fresh DTT was added to achieve a concentration of 20 mM. Then, 100 μl of the mixture was applied to each section, and parafilm coverslips were applied. Hybridization was carried out in humid chambers overnight at 55°C. Coverslips were removed in 5× SSC/10 mM DTT at 55°C. Sections were washed three times in wash buffer (2× SSC/1 mM EDTA/10 mM 2-mercaptoethanol) for 5 min at room temperature. Subsequently, they were treated with 20 mg/ml of RNase A in 500 mM NaCl/10 mM Tris, pH 8.0, for 30 min at room temperature. This was followed by two 5-min changes of wash buffer and a high stringency wash in 4 liters of a wash solution containing 0.1× SSC/1 mM EDTA/10 mM 2-mercaptoethanol (2 h at 55°C). Slides were then washed for 5 min at room temperature in 0.5× SSC without 2-mercaptoethanol or EDTA. Finally, sections were dehydrated with ethanol and air dried. The slides were exposed to Ilford K5D emulsion and stored in the dark at 4°C until developed after 3–10 days of exposure.
RNase Protection Assay (RPA).
RPA experiments were carried out using an RNA probe containing an MUC 2-specific sequence as described (8).
Cloning and Sequencing of the 5′ Flanking Region of the Human MUC 2 Gene, Plasmid Construction, Transfection, and Luciferase Assay.
The 5′ flanking region of the human MUC 2 gene was cloned by screening a human placental lFIXII genomic library using the 5′ region of human MUC 2 cDNA as the probe (9). The 5′ flanking region was sequenced by dideoxy sequencing. Deletional mutants of the 5′ flanking region DNA were obtained by combining restriction digestion of the upstream region of the gene and PCR amplification. The restriction DNA fragments or PCR-amplified fragments were ligated into a luciferase reporter gene. All junctions and identifications of the DNA sequences in the chimeric constructs were confirmed by DNA sequencing. The expression plasmid pREP4.7kbCFTR was obtained from D. Gruenert. Transfection was performed by a standard electroporation method as described (10). P. aeruginosa culture supernatant, LPS, and lipid A were added to the transfected cells 42 h after transfection. After 6 h, the cells were harvested for luciferase assay. All transfections were carried out in triplicate. Luciferase activity was normalized with respect to β-galactosidase activity.
RESULTS AND DISCUSSION
The Mucin MUC 2 mRNA Level Was Highly Elevated in CF Airways.
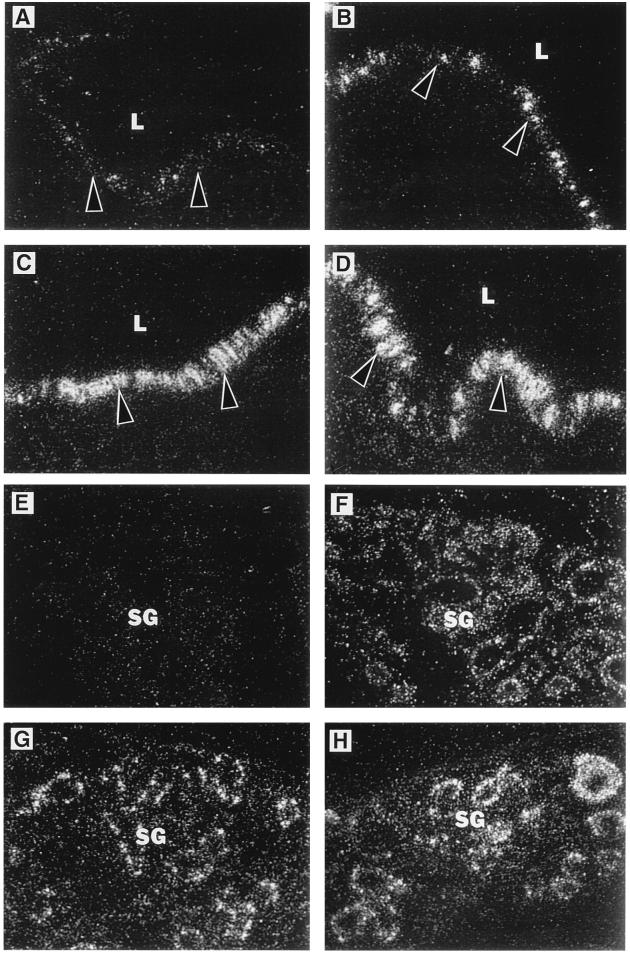
As a first step in evaluating the possibility that P. aeruginosa up-regulates mucin synthesis in CF airways, we monitored airway mucin mRNA levels in CF patients vs. controls. Using a probe for the human MUC 2 mucin gene (9, 11), we showed that a greater proportion of cells expressed MUC 2 in CF than in non-CF airways and that the level of expression per cell was higher (Fig. 1 A, C, E, and G). The results shown in Fig. 1 are typical of four out of four CF and four out of four non-CF individual cases and suggest that factor(s) present in the CF airway up-regulate MUC 2 mRNA. All of the CF tissues we examined were infected with P. aeruginosa, so this Gram-negative bacterium that chronically colonizes the airways of ≈80% of CF patients (12) is a potential source of such factors.
Figure 1.
In situ hybridization analysis of MUC 2 mRNA expression in non-CF and CF human bronchial explants. In situ hybridization was performed with the human airway mucin 1 anti-sense probe recognizing human MUC 2 mRNA as described (7). MUC 2 mRNA expression is shown in vehicle control-treated and P. aeruginosa-treated bronchial explant surface epithelia from non-CF (A and B) and CF (C and D) individuals and in vehicle control-treated and P. aeruginosa-treated bronchial explant submucosal glands from non-CF (E and F) and CF (G and H) individuals. Arrowheads in A–D indicate the position of the epithelial basement membrane. In these experiments, both non-CF and CF bronchial explants were exposed for 6 h to P. aeruginosa culture supernatants. Similar results were observed in bronchial explants from four CF and four non-CF individuals treated with P. aeruginosa strain PAO1 or PA103. L, airway lumen; SG, submucosal glands.
P. aeruginosa Up-Regulated MUC 2 mRNA in Airways.
We directly tested the hypothesis that P. aeruginosa-associated factors up-regulate mucin mRNA by exposing human bronchial explants to P. aeruginosa (strains PAO1 and PA103) culture supernatant or vehicle control (6 h) and by performing MUC 2 in situ hybridization. We found that P. aeruginosa culture supernatant greatly increased MUC 2 expression in both the surface epithelium and submucosal glands of the explants (Fig. 1), confirming that P. aeruginosa-associated factors do up-regulate MUC 2 mRNA. The constitutive expression of MUC 2 mRNA in CF bronchial explants was high relative to that in non-CF bronchial explants, and the CF explants showed relatively small increments in MUC 2 expression in response to exogenous P. aeruginosa. This may indicate that MUC 2 expression had been stimulated nearly maximally by endogenous factors in the CF explants.
P. aeruginosa Directly Up-Regulated MUC 2 mRNA in Epithelial Cells.
In explant tissue, MUC 2 up-regulation could have resulted from either a direct effect of bacterial products on epithelial cells or an indirect effect mediated by resident inflammatory cells. To test the hypothesis that the effect was direct, we applied P. aeruginosa culture supernatant to cultured epithelial cells and measured MUC 2 mRNA by RNase protection assay. Because we were interested in the potential generality of the effect, we assayed a variety of epithelial mucin-expressing cell lines. Results from NCIH 292 (human airway epithelial) and HM3 (human colon epithelial) cells are shown in Fig. 2. The P. aeruginosa culture supernatant increased MUC 2 mRNA levels in mucin-expressing epithelial cell lines but not in the nonmucin-expressing human adult lung fibroblast cell line 16 LU. These findings indicate that P. aeruginosa products act directly and selectively on mucin-expressing cells to up-regulate MUC 2 mRNA.
Figure 2.
RPA analysis of MUC 2 mRNA expression in human MUC 2-expressing epithelial cell lines. Up-regulation of MUC 2 mRNA by P. aeruginosa culture supernatants occurred in both HM3 (human colon epithelial) and NCIH292 (human airway epithelial) cells. Cells were treated with P. aeruginosa culture supernatants (PA) or vehicle (Con) for 6 h before cell lysis and RNA extraction. The results are typical of four separate experiments for each cell line. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Transcriptional Activation of MUC 2 by P. aeruginosa.
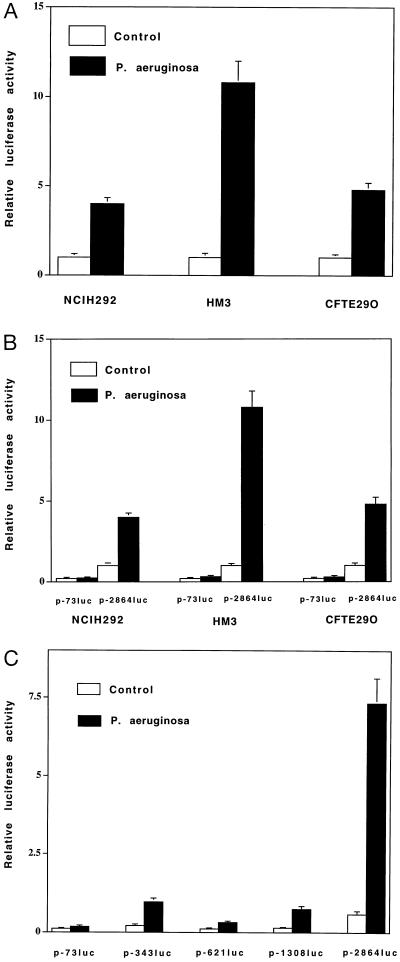
We next performed experiments to determine whether transcriptional control mechanisms were involved in the observed MUC 2 up-regulation. We transfected the epithelial cell lines mentioned above plus CFTE29O (a human airway CF epithelial cell line homozygous for ΔF508 CFTR) with an expression vector containing 2.8 kb of the MUC 2 5′ flanking region fused to a luciferase reporter gene. When we exposed the transfected cells to P. aeruginosa culture supernatant (6 h), luciferase activity increased severalfold (Fig. 3A). This suggests the presence of P. aeruginosa response elements that mediate the steady-state increases in MUC 2 mRNA described above. To localize these elements, we transfected the same three epithelial cell lines with deletion mutants of the 2.8-kb MUC 2 luciferase reporter gene and tested transcriptional activity in the presence and absence of P. aeruginosa culture supernatant. We detected P. aeruginosa response elements between −2864 and −73 bp in all three cell lines (Fig. 3B). To further define these, we analyzed a larger panel of deletion mutants in HM3, the cell line yielding the strongest P. aeruginosa response. This revealed response elements in the regions −343 to −73 bp and −2864 to −1308 bp (Fig. 3C).
Figure 3.
Up-regulation of MUC 2 transcriptional activity by P. aeruginosa. (A) A 2.8-kb DNA fragment of the 5′ flanking region of the human MUC 2 gene cloned into a luciferase reporter gene (p-2864luc) was transfected into NCIH292, HM3, and CFTE29O cells. Luciferase activity was then assessed in P. aeruginosa-treated and nontreated cells. Induction by P. aeruginosa was detected in all cell lines. (B) Comparison of the P. aeruginosa responsiveness of p-2864luc and p-73luc in NCIH292, HM3, and CFTE29O cells. Response elements reside between −2864 and −73 bp. (C) Comparison of the P. aeruginosa responsiveness of p-73luc, p-343luc, p-621luc, p-1308luc, and p-2864luc in HM3 cells. Transfected cells were treated with either P. aeruginosa culture supernatants or vehicle for 6 h before cell lysis. All transfections were carried out in triplicate. Values represent means ± SD (n = 5). Luciferase activity was normalized with respect to β-galactosidase activity.
Up-Regulation of MUC 2 by P. aeruginosa LPS.
We next addressed the question of which components of P. aeruginosa are responsible for MUC 2 up-regulation. Via the screening of P. aeruginosa mutants, it has been found that diverse products of this organism can affect host gene expression (13). We assessed the role of some of these in MUC 2 up-regulation by testing the potency of culture supernatant from bacterial mutants PAOR1 (deficient in production of the P. aeruginosa autoinducer, elastase, alkaline protease, and neuraminidase) (14–16), AK1152 (deficient in production of pilin and flagellin) (17), and PAO/NP (deficient in production of pilin) (13). That these media were as potent as that of wild type essentially excluded a role for the mutant gene products (data not shown).
To obtain additional information regarding the molecular source of MUC 2 stimulatory activity, we analyzed the activity in P. aeruginosa culture supernatant with respect to molecular size as well as to heat and enzyme sensitivity. Fractionation of wild-type culture supernatant using Centricon filters (Amicon) with graded molecular weight cut-offs revealed stimulatory activity across a wide range of molecular sizes. Further analysis showed that the activity was resistant to heat, proteolysis, and DNA digestion (data not shown). Although not diagnostic of any specific molecule, these physicochemical findings are inconsistent with properties of protein and DNA but are consistent with properties of polysaccharides and lipids.
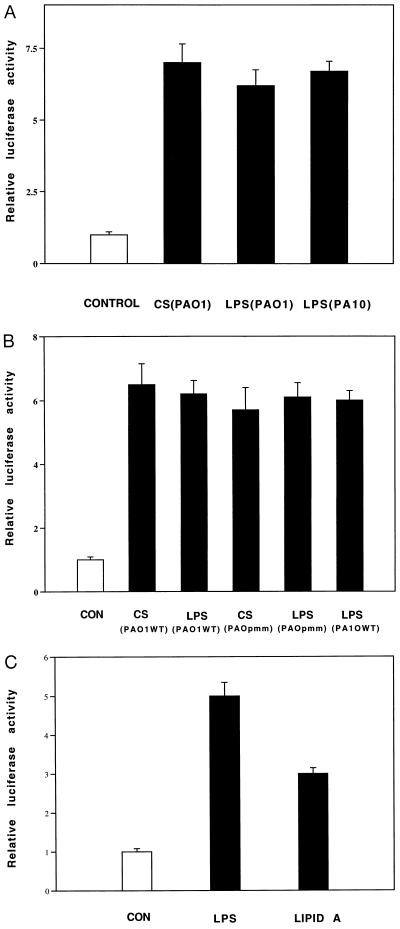
Preeminent among bacterial polysaccharides is LPS, the major outer membrane component of Gram-negative bacteria and a potent activator of host defense responses (18). Based on the results of the physicochemical assays described above, we hypothesized that LPS was involved in MUC 2 up-regulation. Indeed, when we tested the effects of LPS (obtained commercially or purified from P. aeruginosa) on steady-state levels of MUC 2 mRNA (data not shown) and on MUC 2 transcriptional activity in cells transfected with the MUC 2–luciferase expression vector, we observed a strong response (Fig. 4A). Taken together, our results indicate that LPS is a major factor mediating P. aeruginosa up-regulation of MUC 2 transcription.
Figure 4.
Effect of P. aeruginosa LPS on MUC 2 transcriptional activity. HM3 cells were transfected with p-2864luc. (A) After 42 h, the cells were exposed to culture supernatants (CS) and LPS (5 μg/ml) purified from PAO1 and PA10 (serotype 10) (Sigma). (B) As in A, the cells were exposed to culture supernatants or purified LPS from PAO1 (wild-type) or PAO-pmm (algC) mutants. (C) The cells were exposed to LPS (5 μg/ml) (P. aeruginosa serotype 10) and E. coli lipid A (5 μg/ml) (Sigma). After 6 h, the cells were harvested for luciferase activity measurement. All transfections were carried out in triplicate. Values represent means ± SD (n = 4). Luciferase activity was normalized with respect to β-galactosidase.
Structurally, LPS consists of a variable polysaccharide domain covalently linked to an invariable, diglucosamine-based, acylated phospholipid, lipid A. Although many biological effects of LPS are mediated by lipid A and the core oligosaccharide portion of the molecule, others require a complete LPS complex (19, 20). To examine this with respect to mucin up-regulation, we obtained culture supernatant from a P. aeruginosa mutant (PAO-pmm) deficient in synthesis of the LPS variable polysaccharide domain and compared its activity to that of wild type (6). The two supernatants (and LPS purified from each of them) were equipotent (Fig. 4B), suggesting that lipid A and/or its linked core oligosaccharide is sufficient to induce LPS-mediated MUC 2 transcriptional up-regulation. An experiment showing that lipid A (purified from Escherichia coli) could essentially mimic the LPS effect (Fig. 4C) indicated that lipid A was a key factor in the MUC 2 stimulation and that lipid A from diverse bacterial species was potent in this respect.
Tyrosine Kinase Inhibitors Abolished MUC 2 Induction by P. aeruginosa.
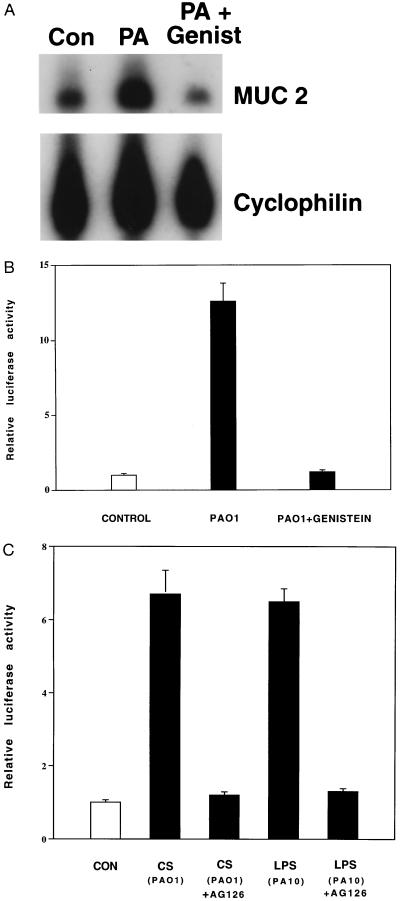
Having identified LPS as a bacterial “trigger” for MUC 2 transcriptional up-regulation and having localized P. aeruginosa-inducible elements of the MUC 2 5′ flanking region directly mediating this effect, we next sought information regarding the intracellular signaling pathway linking these elements. In experiments aimed at assessing the role of protein kinase activity, we found that preincubation of cells with the protein tyrosine kinase inhibitor genistein for 2 h before P. aeruginosa exposure abolished increases in MUC 2 steady-state mRNA and transcriptional activity (Fig. 5 A and B). This suggested that protein tyrosine phosphorylation was required for the response. Preincubation of cells with the more selective tyrosine kinase inhibitor tyrphostin AG 126 also abolished P. aeruginosa- and LPS-induced up-regulation of MUC 2 transcription (Fig. 5C), confirming the requirement for tyrosine phosphorylation. Currently, the best known substrate for tyrphostin AG 126 is the LPS-activated tyrosine kinase p42MAPK (21). Taken together with our data implicating LPS in MUC 2 up-regulation, the tyrphostin AG 126 data suggest that the LPS-activated tyrosine kinase p42MAPK is an integral part of the signal transduction pathway up-regulating MUC 2 transcription.
Figure 5.
Inhibition of P. aeruginosa-induced MUC 2 up-regulation by tyrosine kinase inhibitors. (A) HM3 cells were or were not pretreated with genistein (Genist) (100 μg/ml) (Sigma) for 2 h and then were exposed to P. aeruginosa PAO1 culture supernatants (PA) or vehicle (Con) for 6 h before RNA extraction and RPA analysis. The results are typical of four separate experiments. (B) HM3 cells were transfected with p-2864luc. After 40 h, the cells were pretreated with genistein (100 μg/ml) for 2 h and then were exposed to P. aeruginosa culture supernatants (PAO1) for 6 h before harvesting for luciferase analysis. (C) Forty hours after being transfected with p-2864luc, HM3 cells were pretreated with tyrphostin AG 126 (25 μM) (Calbiochem) for 3 h and then were exposed to PAO1 culture supernatant (CS) or LPS from PA10 (serotype 10) (5 μg/ml) for 6 h before harvesting. Luciferase activity was measured as described above. All transfections in B and C were carried out in triplicate. Values represent means ± SD (n = 4). Luciferase activity was normalized with respect to β-galactosidase.
CF Epithelial Cells Were Not Hypersusceptible to P. aeruginosa with Respect to MUC 2 Induction.
Previously reported data revealed that CFTR mutant epithelial cells are hypersusceptible to some biological effects of P. aeruginosa (13). To examine the possibility that this is also true of MUC 2 transcriptional up-regulation, we compared the magnitude of P. aeruginosa responses in CFTR mutant cells that had vs. those that had not been complemented by transfection with wild-type CFTR expression plasmid. We found no evidence for hypersusceptibility (Fig. 6). Although these data might be interpreted to mean that lung disease such as that seen in CF patients could occur in non-CF individuals with P. aeruginosa lung infections, this is not the case. Militating against the possibility are the facts that (i) non-CF individuals virtually never contract P. aeruginosa lung infection of the severity seen in CF patients, and infection in non-CF lungs does not localize to airways, and (ii) P. aeruginosa-stimulated mucin is, in CF lungs, dissolved in an airway lining fluid that is underhydrated (5) with respect to that in normal lungs. These two factors likely increase airway mucus plugging in CF vs. non-CF airways.
Figure 6.
Effect of P. aeruginosa culture supernatant on MUC 2 transcriptional activity in CFTE 29O ΔF508 cells cotransfected with p-2864luc ± the CFTR wild-type expression plasmid pREP4.7kbCFTR. Forty-two hours after transfection, PAO1 culture supernatant (CS) was added to cells; 6 h later, the cells were harvested for luciferase activity measurement. All transfections were carried out in triplicate. Values represent means ± SD (n = 4). Luciferase activity was normalized with respect to β-galactosidase.
From what we know now about the CF lung, it is evident that pathogenesis does not emanate directly from the CFTR mutation by a linear sequence of events. Instead, by interfering with a variety of physiological mechanisms (2–4), the mutation engenders chronic bacterial infection. Infection, by the mechanisms outlined above, triggers mucin overproduction. The latter, in concert with dehydration (5) also engendered by the CFTR mutation, leads to airway mucus obstruction and lung failure. The data shown here for the first time reveal the capacity for P. aeruginosa to directly stimulate mucin production and thereby offer a rational explanation for why mucus plugging and deterioration of lung function occur relentlessly subsequent to the onset of infection (12). These data suggest that, by directly up-regulating mucin transcription in airway epithelial cells, P. aeruginosa may greatly increase the mucin load discharged into the CF airway lumen. This is especially true in light of the fact that we have recently observed profound up-regulation of the human MUC 5 as well as the MUC 2 gene by P. aeruginosa (8).
Mucus not only impairs airflow and O2/CO2 exchange but, by physically enmeshing bacteria (13) as well as providing nutrition and protection from bactericidal agents, also promotes chronic infection. Thus, the up-regulation of mucin transcription by P. aeruginosa is at the crux of a vicious cycle of chronic infection and mucus obstruction that kills CF patients. The inhibition of this up-regulation by LPS antagonists and/or tyrosine kinase inhibitors would constitute a new therapeutic strategy to reduce morbidity and mortality in CF patients (22, 23).
Acknowledgments
We thank D. Gruenert for providing the CFTE29O cells and CFTR expression plasmid pREP4.7kbCFTR, M. Lim and G. Glugoski for performing some of the in situ hybridization studies, C. Young for performing some of the RPA experiments, I. Ueki and J. Lausier for providing some of the human bronchial explant material, and S. Fleiszig for helpful discussion. This work was supported by National Institutes of Health grants to C.B. (HL 43762) and to C.B. and J.A.N. (HL 24136) and by a National Institute of Diabetes and Digestive and Kidney Diseases–University of California, San Francisco, Gene Therapy Core Center grant.
Footnotes
Abbreviations: CF, cystic fibrosis; CFTR, CF transmembrane conductance regulator; LPS, lipopolysaccharide; MUC 2, mucin MUC 2 gene; RPA, RNase protection assay.
Data deposition: The sequence reported in this paper has been deposited in the GenBank/European Molecular Biology Laboratory data base (accession no. U67167U67167).
References
- 1.Collins F S, Wilson J M. Nature (London) 1992;358:708–709. doi: 10.1038/358708a0. [DOI] [PubMed] [Google Scholar]
- 2.Saiman L, Prince A. J Clin Invest. 1993;92:1875–1880. doi: 10.1172/JCI116779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pier G B, Grout M, Zaidi T S, Olsen J C, Johnson L G, Yankaskas J R, Goldberg J B. Science. 1996;271:64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 5.Matthews L. Am Rev Respir Dis. 1963;88:199–207. doi: 10.1164/arrd.1963.88.2.199. [DOI] [PubMed] [Google Scholar]
- 6.Coyne M J, Kristin J R, Russell S, Coyle C L, Goldberg J B. J Bacteriol. 1994;176:3500–3507. doi: 10.1128/jb.176.12.3500-3507.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dohrman A, Basbaum C B. Exp Lung Res. 1994;20:367–382. doi: 10.3109/01902149409064393. [DOI] [PubMed] [Google Scholar]
- 8.Dohrman A, Basbaum C B. Respir Crit Care Med. 1995;151:A160. (abstr.). [Google Scholar]
- 9.Gum J R, Hicks J W, Toribara N W, Siddiki B, Kim Y S. J Biol Chem. 1994;269:2440–2446. [PubMed] [Google Scholar]
- 10.Hiro K, Takeuchi K, Ohmori H, Li J D, Gallup M, Basbaum C B. J Cell Biochem. 1996;61:350–362. doi: 10.1002/(SICI)1097-4644(19960601)61:3%3C350::AID-JCB3%3E3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 11.Gum J R, Byrd J C, Hicks J W, Toribara N W, Lamport D T A, Kim Y S. J Biol Chem. 1989;264:6480–6487. [PubMed] [Google Scholar]
- 12.Boat T, Boucher R. In: Textbook of Respiratory Medicine. Murray J, Nadel J, editors. Philadelphia: Saunders; 1994. pp. 1418–1450. [Google Scholar]
- 13.DiMango E, Zar H J, Bryan R, Prince A. J Clin Invest. 1995;96:2204–2210. doi: 10.1172/JCI118275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gambello M J, Iglewski B H. J Bacteriol. 1991;173:3000–3009. doi: 10.1128/jb.173.9.3000-3009.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearson J P, Gray K M, Passador L, Tucker K D, Eberhard A, Iglewski B H, Greenberg E P. Proc Natl Acad Sci USA. 1994;91:197–201. doi: 10.1073/pnas.91.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cacalano G, Kays M, Saiman L, Prince A. J Clin Invest. 1992;89:1866–1874. doi: 10.1172/JCI115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drake D, Montie T C. J Gen Microbiol. 1988;134:43–52. doi: 10.1099/00221287-134-1-43. [DOI] [PubMed] [Google Scholar]
- 18.Weinstein S L, Sanghera G S, Lemke K, DeFranco A L, Pelech S L. J Biol Chem. 1992;267:14955–14962. [PubMed] [Google Scholar]
- 19.Ractz C. Annu Rev Biochem. 1990;59:129–141. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- 20.Tang H B, DiMango E, Bryan R, Gambello M, Iglewski B H, Goldberg J B, Prince A. Infect Immun. 1996;64:37–43. doi: 10.1128/iai.64.1.37-43.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novogrodsky A, Vanichkin A, Patya M, Gazit A, Osherov N, Levitzki A. Science. 1996;264:1319–1322. doi: 10.1126/science.8191285. [DOI] [PubMed] [Google Scholar]
- 22.Lynn W A, Golenboch D T. Immunol Today. 1992;13:271–276. doi: 10.1016/0167-5699(92)90009-V. [DOI] [PubMed] [Google Scholar]
- 23.Levitzki A, Gazit A. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]