Abstract
On the histone H3 tail, Lys 9 and Lys 27 are both methylation sites associated with epigenetic repression, and reside within a highly related sequence motif ARKS. Here we show that the chromodomain proteins Polycomb (Pc) and HP1 (heterochromatin protein 1) are highly discriminatory for binding to these sites in vivo and in vitro. In Drosophila S2 cells, and on polytene chromosomes, methyl-Lys 27 and Pc are both excluded from areas that are enriched in methyl-Lys 9 and HP1. Swapping of the chromodomain regions of Pc and HP1 is sufficient for switching the nuclear localization patterns of these factors, indicating a role for their chromodomains in both target site binding and discrimination. To better understand the molecular basis for the selection of methyl-lysine binding sites, we solved the 1.8 Å structure of the Pc chromodomain in complex with a H3 peptide bearing trimethyl-Lys 27, and compared it with our previously determined structure of the HP1 chromodomain in complex with a H3 peptide bearing trimethyl-Lys 9. The Pc chromodomain distinguishes its methylation target on the H3 tail via an extended recognition groove that binds five additional residues preceding the ARKS motif.
Keywords: Polycomb, HP1, heterochromatin, histone H3, lysine methylation, epigenetic
Chromatin structure contains the molecular imprint underlying cell memory and epigenetic inheritance, and emerging evidence suggests that covalent modifications of histones play a major role as carriers of epigenetic information (Felsenfeld and Groudine 2003). Histone modifications can be highly reversible, such as histone acetylation, or more stable, such as histone (lysine) methylation (Zhang and Reinberg 2001; Lachner and Jenuwein 2002). Thus, a wide range of chromatin-based regulatory options is available. These include dynamic marks permitting rapid changes in gene expression in response to physiological and environmental stimuli as well as more permanent indexing systems required for the passage of heritable patterns of epigenetic information from one cell generation to the next (Fischle et al. 2003). The identification of enzyme systems responsible for the steady-state balance of posttranslational histone modifications, together with the discovery of binding modules that “read” covalent marks on histones, have been key for our present understanding of gene regulation in the context of the chromatin polymer.
Bromodomains have been the first modules implicated in the read-out of histone marks. They show affinity for acetylated lysines in histone and nonhistone proteins (for review, see Zeng and Zhou 2002), and local recruitment of bromodomain factors to certain regions of chromatin might function in mediating acetyl-histone-encoded antisilencing (Ladurner et al. 2003). In contrast, a second conserved module found in a variety of chromosomal proteins, the chromodomain, has been implicated in binding to methylated lysines on the histone tails (Bannister et al. 2001; Jacobs et al. 2001; Lachner et al. 2001). Indeed, recently a biochemical pathway of gene repression by heterochromatin assembly, involving methylation of Lys 9 of H3 by SET-type histone methyltransferases (HMTs), and the read-out of this methylation mark by the chromodomain of HP1 (heterochromatin protein 1), has been established (Schotta et al. 2002; Snowden et al. 2002; Cheutin et al. 2003). Furthermore, the three-dimensional structure of HP1 revealed that three “caging” aromatic residues are necessary for methyl-Lys 9 binding of this domain (Jacobs and Khorasanizadeh 2002; Nielsen et al. 2002). Many, but not all chromodomain proteins identified to date, contain such aromatic residues at conserved positions (Jacobs and Khorasanizadeh 2002). However, it is unclear if these additional chromodomains indeed bind to methylated lysines and if they have preferences for specific methyl marks on histones or other proteins.
HP1 is a conserved chromosomal protein that participates in chromatin packaging and gene silencing (for review, see Eissenberg and Elgin 2000). Loss of HP1 leads to lethality in Drosophila and correlates with metastasis in human breast cancer cells (Kirschmann et al. 2000). Factors of the Polycomb group (PcG) of proteins are part of a widely conserved cell memory system that controls repressed transcriptional states of many loci in the genome, including developmentally and cell-cycle-regulated genes. The PcG proteins were first identified in the fruit fly Drosophila melanogaster, but homologs have been identified in all higher organisms (for review, see Jacobs and van Lohuizen 2002; Orlando 2003). These proteins are required for long-term transcriptional silencing of the Drosophila homeotic genes, which are required for proper embryonic development. In mammalian systems, PcG repressors are implicated in hematopoesis, X inactivation, B-cell development and control of cell proliferation. Mutations in PcG proteins have also been recently linked to cancers of the immune system and prostate (for review, see Simon 2003). However, the mechanisms by which PcG proteins repress transcription are largely unknown. In Drosophila, Polycomb response elements (PRE) have been identified and implicated for PcG targeting. However, such DNA elements have been elusive in mammalian systems. The PcG proteins are known to be present in multiprotein complexes. The best-characterized complex is Polycomb-repressive complex (PRC) 1, which contains the Polycomb, Polycomb-like, Polyhomeotic, Posterior Sex Combs, and Sex Combs on Midleg proteins, among others (for review, see Simon and Tamkun 2002). The gene-repressing activity of the PRC1 complex has been suggested to involve activities that render target regions resistant to remodeling by chromatin-remodeling complexes (Shao et al. 1999).
Recent reports have provided breakthrough evidence that a second PcG complex, the Esc-E(z) complex, contains HMT activity. This activity is dependent on the E(z) (enhancer of zeste) SET domain protein, and the complex has been reported to preferentially methylate Lys 27 and Lys 9 on the H3 tail. Furthermore, it has been suggested that the PcG chromodomain protein Polycomb (Pc) could act as a binding module for methyl-Lys 9 and methyl-Lys 27 in the H3 tail, thereby critically mediating the targeting of PcG complexes to different sites of the epigenome (Cao et al. 2002; Czermin et al. 2002; Kuzmichev et al. 2002; Muller et al. 2002). Interestingly, when the chromodomain of HP1 was substituted by the chromodomain of Pc, the chimeric HP1Pc was recruited to PcG-binding sites on polytene chromosomes (Platero et al. 1995). This implies that either the chromodomain is sufficient to recognize and be recruited to different methyl marks on the H3 tail, or the Pc chromodomain specifies critical interactions with other PcG components that mediate recruitment to PcG sites. So far, the extent by which Pc binds preferentially to either repressive methyl-Lys 9 or methyl-Lys 27 marks on the H3 tail is unclear. Furthermore, the role of chromodomain binding to methyl marks for recruitment to different target sites has not been established. Equally unclear is whether any potential discrimination of Pc or HP1 for one lysine over another is an intrinsic structural feature of their chromodomains.
Results
Specific binding of Pc to methyl-Lys 27
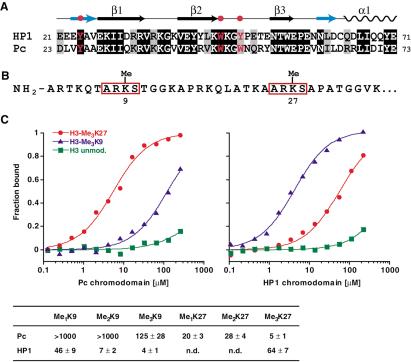
The chromodomains of Pc and HP1 are highly conserved (54% identity in protein sequence; see Fig. 1A). Moreover, the Pc chromodomain contains three “caging” aromatic residues that were shown in HP1 to be necessary for methyl-Lys 9 binding (Jacobs and Khorasanizadeh 2002). The amino acid sequences immediately surrounding Lys 9 and Lys 27 in the H3 tail are very similar as shown in Figure 1B and, in particular, share a consensus sequence ARKS. However, the residues flanking this consensus motif are unrelated. To determine the extent by which Pc and HP1 are able to discriminate between the Lys 9 and Lys 27 methylation sites, we used a set of synthetic methylated H3 peptides to measure the relative binding affinities of their chromodomains to these histone H3 tail segments. Fluorescence polarization measurements indicated a clear preference of the chromodomain of Pc for the trimethylated Lys 27 site. Specifically, the dissociation constant for the trimethyl-Lys 27 peptide was 5 μM, and that for the trimethyl-Lys 9 peptide was 125 μM (Fig. 1C). In contrast, the HP1 chromodomain bound to the trimethyl-Lys 9 peptide with an affinity of 4 μM and to the trimethyl-Lys 27 peptide with an affinity of 64 μM (Fig. 1C; Jacobs and Khorasanizadeh 2002). These data indicate that both protein modules discriminate methyl marks effectively, with Pc showing a 25-fold selectivity and HP1 showing a 16-fold selectivity for the cognate versus noncognate target sequences.
Figure 1.
Preferential binding of Pc and HP1 chromodomains to different methyl-lysines on the histone H3 tail. (A) Sequence alignment of the chromodomains of HP1 and Pc. Three conserved aromatic residues forming an aromatic cage for methyl-lysine recognition in HP1 are highlighted. Secondary structure elements of the HP1 chromodomain fold are illustrated on top. (B) Sequence of the N terminus (residues 1-36) of histone H3. The “neighborhoods” of the Lys 9 and Lys 27 methylation sites are very similar; identical sequence stretches surrounding both sites are boxed. (C) Binding of the chromodomains of Pc (residues 1-98) and HP1 (residues 17-76) to different methylated and unmodified H3 peptides in fluorescence polarization assays. KD (μM) values are listed in the bottom. Note that neither chromodomain interacts with the unmodified H3 tail.
We also investigated to what extent the degree of methylation affected target selection. Binding of Pc to a peptide with mono- or dimethylated Lys 27 peptides was about five times weaker than binding to the trimethylated Lys 27 peptide, but still much stronger than binding to the trimethylated Lys 9 peptide (Fig. 1C). Furthermore, no significant interactions between the Pc chromodomain and mono- or dimethylated Lys 9 peptides were observed. HP1 binding to dimethyl- and monomethyl-Lys 9 was weakened 2-fold and 15-fold, respectively. These results indicate that the degree of methylation does affect the binding of both chromodomains to their target sites and that the trimethyl-lysine is the preferred level of modification for both proteins in vitro. In the context of trimethyl-lysine, each protein shows clear discrimination for its cognate site, likely because of differences in sequence context of the trimethyl-Lys in H3 as well as the binding groove of the chromodomain (see below).
Colocalization of Pc and H3 Lys 27 methylation on polytene chromosomes
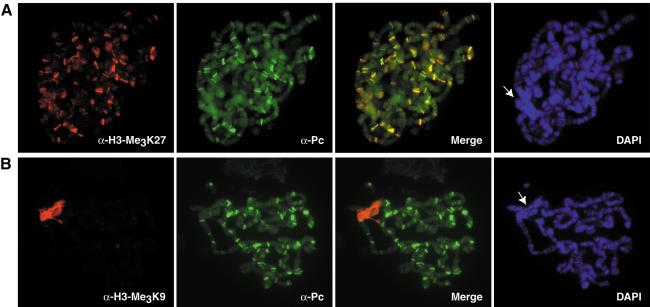
Using immunofluorescence staining, we have previously shown that the HP1 protein is localized almost exclusively to the chromocentric heterochromatin of Drosophila salivary gland polytene chromosomes, a chromosomal domain highly enriched in H3 Lys 9 methylation (Jacobs et al. 2001). To investigate the specific localization pattern of the Pc protein and to correlate its distribution with chromosomal regions of H3 Lys 9 and H3 Lys 27 methylation, we performed similar immunostaining experiments using newly developed anti-H3-Me3K27-specific antibodies in combination with antibodies specific for Pc. As shown in Figure 2A, the anti-H3-Me3K27-specific antibodies recognized many bands on the arms of polytene chromosomes. A weaker immunofluorescence signal for this modification was detected around the chromocentric regions. Whether the lower staining in this area represents a low level of H3 Lys 27 trimethylation at the chromocenter or might be caused by slight cross-reactivity of the antibodies with the H3 Lys 9 methyl mark is unknown (data not shown; see Materials and Methods). Importantly, the bands labeled by the anti-H3-Me3K27-specific antibodies were highly correlated with bands detected by anti-Pc-specific antibodies. Indeed, >90% of bands labeled by these antibodies showed a clear overlap (Fig. 2A, merged image). In contrast, antibodies specific for H3-Me3K9 only labeled the chromocenter, but did not stain regions overlapping with the anti-Pc-specific antibodies (Fig. 2B). Similar results were obtained using anti-H3-Me2K9-specific antibodies (data not shown). The colocalization of H3 Lys 27 trimethylation with Pc complex proteins was independently verified by double labeling experiments using an anti-Psc (posterior sex comb)-specific monoclonal antibody in combination with the anti-H3-Me3K27-specific polyclonal antibodies (data not shown). The immunostaining experiments are consistent with recruitment of Pc to regions of H3 Lys 27 trimethylation but not to regions of H3 Lys 9 trimethylation. This interpretation is in excellent agreement with the observed binding preferences of the chromodomains of HP1 and Pc for methylated H3 tails in vitro.
Figure 2.
Colocalization of Pc with H3 Lys 27 trimethylation, but not H3 Lys 9 trimethylation on polytene chromosomes. (A) Immunostaining of salivary gland polytene chromosomes with anti-H3-Me3K27-specific antibodies and anti-Pc antibodies. Many bands on the arms of the polytene chromosomes are labeled by both antibodies (as shown by yellow bands in the merged image), indicating colocalization of the Pc protein with sites of H3 Lys 27 trimethylation. It is not clear whether the weaker signal of H3 Lys 27 trimethylation occasionally observed around the chromocentric regions represents a true accumulation of this modification or might be caused by slight cross-reactivity of the antibodies with the H3 Lys 9 methyl mark. DNA was stained with DAPI, and the arrow points to the chromocenter. (B) Double labeling with anti-H3-Me3K9-specific antibodies and anti-Pc-specific antibodies. The anti-H3-Me3K9-specific antibodies stain mainly the chromocenter (denoted by an arrow in the DAPI staining), but not the many bands where the Pc protein is localized.
Different subnuclear localizations of Pc and HP1 in S2 cells are correlated with different histone H3 methyl marks
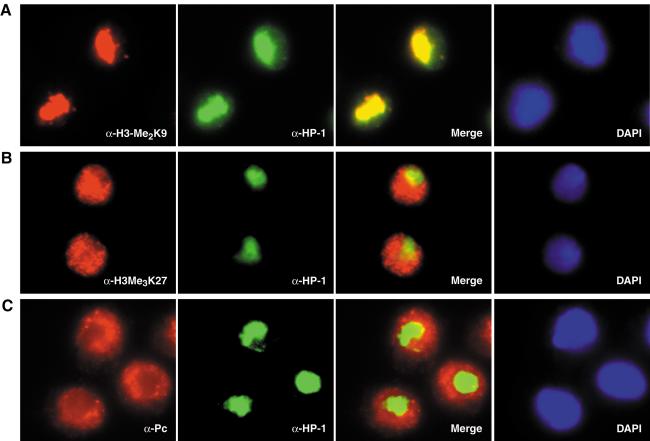
To further analyze and compare the localization of histone H3 methylation on Lys 9 and Lys 27 with that of the HP1 and Pc proteins in vivo, we performed indirect immunofluorescence studies on Drosophila Schneider S2 cells. In these diploid male cells, antibodies specific for H3 dimethylated or trimethylated on Lys 9 accumulated in defined, often more internal, subnuclear regions (Fig. 3A). In contrast, immunostaining with antibodies specific for H3 trimethylated on Lys 27 showed a more diffuse, external nuclear staining pattern, which almost decorated the whole cell nucleus (Fig. 3B). Costaining with antibodies specific for HP1 showed a complete overlap with the anti-H3-Me2K9 signal as indicated by the yellow spots in the merged image (Fig. 3A). These findings are in good agreement with previously published observations on other cellular systems and reinforce the idea that HP1 is recruited to sites of Lys 9 methylation in vivo (Moazed 2001; Cheutin et al. 2003). However, little, if any, significant overlap was detected between the anti-HP1 and anti-H3-Me3K27 immunostainings. HP1 is rather excluded from regions enriched in Lys 27 methylation in this cell line (Fig. 3B). Furthermore, Pc showed a subnuclear distribution very similar to the anti-H3-Me3K27 signal. Again, almost no overlap in staining with HP1 was detected (Fig. 3C). Thus, Pc-mediated gene silencing and H3-Lys 27 methylation appear to be similarly distributed in diploid S2 cells. Both are mainly localized to areas distinct from the heterochromatic regions marked by enrichment in HP1 and H3-Lys 9 methylation.
Figure 3.
Localization of HP1, Pc, and repressive H3 methyl-lysine marks to distinct regions of the nucleus in S2 cells. (A) Immunostaining of S2 cells with anti-H3-Me2K9-specific (red) and anti-HP1-specific (green) antibodies shows colocalization to putatively heterochromatic regions inside the cell nucleus. Coimmunostaining of anti-HP1-specific antibodies with anti-H3-Me3K27-specific (B) or anti-Pc-specific (C) antibodies, in contrast, shows strict exclusion from nuclear regions enriched in HP1. DNA inside the cell nucleus was stained with DAPI.
Importance of the different chromodomains of Pc and HP1 for distinct subnuclear targeting
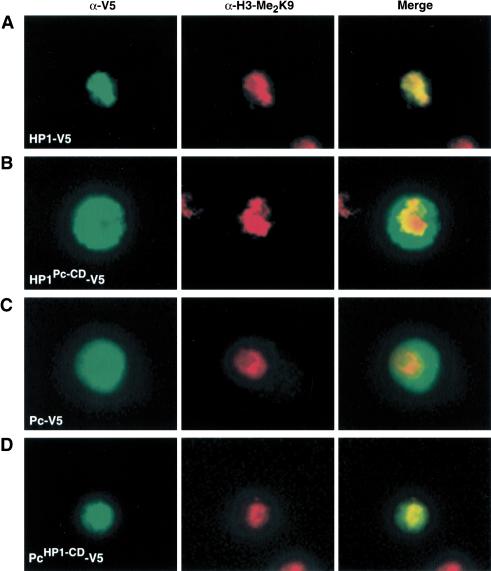
Based on the different binding preferences of the chromodomains of Pc and HP1 for selective methyl marks on H3 tail peptides in vitro, and the distinct cellular localization patterns of Pc and HP1, we next wanted to test if the chromodomains of Pc and HP1 are sufficient for subnuclear targeting in vivo. Therefore, we generated chimeric fusion protein constructs, replacing the chromodomain of Pc with that of HP1 (PcHP1-CD-V5) and the chromodomain of HP1 with that of Pc (HP1Pc-CD-V5). These chimeric fusion protein constructs and their wild-type counterparts were transiently transfected into S2 cells. The cellular distribution of the fusion proteins was then analyzed by immunofluorescence using antibodies directed against the common V5 tag. Equal expression levels of the fusion proteins were confirmed by Western blotting (data not shown). As shown in Figure 4A, the transiently expressed HP1-V5 fusion protein showed a subnuclear staining pattern very similar to that observed for the endogenous HP1 protein (see Fig. 3A). In addition, good overlap with heterochromatic regions stained by the anti-H3-Me2K9-specific antibodies was detected. In contrast, the HP1Pc-CD-V5 chimeric fusion protein did not show a focal localization, but was rather dispersed throughout almost the whole cell nucleus in many cells inspected. Very little colocalization with H3 dimethylated on Lys 9 was detected (Fig. 4B). Conversely, the Pc-V5 fusion protein localized to regions outside of the heterochromatic domains stained by the anti-H3-Me2K9-specific antibodies, similar to the endogenous Pc protein (Fig. 4C). Swapping the chromodomain of Pc with that of HP1 resulted in a focal subnuclear distribution reminiscent of that of HP1 (PcHP1-CD-V5; Fig. 4D). Furthermore, this chimeric fusion protein was often closely associated with the heterochromatic regions stained by the anti-H3-Me2K9-specific antibodies (Fig. 4D). The importance of the chromodomain for Pc localization was further emphasized by the loss of specific subnuclear targeting of an Ile 69-Asp 70 deletion mutant, which had previously been identified in an unbiased genetic screen (Messmer et al. 1992; data not shown). In our in vitro binding assays, this mutant Pc chromodomain was not able to interact with the trimethyl-Lys 27 H3 peptide presumably because of loss of necessary structure for peptide binding (see below). Our chromodomain swapping experiments implicate an important role for the different chromodomains of Pc and HP1 in both target site binding and discrimination. They further emphasize the critical targeting role of the chromodomains for the biological function of these factors.
Figure 4.
Importance of the different chromodomains of Pc and HP1 for distinct subnuclear targeting in vivo. (A) Full-length HP1 fused to a V5-tag was transiently expressed in S2 cells. Immunostaining with antibodies specific for the V5-tag (green) shows colocalization with H3 dimethylated on Lys 9 (red). (B) The chromodomain of HP1 was replaced by the chromodomain of Pc (HP1Pc-CD-V5). Coimmunostaining with anti-V5- and anti-H3-Me2K9-specific antibodies shows exclusion of this chimeric fusion protein from the heterochromatic regions enriched in H3 Lys 9 dimethylation. (C) A transiently expressed Pc-V5 fusion protein localizes to regions that are low in anti-H3-Me2K9 staining. (D) Replacement of the Pc chromodomain with that of HP1 (PcHP1-CD-V5) results in recruitment of this fusion protein to regions enriched in H3 Lys 9 dimethylation. Exemplary cells of a broader spectrum of phenotypes are shown.
Overall structure of the Pc chromodomain-methyl-Lys 27 H3 peptide complex
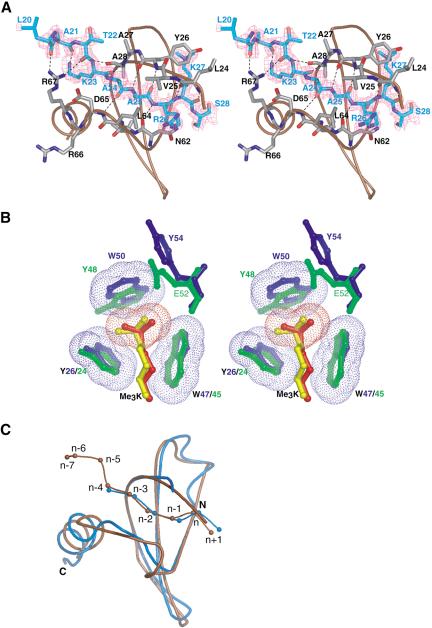
To visualize how the Pc protein binds the methyl-Lys 27-containing histone H3 tail, we crystallized its chromodomain in complex with a synthetic peptide corresponding to residues 15-32 of histone H3 with a trimethyl-lysine at residue 27. The crystals diffracted to 1.8 Å resolution, and the structure was solved using molecular replacement. Table 1 summarizes the quality of the X-ray diffraction data and the structure refinement parameters. The electron density map was interpretable throughout the entire chromodomain region (residues 23-73). Analysis of the |2Fo - Fc| and |Fo - Fc| difference maps clearly indicated electron density for the bound position of the H3 peptide in the complex as shown in Figure 5A. The bound H3 peptide density observed corresponds to residues 20-28, including clear density for trimethyl-Lys 27.
Table 1.
Crystallographic data and refinement information
| Resolutiona | 50.0-1.76 (1.82-1.76) |
| Wavelength (Å) | 0.97934 |
| Unique reflections | 9494 |
| Completeness (%)a | 93.7 (74.2) |
| Average I/σa | 32.6 (2.95) |
| Average redundancy | 4.1 (1.7) |
| Rsym (%)a,b | 3.3 (21.2) |
| Rcryst/Rfree (%)c,d | 22.33/25.23 |
| RMSD | |
| Bonds (Å) | 0.00574 |
| Angles (°) | 1.17 |
| Average B-factors | |
| Protein (Å2) | 23.26 |
| Water (Å2) | 50.76 |
Numbers in parentheses are for the highest resolution shell.
Rsym = ∑|Ih - 〈Ih〉|/∑Ih, where 〈Ih〉 is the average intensity over symmetry equivalent reflections.
Rcryst = ∑|Fo - Fc|/∑Fo, where summation is over the data used for refinement.
Rfree was calculated using 10% of data excluded from refinement.
Figure 5.
Structure of the Pc chromodomain (residues 15-77) in complex with the trimethylated-Lys 27 H3 tail (residues 15-32) at 1.8 Å resolution. (A) Stereo diagram of an |Fo - Fc| simulated annealing omit map contoured at 2.5σ in which the H3 tail peptide was omitted for map calculation. Side chains that make critical contacts are depicted; the chromodomain backbone is in brown, and the electron density of the H3 peptide is in magenta. Residues 15-19 and 29-32 of the peptide appear to be unstructured, suggesting that only Leu 20-Ser 28 of the histone H3 tail participate in binding to the chromodomain. Broken lines indicate intermolecular hydrogen bonds. (B) The chromodomains of both Pc (blue) and HP1 (green) contain three aromatic residues that form superimposable cages around the methyl-ammonium groups of Lys 27 (red) and Lys 9 (yellow), respectively. The van der Waals radii of the Pc aromatic rings and the methyl-Lys 27 methyl-ammonium atoms are shown. (C) Superposition of the backbone structures of the Pc (brown) and HP1 (blue) chromodomains bound to Me3K27 and Me3K9 H3 tail peptides, respectively.
The histone peptide forms a β-strand structure that lies between two β-strands from one face of the chromodomain, completing a three-stranded sheet and the overall β-sandwich architecture of the protein. We previously observed this binding mode in the structure of the HP1 chromodomain in complex with H3-peptide-bearing trimethyl-Lys 9. The methyl-Lys 27 interacts with the Pc protein via a cation-π interaction reminiscent of the mode seen in the HP1 chromodomain interaction with methyl-Lys 9 (Jacobs and Khorasanizadeh 2002). The aromatic cages of the two complexes superimpose with an RMSD of 0.7 Å, supporting our previous prediction that aromatic cages in diverse chromodomains act as a recognition substructure for methyl-lysines in target sequences (Jacobs and Khorasanizadeh 2002). Within the aromatic cage, one of the residues of the Pc chromodomain, Trp 50, is different from its counterpart in HP1, Tyr 48 (Fig. 5B). Mutation of Trp 50 to Tyr does not change the affinity of Pc for its target peptide significantly (KD = 7 μM for the binding of the W50Y variant to trimethyl-Lys 27 H3 peptide). Other architectural features of the HP1 and Pc chromodomains are highly related. Comparison of the HP1 and Pc complex structures reveals that the chromodomains and H3 tail peptides superimpose with an RMSD of 0.74 Å and 1.0 Å over all of their overlapping Cα atoms, respectively (Fig. 5C).
Molecular basis for discrimination of methyl-Lys 9 and methyl-Lys 27 by Pc and HP1
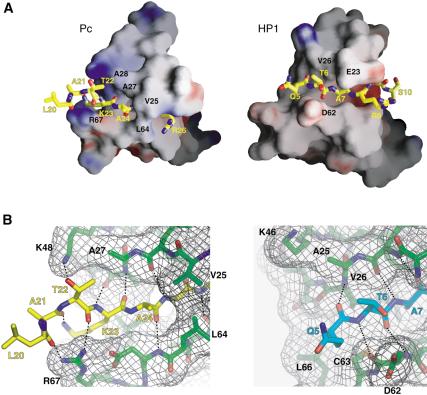
Despite the fact that the chromodomains of HP1 and Pc are similarly structured, their peptide-binding grooves show distinct features (Fig. 6A). The most striking difference is the extent of peptide-protein interactions in these two complexes. A total of six residues of the H3 tail were observed and ordered in the HP1 complex (Jacobs and Khorasanizadeh 2002). These correspond to the sequence stretch Gln 5 to Ser 10. In the structure of Pc complex with trimethyl-Lys 27 H3, a total of nine residues corresponding to the sequence stretch Leu 20 to Ser 28 are observed. As a result of a more extended peptide-binding groove in the Pc protein, its interactions with the Lys 27 site bury 1482 Å2, whereas the interaction of HP1 with the Lys 9 site buries 1063 Å2. The remaining peptide residues in each case were present in the crystals as confirmed by mass spectrometry, but are unobserved because of disorder, presumably caused by their lack of binding interaction with the chromodomain surface.
Figure 6.
Structural basis for the recognition of methyl-Lys 27 by the Pc chromodomain. (A) Surface depictions of the Pc and HP1 chromodomain H3 tail-binding interfaces. (B) Closeup view of the surface of the Pc chromodomain (left) that interacts with residues 20-24 of the H3 tail, and the surface of the HP1 chromodomain (right) that interacts with residues 5-7 of the H3 tail.
The two complexes show differences in the recognition of the n - 4 position (Gln 5 vs. Lys 23), where n corresponds to the methyl-lysine (see Fig. 1B for the H3 sequence). This appears to have important consequences for the Pc peptide interactions. Within the peptide, Lys 23 donates a hydrogen bond to the backbone carbonyl of Ala 21, thus stabilizing the extended conformation bound in the Pc groove (Fig. 6B). Gln 5 does not form a similar stabilizing interaction when bound to the HP1 chromodomain. Another important difference is the n - 5 position of the peptides (Thr 22 vs. Lys 4). In the Pc complex, Thr 22 of the peptide is appropriately positioned to donate a hydrogen bond to the side chain of a conserved chromodomain residue, Lys 48. A lysine in this position of the peptide would not be able to make this interaction, and in fact would cause charge repulsion with the lysine of the chromodomain. In addition, Thr 22 together with Leu 20 of the peptide form backbone hydrogen bonds with the Arg 67 side chain of the Pc protein. This interaction would not be possible with the HP1 protein, as Arg 67 is not conserved and is instead substituted by Asp 62 in HP1. These observations indicate that the n - 4 through n - 7 positions interact with the Pc protein through several backbone contacts. As such, these do not provide specific recognition per se, but stabilize the overall strand conformation of the H3 tail. As an important consequence, the side chain of n - 5 is oriented to hydrogen-bond with the Pc protein, and the side chains of n - 4 and n - 6 are allowed to form complementary van der Waals contacts on the Pc surface. Together these interactions provide the necessary specificity for discrimination of residues n - 4 through n - 7 by the Pc protein.
Whereas the Pc chromodomain recognizes an extended surface encompassing n - 4 through n - 7 residues, HP1 appears to be more discriminating for residues in the immediate vicinity of the methyl-Lys corresponding to the n - 1 through n - 3 residues (TARK9 vs. AARK27). This is shown through mutagenesis studies in which the n - 3 residue alone is changed from one target site to the other. Mutation of Thr 5 to Ala (corresponding to residue 24) reduced the peptide-binding affinity of HP1 by sixfold (KD = 27 ± 4 μM for the binding of HP1 to the T5A variant of a trimethyl-Lys 9 H3 peptide). Conversely, when Ala 24 is changed to Thr, the peptide-binding affinity of Pc did not change significantly (KD = 8 ± 1 μM for the binding of Pc to the A24T variant of a trimethyl-Lys 27 H3 peptide). Together, these results indicate that the HP1 protein is much more discriminating than Pc for the residue in the n - 3 position.
Discussion
Understanding the biological role of posttranslational histone modifications requires understanding the mechanisms by which these marks are selectively recognized by conserved protein modules. The following conclusions can be drawn from the work presented here: Two very similar chromodomains of Pc and HP1 interact differently with two very similar methyl-lysine marks in the histone H3 tail, methyl-Lys 9 and methyl-Lys 27. The inverse preference of target binding observed in vitro is reflected in nonoverlapping localization and recruitment of these factors to differentially modified regions of chromatin in vivo. Our newly solved structure of the Pc chromodomain in complex with a target H3-peptide-bearing methyl-Lys 27 confirms our previous finding that clustering of three chromodomain aromatic residues forms an aromatic cage for methyl-lysine binding. Furthermore, comparison of the structures of Pc and HP1 complexes shows how their chromodomains have evolved to discriminate related but distinct regions of the histone H3 tail.
Structural aspects of chromodomain methyl-lysine binding
In both the HP1 and Pc complexes, histone tail interactions are stabilized by the formation of hydrogen bonds and a complementary surface, whereas the recognition of the methyl-lysine mark is mediated by an aromatic cage consisting of three residues. An intriguing feature of our structural analyses of the HP1 and Pc methyl-lysine H3 tail complexes is that the recognition of specific target sites is highly restricted to the N-terminal “faces” of the Lys 9- and Lys 27-binding sites. No residues C-terminal to n + 1 (Ser 10 or Ser 28) are seen in the three-dimensional structures. Therefore, the specificity of interaction has to be derived from hydrophobic and van der Waals interactions between the chromodomain and residues not only N-terminal to the methyl-lysine mark but also N-terminal to the n - 2 residue because both Lys 9 and Lys 27 are embedded into the same sequence motif ARKS. Whereas the Pc chromodomain forms an extended groove that discriminates its target via the n - 4 to n - 7 residues of the H3 tail, the HP1 chromodomain does not form stable interactions with the corresponding residues in its target, but is more discriminating for the n - 3 position. This interpretation of the complex structures is consistent with our mutagenesis analysis of the H3 tail motif surrounding the Lys 9 and Lys 27 methyl marks, where mutation of the n - 3 position had a significant effect on the binding of HP1 to its cognate target, but did not impair Pc affinity for methyl-Lys 27. Therefore, we conclude that more than a single key residue is important for target selection by the chromodomains of HP1 and Pc. This asymmetric and selective binding to a cognate mark and its surrounding residues is reminiscent of SH2 domains and other cellular signaling docking modules (Pawson et al. 2001). Future structural studies of other chromodomains that recognize a methyl-lysine in distantly related sequence context should provide additional insights about the target selectivity. For example, Lys 4 in histone H3 can also be methylated, and the sequence context of this methyl mark is very different from Lys 9 and Lys 27 (Fig. 1B).
Interestingly, the interacting peptide regions of the H3 tail that are in contact with the chromodomains in both, the Pc and HP1 complexes, contain additional residues known to be posttranslationally modified. These include phospho marks on Ser 10 and Ser 28 and an acetyl mark on Lys 23. At present it is not clear to what extent combinations of these marks with the methyl-lysine marks of Lys 9 and Lys 27 exist. However, additional modifications could influence the binding affinity and selectivity of chromodomain modules to methyl-lysine marks additively, synergistically, or antagonistically. Therefore, the analysis of such effects will be important for future biochemical and structural analyses and should help to delineate the regulation of modules binding to histone marks (Strahl and Allis 2000; Jenuwein and Allis 2001).
Polycomb, HP1, and targeting of chromatin modifiers
Our studies show a clear preference of the Pc chromodomain for the H3 Lys 27 methyl mark. How does this activity of Pc contribute to PcG function? In the case of the formation of heterochromatin and the initially genetically defined pathway of suppression of variegation, it has been suggested that methylation of H3 on Lys 9 by Suv3-9 generates a docking site for the HP1 (also known as Suv2-5) chromodomain. Further recruitment of Suv3-9 by the chromo shadow domain of HP1 has been postulated to lead to a perpetuation and spreading of a heterochromatic domain until blocked by yet unknown mechanisms (Bannister et al. 2001; Dillon and Festenstein 2002; Grewal and Elgin 2002; Snowden et al. 2002). Similarly, Esc-E(z)-dependent methylation of Lys 27 (and possibly Lys 9) and consecutive recruitment of Pc and Pc-containing complexes might contribute to the stability of the PcG complex, particularly in the early stages of assembly at a PRE by permitting complex formation to spread to neighboring sequences (Poux et al. 2001a,b). This interpretation of the specific binding of Pc to methyl-Lys 27 is in agreement with studies demonstrating loss of chromosome binding for several components of PRC1 upon inactivation of E(z) (Rastelli et al. 1993) and is consistent with several other in vivo results that imply synergy between these complexes (Simon and Tamkun 2002). However, it is unclear at present if dynamic perpetuated spreading of a Lys 27 mark indeed exists and is dependent on Pc recruitment and Esc-E(z) enzymatic activity. Other possible functions for the binding of Pc to the Lys 27 methyl mark include a more static maintenance effect that could contribute to epigenetic memory. In this model, the recruitment of Esc-E(z) would be independent of and precede any involvement of Pc binding. Alternatively, although complex recruitment could be constitutive, the decision to repress or not could depend on an epigenetic switch mediated by Lys 27 methylation and its interaction with local Pc/PRC1 (Breiling et al. 2001; Czermin et al. 2002).
However, alternative routes and mechanisms of Pc and PcG recruitment to local sites of chromatin besides recognition of Lys 27 methylation might also exist. For example, studies involving the localization of a chimeric HP1 protein containing the chromodomain of Pc on polytene chromosomes implied critical interactions of the Pc chromodomain with other PcG components that are recruited to PcG sites (Platero et al. 1995, 1996). Therefore, the particular localization patterns observed for the wild-type and chimeric proteins in our swapping experiments might be the result of additive effects, including other targeting mechanisms besides methyl-lysine binding. Nevertheless, it is intriguing to note that the subnuclear localization patterns for wild-type and chimeric Pc and HP1 proteins are coincident with the localization of specifically recognized methyl-lysine marks on the histone H3 tail. It is unclear to what extent additional regions of the proteins C-terminal to the chromodomains could contribute to subnuclear localization and function. For example, different studies have implicated the C-terminal chromo shadow domain and hinge regions of HP1 in addition to the chromodomain in the specific subnuclear targeting of this factor (Smothers and Henikoff 2001; Muchardt et al. 2002; Cheutin et al. 2003). However, a C-terminal truncation of Pc did not affect its specific chromosomal localization (Messmer et al. 1992). Additional studies will have to address the exact contribution of the chromodomains and their recognition and binding of particular methyl-lysine marks to the specific functions of the Pc and HP1 proteins.
Materials and methods
Antibodies
Polyclonal antibodies against H3-K9 (Me)2 and H3-K9 (Me)3 were from Upstate Biotech, Inc.; the monoclonal antibody against HP1 was obtained from the Hybridoma Bank at the University of Iowa. Antibodies against H3-Me3K27 (Silva et al. 2003), Pc (Messmer et al. 1992), and Psc (Martin and Adler 1993) were kind gifts from Thomas Jenuwein (Research Institute of Molecular Pathology, The Vienna Biocenter, Vienna, Austria), Renato Paro (ZMBH, University of Heidelberg, Heidelberg, Germany), and Paul Adler (Biology Department and Cancer Center, University of Virginia, Charlottesville, VA), respectively. The monoclonal anti-V5 antibody was purchased from Invitrogen.
Peptide preparation
Synthetic peptides of the H3 tail were prepared at the Core Facility of Baylor College of Medicine. Peptides corresponding to the Lys 9 and Lys 27 regions include residues 1-15 and 15-32, respectively (see Fig. 1A). Unmodified as well as modified lysines (mono-, di-, and trimethylation) were incorporated at the Lys 9 and Lys 27 positions. A nonnative Tyr residue at the C terminus of each peptide was used for concentration determination by UV absorption measurements. Peptides were labeled with fluorescein as previously described (Jacobs et al. 2001).
Molecular biology
For binding studies, the chromodomain of Pc (residues 1-90) was amplified by PCR and cloned into the BamHI/NdeI sites of the pET16b vector (Novagen). For crystallization, Pc residues 15-77 were fused to a His6-tag by PCR and cloned into the BamHI/NdeI sites of the pET11a vector. The construct for the expression of the chromodomain of HP1 (residues 17-76) has been described previously (Jacobs and Khorasanizadeh 2002). Full-length Drosophila HP1 (residues 1-206) and Pc (1-390) were PCR-amplified and cloned into the EcoRI/XhoI sites of the pMT/V5-His A vector (Invitrogen). The chromodomain of HP1 (residues 23-77) and Pc (residues 21-74) were swapped using staggered PCR by incorporation of overlapping oligonucleotides. Chimeric cDNAs were then cloned into the pMT/V5-His A vector. Site-directed mutagenesis was performed according to the QuikChange protocol (Stratagene).
Binding assays
Fusion proteins with N-terminal His-tag were expressed in Escherichia coli strain BL21 (DE3) (Novagen) and purified by Ni2+-affinity chromatography (QIAGEN). Protein concentrations were determined by absorbance spectroscopy using predicted extinction coefficients (for Pc chromodomain, ε280 = 22,190 M-1 cm-1; for HP1 chromodomain, ε280 = 17,780 M-1 cm-1). Peptide concentrations were determined using absorbance spectroscopy (extinction coefficient for tyrosine, ε280 = 1280 M-1 cm-1; extinction coefficient for fluorescinated peptides, ε492 = 68,000 M-1 cm-1). Fluorescence polarization binding assays were performed under conditions of 20 mM imidazole (pH 7.0), 25 mM NaCl, and in the presence of 100 nM fluorescein-labeled peptide following a previously described protocol (Jacobs et al. 2001). Data were obtained using a Teacan Polarion 96-well plate reader by setting it on automatic gain and 100 flashes. Sample plates were kept on ice until fluorescence reading at room temperature.
S2 cell transfection and immunofluorescence
S2 cells were grown at room temperature in Schneider's Drosophila medium (Invitrogen) supplemented with 10% FBS. Cells were transfected using the calcium phosphate method as instructed by the manufacturer (Invitrogen). Expression of fusion proteins was induced by adding 250 μM CuSO4 for 12 h. For immunofluorescence staining, ∼5 × 106 cells were spun onto glass coverslips in 6-well tissue culture dishes (2000 rpm, 4 min). Cells were fixed in solution I (1× PBS, 3.7% formaldehyde, 1% Triton X-100, 2% NP-40) for 10 min, washed in 1× PBST (PBS with 1% Triton X-100) three times for 10 min. Slides were blocked for 1 h and incubated with the indicated primary antibodies overnight in a humidified atmosphere. Dilutions for primary antibodies were anti-H3-Me2K9 (1:500), anti-H3-Me3K27 (1:500, preabsorbed with H3-Me3K9 peptide at 5 μg/mL), anti-HP1 (1:500), anti-Pc (1:400), anti-Psc (1:100), and anti-V5 (1:500). Slides were washed in 1× PBST three times for 10 min and incubated with the appropriate secondary antibodies for 2 h in a humidified atmosphere. After washing in 1× PBST, DNA was stained with DAPI (1 μg/mL) for 10 sec. Pictures were taken on a Zeiss Axiopod II equipped with a 60× lens. Cells with intermediate levels of fusion protein expression were selected.
Polytene chromosome immunofluorescence
Staining of polytene chromosomes was performed essentially as previously described (Jin et al. 2000). In brief, salivary glands from third instar larvae were dissected in 1× PBS, fixed in solution I for 60 sec, followed by incubation in solution II (50% glacial acetic acid, 3.7% formaldehyde in H2O) for 2 min. Slides were transferred to solution III (50% acetic acid, 16.7% lactic acid in H2O) for 2 min. The fixed salivary glands were squashed, frozen in liquid N2, dehydrated in 95% ethanol, and washed two times in 1× PBST, 30 min each. Immunostaining was essentially performed as described for S2 cells. For sequential double labeling using two polyclonal antibodies from rabbits, the first primary antibodies (anti-Pc, 1:400) were incubated with the polytene tissue at room temperature for 2 h. After washing (1× PBST, three times for 10 min), FITC-conjugated goat anti-rabbit secondary antibodies (1:200, Fab fragment) were applied at room temperature for 1 h. After washing (1× PBST, three times for 10 min), slides were blocked with the corresponding unlabeled goat anti-rabbit antibodies (Fab fragment at 70 μg/mL, at room temperature for 2 h). After washing, the second primary antibodies (either anti-H3-Me3K9 or anti-H3-Me3K27) were applied (at room temperature for 2 h), followed by incubation with Cy3-conjugated goat anti-rabbit secondary antibodies.
Crystallization, data collection, and refinement
Purified Pc chromodomain (residues 15-77) was dialyzed into 20 mM Tris-HCl (pH 8.0), 50 mM NaCl, and concentrated to 5 mg/mL before addition of H3-Me3K27 peptide to reach a final protein-to-peptide molar ratio of 1:5. Single crystals in space group I212121 (a = 32.12, b = 75.81, c = 80.46) were grown by the hanging drop vapor diffusion method at 10°C or 4°C by mixing 1.5 μL of the protein-peptide solution with 1.5 μL of a reservoir solution containing 0.1 M Tris-HCl (pH 8.5), 0.2 M Li2SO4, and 30% polyethylene glycol 4000. Crystals were cryoprotected in the same solution supplemented with 25% ethylene glycol and flash-frozen in liquid nitrogen for data collection at the Advanced Photon Source Beamline SBC 19-ID. Data were processed and scaled with HKL2000 (Otwinowski and Minor 1997). Phases were solved by molecular replacement with MOLREP (Vagin and Teplyakov 1997) using the HP1 chromodomain crystal structure (PDB code 1KNA) as the model. MOLREP produced a clear solution with a correlation coefficient of 0.31 and Rcrystal of 0.52. Rigid-body refinement of this solution using REFMAC5 (Murshudov et al. 1997) reduced the Rfactor to 0.42 and Rfree to 0.46. This model was then submitted to ARP/wARP (Perrakis et al. 1999) to be used only as a source of phases for automatic main-chain tracing and side-chain docking as well as refinement. ARP/wARP successfully traced residues 25-73 of the Pc chromodomain and 21-28 of the H3 tail, reducing the Rcrystal and Rfree to 0.27 and 0.36, respectively. Residues 23 and 24 of Pc and 20 of H3 were manually built as there was clear density present in a simulated annealing composite omit map calculated using CNS (Brunger et al. 1998). Subsequent rounds of manual rebuilding and refinement using O (Jones and Kjeldgaard 1994) and CNS and addition of water molecules led to the converged Rcrystal and Rfree values reported in Table 1.
Coordinates
Atomic coordinates and structure factors have been deposited in the Protein Data Bank (accession code 1PDQ).
Acknowledgments
We thank Drs. Thomas Jenuwein, Yi Zhang, Ru Cao, Renato Paro, and Paul Adler for providing crucial reagents used in these studies and Dr. Fraydoon Rastinejad for assistance with crystallography. W.F. is a Robert Black fellow of the Damon Runyon Cancer Research Foundation. This work was supported by NIH grants to S.K. (GM116635) and C.D.A. (GM63959, GM53512).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Corresponding authors.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1110503.
References
- Bannister A.J., Zegerman, P., Partridge, J.F., Miska, E.A., Thomas, J.O., Allshire, R.C., and Kouzarides, T. 2001. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410: 120-124. [DOI] [PubMed] [Google Scholar]
- Breiling A., Turner, B.M., Bianchi, M.E., and Orlando, V. 2001. General transcription factors bind promoters repressed by Polycomb group proteins. Nature 412: 651-655. [DOI] [PubMed] [Google Scholar]
- Brunger A.T., Adams, P.D., Clore, G.M., DeLano, W.L., Gros, P., Grosse-Kunstleve, R.W., Jiang, J.S., Kuszewski, J., Nilges, M., Pannu, N.S., et al. 1998. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Cryst. D 54: 905-921. [DOI] [PubMed] [Google Scholar]
- Cao R., Wang, L., Wang, H., Xia, L., Erdjument-Bromage, H., Tempst, P., Jones, R.S., and Zhang, Y. 2002. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298: 1039-1043. [DOI] [PubMed] [Google Scholar]
- Cheutin T., McNairn, A.J., Jenuwein, T., Gilbert, D.M., Singh, P.B., and Misteli, T. 2003. Maintenance of stable heterochromatin domains by dynamic HP1 binding. Science 299: 721-725. [DOI] [PubMed] [Google Scholar]
- Czermin B., Melfi, R., McCabe, D., Seitz, V., Imhof, A., and Pirrotta, V. 2002. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 111: 185-196. [DOI] [PubMed] [Google Scholar]
- Dillon N. and Festenstein, R. 2002. Unravelling heterochromatin: Competition between positive and negative factors regulates accessibility. Trends Genet. 18: 252-258. [DOI] [PubMed] [Google Scholar]
- Eissenberg J.C. and Elgin, S.C.R. 2000. The HP1 protein family: Getting a grip on chromatin. Curr. Opin. Genet. Dev. 10: 204-210. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. and Groudine, M. 2003. Controlling the double helix. Nature 421: 448-453. [DOI] [PubMed] [Google Scholar]
- Fischle W., Wang, Y., and Allis, C.D. 2003. Histone and chromatin cross-talk. Curr. Opin. Cell Biol. 15: 172-183. [DOI] [PubMed] [Google Scholar]
- Grewal S.I. and Elgin, S.C. 2002. Heterochromatin: New possibilities for the inheritance of structure. Curr. Opin. Genet. Dev. 12: 178-187. [DOI] [PubMed] [Google Scholar]
- Jacobs J.J. and van Lohuizen, M. 2002. Polycomb repression: From cellular memory to cellular proliferation and cancer. Biochim. Biophys. Acta 1602: 151-161. [DOI] [PubMed] [Google Scholar]
- Jacobs S.A. and Khorasanizadeh, S. 2002. Structure of the HP1 chromodomain bound to a lysine 9-methylated histone H3 tail. Science 295: 2080-2083. [DOI] [PubMed] [Google Scholar]
- Jacobs S.A., Taverna, S.D., Zhang, Y., Briggs, S.D., Li, J., Eissenberg, J.C., Allis, C.D., and Khorasanizadeh, S. 2001. Specificity of the HP1 chromo domain for the methylated N-terminus of histone H3. EMBO J. 20: 5232-5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T. and Allis, C.D. 2001. Translating the histone code. Science 293: 1074-1080. [DOI] [PubMed] [Google Scholar]
- Jin Y., Wang, Y., Johansen, J., and Johansen, K.M. 2000. JIL-1, a chromosomal kinase implicated in regulation of chromatin structure, associates with the male specific lethal (MSL) dosage compensation complex. J. Cell Biol. 149: 1005-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T.A. and Kjeldgaard, M. 1994. O version 5.9. Uppsala University, Uppsala, Sweden.
- Kirschmann D.A., Lininger, R.A., Gardner, L.M., Seftor, E.A., Odero, V.A., Ainsztein, A.M., Earnshaw, W.C., Wallrath, L.L., and Hendrix, M.J. 2000. Down-regulation of HP1Hsα expression is associated with the metastatic phenotype in breast cancer. Cancer Res. 60: 3359-3363. [PubMed] [Google Scholar]
- Kuzmichev A., Nishioka, K., Erdjument-Bromage, H., Tempst, P., and Reinberg, D. 2002. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes & Dev. 16: 2893-2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachner M. and Jenuwein, T. 2002. The many faces of histone lysine methylation. Curr. Opin. Cell Biol. 14: 286-298. [DOI] [PubMed] [Google Scholar]
- Lachner M., O'Carroll, D., Rea, S., Mechtler, K., and Jenuwein, T. 2001. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410: 116-120. [DOI] [PubMed] [Google Scholar]
- Ladurner A.G., Inouye, C., Jain, R., and Tjian, R. 2003. Bromodomains mediate an acetyl-histone encoded antisilencing function at heterochromatin boundaries. Mol. Cell 11: 365-376. [DOI] [PubMed] [Google Scholar]
- Martin E.C. and Adler, P.N. 1993. The Polycomb group gene Posterior Sex Combs encodes a chromosomal protein. Development 117: 641-655. [DOI] [PubMed] [Google Scholar]
- Messmer S., Franke, A., and Paro, R. 1992. Analysis of the functional role of the Polycomb chromo domain in Drosophila melanogaster. Genes & Dev. 6: 1241-1254. [DOI] [PubMed] [Google Scholar]
- Moazed D. 2001. Common themes in mechanisms of gene silencing. Mol. Cell 8: 489-498. [DOI] [PubMed] [Google Scholar]
- Muchardt C., Guilleme, M., Seeler, J.S., Trouche, D., Dejean, A., and Yaniv, M. 2002. Coordinated methyl and RNA binding is required for heterochromatin localization of mammalian HP1α. EMBO Rep. 3: 975-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller J., Hart, C.M., Francis, N.J., Vargas, M.L., Sengupta, A., Wild, B., Miller, E.L., O'Connor, M.B., Kingston, R.E., and Simon, J.A. 2002. Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell 111: 197-208. [DOI] [PubMed] [Google Scholar]
- Murshudov G.N., Vagin, A.A., and Dodson, E.J. 1997. Refinement of macromolecular structures by the maximum-likelihood method. Acta Cryst. D 53: 240-255. [DOI] [PubMed] [Google Scholar]
- Nielsen P.R., Nietlispach, D., Mott, H.R., Callaghan, J., Bannister, A., Kouzarides, T., Murzin, A.G., Murzina, N.V., and Laue, E.D. 2002. Structure of the HP1 chromodomain bound to histone H3 methylated at lysine 9. Nature 416: 103-107. [DOI] [PubMed] [Google Scholar]
- Orlando V. 2003. Polycomb, epigenomes, and control of cell identity. Cell 112: 599-606. [DOI] [PubMed] [Google Scholar]
- Otwinowski Z. and Minor, W. 1997. Processing of X-ray diffraction data collected in oscillation mode. Meth. Enzymol. 276: 307-326. [DOI] [PubMed] [Google Scholar]
- Pawson T., Gish, G.D., and Nash, P. 2001. SH2 domains, interaction modules and cellular wiring. Trends Cell Biol. 11: 504-511. [DOI] [PubMed] [Google Scholar]
- Perrakis A., Morris, R.J., and Lamzin, V.S. 1999. Automated protein model building with iterative structure refinement. Nat. Struct. Biol. 6: 458-468. [DOI] [PubMed] [Google Scholar]
- Platero J.S., Hartnett, T., and Eissenberg, J.C. 1995. Functional analysis of the chromo domain of HP1. EMBO J. 14: 3977-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platero J.S., Sharp, E.J., Adler, P.N., and Eissenberg, J.C. 1996. In vivo assay for protein-protein interactions using Drosophila chromosomes. Chromosoma 104: 393-404. [DOI] [PubMed] [Google Scholar]
- Poux S., McCabe, D., and Pirrotta, V. 2001a. Recruitment of components of Polycomb Group chromatin complexes in Drosophila. Development 128: 75-85. [DOI] [PubMed] [Google Scholar]
- Poux S., Melfi, R., and Pirrotta, V. 2001b. Establishment of Polycomb silencing requires a transient interaction between PC and ESC. Genes & Dev. 15: 2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastelli L., Chan, C.S., and Pirrotta, V. 1993. Related chromosome binding sites for zeste, suppressors of zeste and Polycomb group proteins in Drosophila and their dependence on Enhancer of zeste function. EMBO J. 12: 1513-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotta G., Ebert, A., Krauss, V., Fischer, A., Hoffmann, J., Rea, S., Jenuwein, T., Dorn, R., and Reuter, G. 2002. Central role of Drosophila SU(VAR)3-9 in histone H3-K9 methylation and heterochromatic gene silencing. EMBO J. 21: 1121-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z., Raible, F., Mollaaghababa, R., Guyon, J.R., Wu, C.T., Bender, W., and Kingston, R.E. 1999. Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell 98: 37-46. [DOI] [PubMed] [Google Scholar]
- Silva J., Mak, W., Zvetkova, I., Appanah, R., Nesterova, T.B., Webster, Z., Peters, A.H., Jenuwein, T., Otte, A.P., and Brockdorff, N. 2003. Establishment of histone H3 methylation on the inactive X chromosome requires transient recruitment of eed-enx1 polycomb group complexes. Dev. Cell 4: 481-495. [DOI] [PubMed] [Google Scholar]
- Simon J.A. 2003. Polycomb group proteins. Curr. Biol. 13: R79-R80. [DOI] [PubMed] [Google Scholar]
- Simon J.A. and Tamkun, J.W. 2002. Programming off and on states in chromatin: Mechanisms of Polycomb and trithorax group complexes. Curr. Opin. Genet. Dev. 12: 210-218. [DOI] [PubMed] [Google Scholar]
- Smothers J.F. and Henikoff, S. 2001. The hinge and chromo shadow domain impart distinct targeting of HP1-like proteins. Mol. Cell. Biol. 21: 2555-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden A.W., Gregory, P.D., Case, C.C., and Pabo, C.O. 2002. Gene-specific targeting of H3K9 methylation is sufficient for initiating repression in vivo. Curr. Biol. 12: 2159-2166. [DOI] [PubMed] [Google Scholar]
- Strahl B.D. and Allis, C.D. 2000. The language of covalent histone modifications. Nature 403: 41-45. [DOI] [PubMed] [Google Scholar]
- Vagin A. and Teplyakov, A. 1997. MOLREP: An automated program for molecular replacement. J. Appl. Cryst. 30: 1022-1025. [Google Scholar]
- Zeng L. and Zhou, M.M. 2002. Bromodomain: An acetyl-lysine binding domain. FEBS Lett. 513: 124-128. [DOI] [PubMed] [Google Scholar]
- Zhang Y. and Reinberg, D. 2001. Transcription regulation by histone methylation: Interplay between different covalent modifications of the core histone tails. Genes & Dev. 15: 2343-2360. [DOI] [PubMed] [Google Scholar]