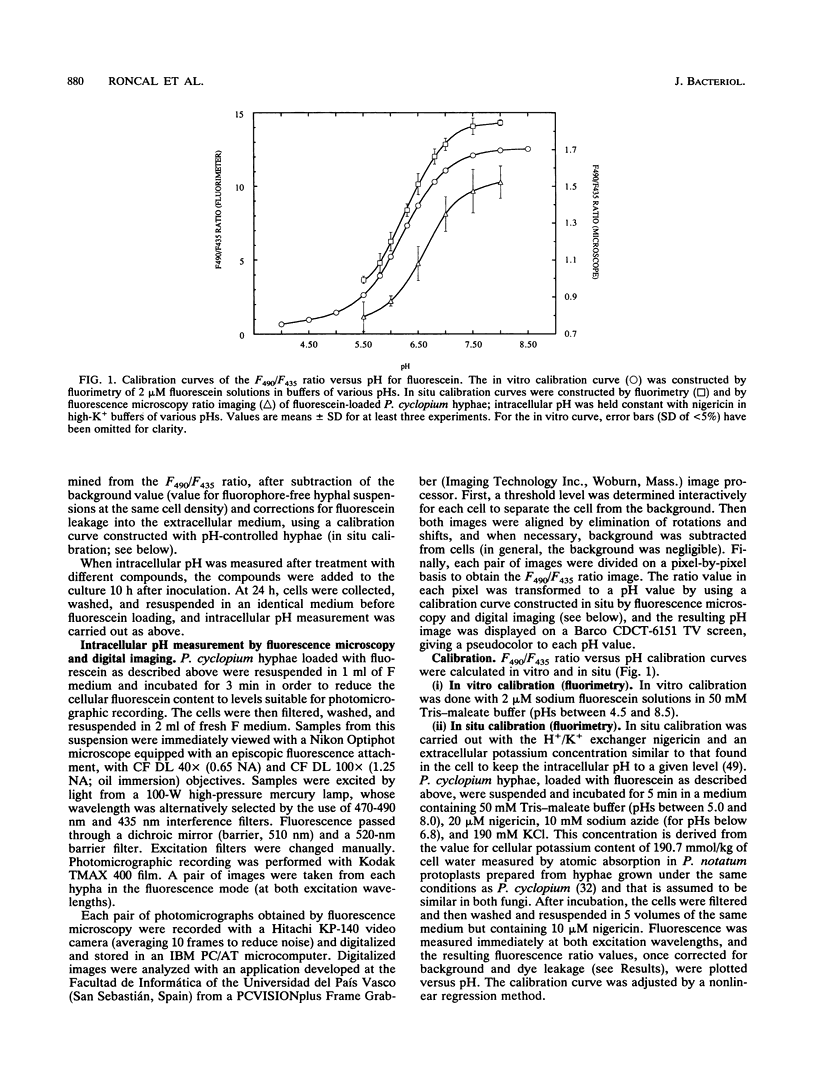
Abstract
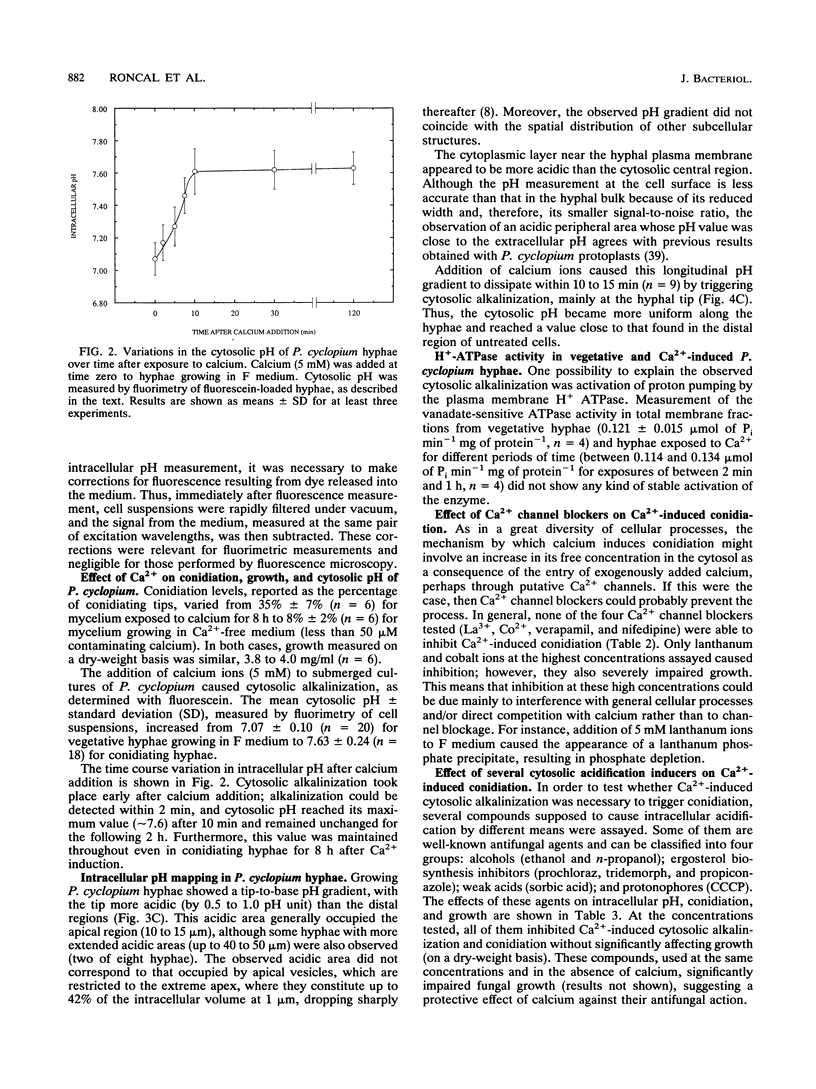
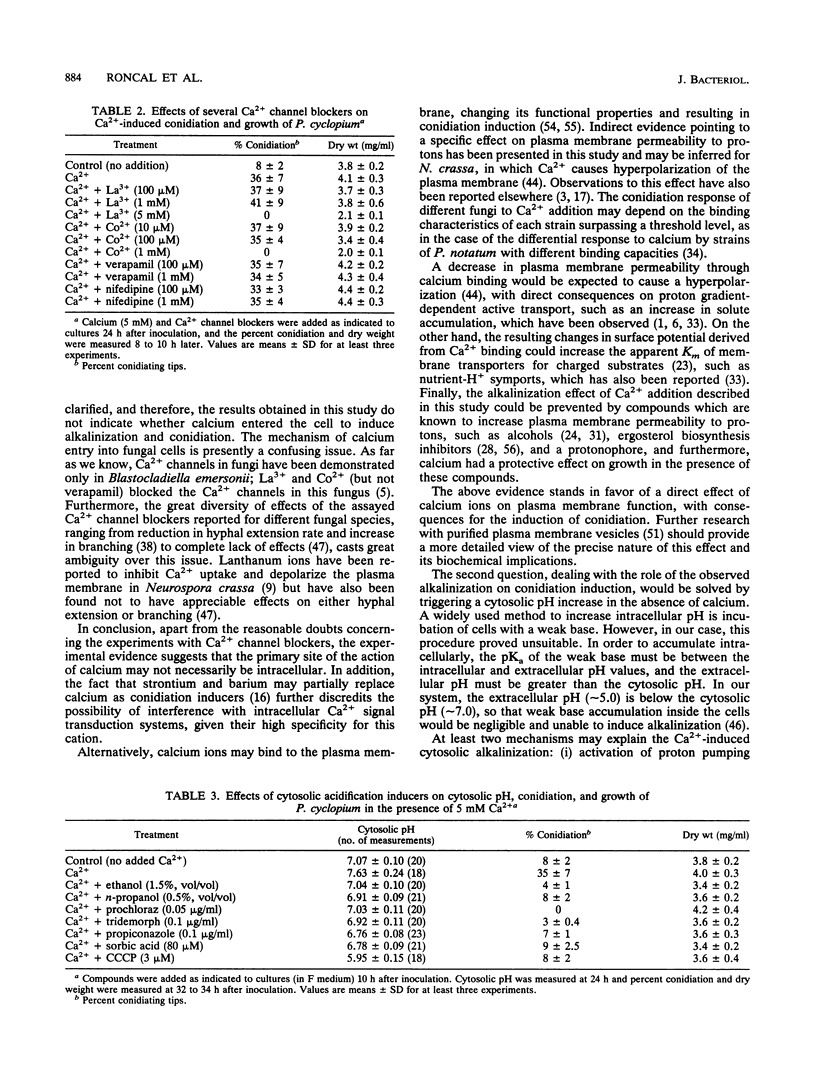
Addition of Ca2+ (1 to 10 mM) to submerged cultures of Penicillium cyclopium induces conidiation. Ca2+ induced an increase in cytosolic pH from approximately 7.00 to > 7.60 in less than 10 min, as determined with the fluorescent pH probe fluorescein. Measurement of the H(+)-ATPase activity in total membrane fractions did not show any stable activation in vivo as a result of Ca2+ treatment. By fluorescence ratio imaging microscopy, it was observed that vegetative hyphae exhibit a tip-to-base pH gradient, with the tip being more acidic. Ca2+ caused this gradient to dissipate within 10 min. The effect of several agents that are supposed to cause internal acidification, by different means, on conidiation was tested. Concentrations of these agents that did not significantly affect growth but inhibited Ca(2+)-induced conidiation also prevented the intracellular alkalinization observed after exposure to the cation. Calcium channel blockers (lanthanum, cobalt, verapamil, and nifedipine) were not able to inhibit Ca(2+)-induced conidiation, although their effect on calcium uptake was not evaluated. However, the combined results point towards externally bound Ca2+ as the primary agent of conidiation induction, causing changes in plasma membrane function which disrupt the pH gradient observed during apical growth.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caldwell J. H., Van Brunt J., Harold F. M. Calcium-dependent anion channel in the water mold, Blastocladiella emersonii. J Membr Biol. 1986;89(1):85–97. doi: 10.1007/BF01870898. [DOI] [PubMed] [Google Scholar]
- Cameron L. E., LéJohn H. B. On the involvement of calcium in amino acid transport and growth of the fungus Achlya. J Biol Chem. 1972 Aug 10;247(15):4729–4739. [PubMed] [Google Scholar]
- Chang A., Slayman C. W. Maturation of the yeast plasma membrane [H+]ATPase involves phosphorylation during intracellular transport. J Cell Biol. 1991 Oct;115(2):289–295. doi: 10.1083/jcb.115.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuppoletti J., Segel I. H. Kinetics of sulfate transport by Penicillium notatum. Interactions of sulfate, protons, and calcium. Biochemistry. 1975 Oct 21;14(21):4712–4718. doi: 10.1021/bi00692a023. [DOI] [PubMed] [Google Scholar]
- Foster J. W., McDaniel L. E., Woodruff H. B., Stokes J. L. Microbiological Aspects of Penicillin: V. Conidiospore Formation in Submerged Cultures of Penicillium Notatum. J Bacteriol. 1945 Sep;50(3):365–368. doi: 10.1128/jb.50.3.365-368.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies R. J., Ugurbil K., den Hollander J. A., Shulman R. G. 31P NMR studies of intracellular pH and phosphate metabolism during cell division cycle of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2125–2129. doi: 10.1073/pnas.78.4.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow N. A., Kropf D. L., Harold F. M. Growing hyphae of Achlya bisexualis generate a longitudinal pH gradient in the surrounding medium. J Gen Microbiol. 1984 Nov;130(11):2967–2974. doi: 10.1099/00221287-130-11-2967. [DOI] [PubMed] [Google Scholar]
- Gow N. A. Transhyphal electrical currents in fungi. J Gen Microbiol. 1984 Dec;130(12):3313–3318. doi: 10.1099/00221287-130-12-3313. [DOI] [PubMed] [Google Scholar]
- Gresík M., Kolarova N., Farkas V. Hyperpolarization and intracellular acidification in Trichoderma viride as a response to illumination. J Gen Microbiol. 1991 Nov;137(11):2605–2609. doi: 10.1099/00221287-137-11-2605. [DOI] [PubMed] [Google Scholar]
- Inouye K. Measurements of intracellular pH and its relevance to cell differentiation in Dictyostelium discoideum. J Cell Sci. 1985 Jun;76:235–245. doi: 10.1242/jcs.76.1.235. [DOI] [PubMed] [Google Scholar]
- Kropf D. L., Caldwell J. H., Gow N. A., Harold F. M. Transcellular ion currents in the water mold Achlya. Amino acid proton symport as a mechanism of current entry. J Cell Biol. 1984 Aug;99(2):486–496. doi: 10.1083/jcb.99.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf D. L., Lupa M. D., Caldwell J. H., Harold F. M. Cell polarity: endogenous ion currents precede and predict branching in the water mold achyla. Science. 1983 Jun 24;220(4604):1385–1387. doi: 10.1126/science.220.4604.1385. [DOI] [PubMed] [Google Scholar]
- Lew R. R. Calcium activates an electrogenic proton pump in neurospora plasma membrane. Plant Physiol. 1989 Sep;91(1):213–216. doi: 10.1104/pp.91.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leão C., Van Uden N. Effects of ethanol and other alkanols on passive proton influx in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 1984 Jul 11;774(1):43–48. doi: 10.1016/0005-2736(84)90272-4. [DOI] [PubMed] [Google Scholar]
- Mercer E. I. Morpholine antifungals and their mode of action. Biochem Soc Trans. 1991 Aug;19(3):788–793. doi: 10.1042/bst0190788. [DOI] [PubMed] [Google Scholar]
- Nations C., Allen R. G., Balin A. K., Reimer R. J., Sohal R. S. Superoxide dismutase activity and glutathione concentration during the calcium-induced differentiation of Physarum polycephalum microplasmodia. J Cell Physiol. 1987 Oct;133(1):181–186. doi: 10.1002/jcp.1041330124. [DOI] [PubMed] [Google Scholar]
- Petrov V. V., Okorokov L. A. Increase of the anion and proton permeability of Saccharomyces carlsbergensis plasmalemma by n-alcohols as a possible cause of its de-energization. Yeast. 1990 Jul-Aug;6(4):311–318. doi: 10.1002/yea.320060404. [DOI] [PubMed] [Google Scholar]
- Potapova T. V., Aslanidi K. B., Belozerskaya T. A., Levina N. N. Transcellular ionic currents studied by intracellular potential recordings in Neurospora crassa hyphae. Transfer of energy from proximal to apical cells. FEBS Lett. 1988 Dec 5;241(1-2):173–176. doi: 10.1016/0014-5793(88)81054-8. [DOI] [PubMed] [Google Scholar]
- Preston R. A., Murphy R. F., Jones E. W. Assay of vacuolar pH in yeast and identification of acidification-defective mutants. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7027–7031. doi: 10.1073/pnas.86.18.7027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos W., Slavík J. Intracellular pH topography of Penicillium cyclopium protoplasts. Maintenance of delta pH by both passive and active mechanisms. Biochim Biophys Acta. 1987 May 12;899(1):67–75. doi: 10.1016/0005-2736(87)90240-9. [DOI] [PubMed] [Google Scholar]
- Rosa M. F., Sá-Correia I. In vivo activation by ethanol of plasma membrane ATPase of Saccharomyces cerevisiae. Appl Environ Microbiol. 1991 Mar;57(3):830–835. doi: 10.1128/aem.57.3.830-835.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLAYMAN C. L., SLAYMAN C. W. Measurement of membrane potentials in Neurospora. Science. 1962 Jun 8;136(3519):876–877. doi: 10.1126/science.136.3519.876. [DOI] [PubMed] [Google Scholar]
- Schreurs W. J., Harold F. M. Transcellular proton current in Achlya bisexualis hyphae: relationship to polarized growth. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1534–1538. doi: 10.1073/pnas.85.5.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R. In vivo glucose activation of the yeast plasma membrane ATPase. FEBS Lett. 1983 May 30;156(1):11–14. doi: 10.1016/0014-5793(83)80237-3. [DOI] [PubMed] [Google Scholar]
- Slavík J. Intracellular pH of yeast cells measured with fluorescent probes. FEBS Lett. 1982 Apr 5;140(1):22–26. doi: 10.1016/0014-5793(82)80512-7. [DOI] [PubMed] [Google Scholar]
- Slayman C. L. Electrical properties of Neurospora crassa. Effects of external cations on the intracellular potential. J Gen Physiol. 1965 Sep;49(1):69–92. doi: 10.1085/jgp.49.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart E., Gow N. A., Bowen D. V. Cytoplasmic alkalinization during germ tube formation in Candida albicans. J Gen Microbiol. 1988 May;134(5):1079–1087. doi: 10.1099/00221287-134-5-1079. [DOI] [PubMed] [Google Scholar]
- Takeuchi Y., Schmid J., Caldwell J. H., Harold F. M. Transcellular ion currents and extension of Neurospora crassa hyphae. J Membr Biol. 1988;101(1):33–41. doi: 10.1007/BF01872817. [DOI] [PubMed] [Google Scholar]
- Thiel R., Schreurs W. J., Harold F. M. Transcellular ion currents during sporangium development in the water mould Achlya bisexualis. J Gen Microbiol. 1988 May;134(5):1089–1097. doi: 10.1099/00221287-134-5-1089. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Ugalde U. O., Hernandez A., Galindo I., Pitt D., Barnes J. C., Wakley G. Preparation of right-side-out plasma membrane vesicles from Penicillium cyclopium: a critical assessment of markers. J Gen Microbiol. 1992 Oct;138(10):2205–2212. doi: 10.1099/00221287-138-10-2205. [DOI] [PubMed] [Google Scholar]
- Ugalde U. O., Virto M. D., Pitt D. Calcium binding and induction of conidiation in protoplasts of Penicillium cyclopium. Antonie Van Leeuwenhoek. 1990 Jan;57(1):43–49. doi: 10.1007/BF00400335. [DOI] [PubMed] [Google Scholar]