Abstract
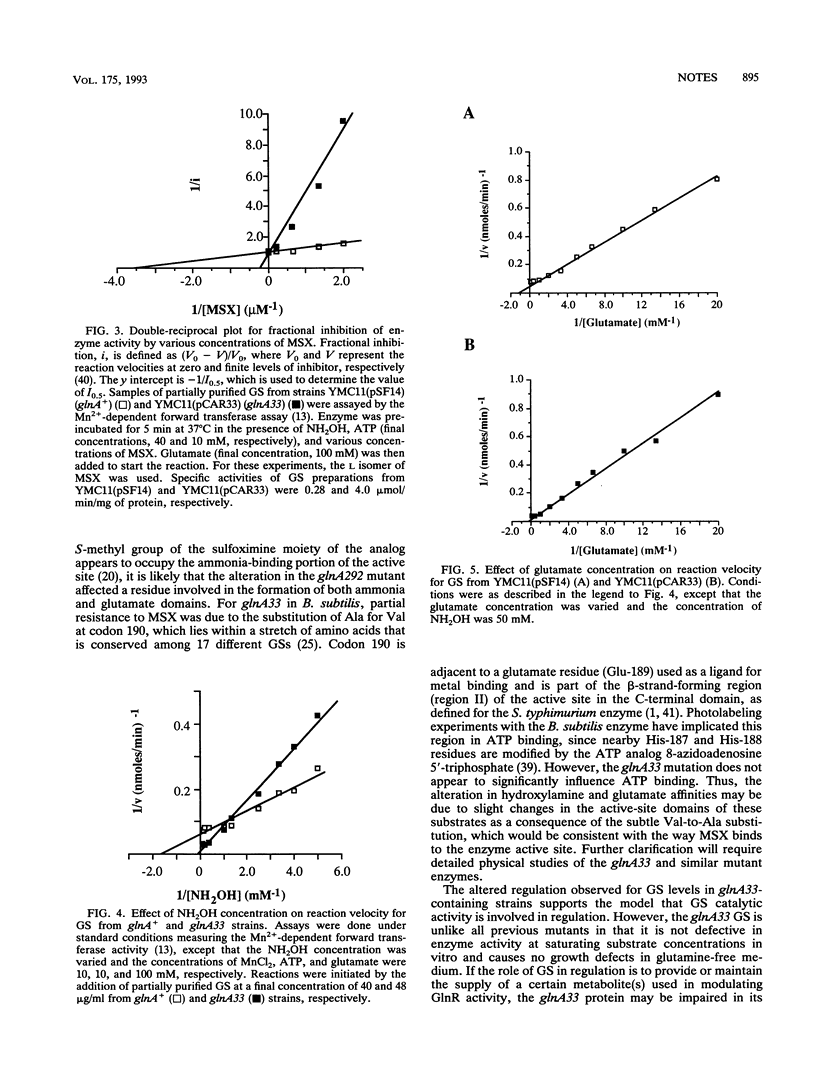
A Bacillus subtilis mutant that produced glutamine synthetase (GS) with altered sensitivity to DL-methionine sulfoximine was isolated. The mutation, designated glnA33, was due to a T.A-to-C.G transition, changing valine to alanine at codon 190 within the active-site C domain. Altered regulation was observed for GS activity and antigen and mRNA levels in a B. subtilis glnA33 strain. The mutant enzyme was 28-fold less sensitive to DL-methionine sulfoximine and had a 13.0-fold-higher Km for hydroxylamine and a 4.8-fold-higher Km for glutamate than wild-type GS did.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almassy R. J., Janson C. A., Hamlin R., Xuong N. H., Eisenberg D. Novel subunit-subunit interactions in the structure of glutamine synthetase. 1986 Sep 25-Oct 1Nature. 323(6086):304–309. doi: 10.1038/323304a0. [DOI] [PubMed] [Google Scholar]
- Backman K., Chen Y. M., Magasanik B. Physical and genetic characterization of the glnA--glnG region of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3743–3747. doi: 10.1073/pnas.78.6.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. R., Aronson A. I. Selection of Bacillus subtilis mutants impaired in ammonia assimilation. J Bacteriol. 1980 Feb;141(2):985–988. doi: 10.1128/jb.141.2.985-988.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D. R., Hoch J. A., Aronson A. I. Alteration of the Bacillus subtilis glutamine synthetase results in overproduction of the enzyme. J Bacteriol. 1977 Sep;131(3):981–987. doi: 10.1128/jb.131.3.981-987.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnen G. E., Cox E. C. Conditional mutator gene in Escherichia coli: isolation, mapping, and effector studies. J Bacteriol. 1974 Feb;117(2):477–487. doi: 10.1128/jb.117.2.477-487.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Ginsburg A., Yeh J., Shelton E., Stadtman E. R. Bacillus subtilis glutamine synthetase. Purification and physical characterization. J Biol Chem. 1970 Oct 25;245(20):5195–5205. [PubMed] [Google Scholar]
- Deuel T. F., Stadtman E. R. Some kinetic properties of Bacillus subtilis glutamine synthetase. J Biol Chem. 1970 Oct 25;245(20):5206–5213. [PubMed] [Google Scholar]
- Fisher S. H., Rosenkrantz M. S., Sonenshein A. L. Glutamine synthetase gene of Bacillus subtilis. Gene. 1984 Dec;32(3):427–438. doi: 10.1016/0378-1119(84)90018-0. [DOI] [PubMed] [Google Scholar]
- Fisher S. H., Sonenshein A. L. Bacillus subtilis glutamine synthetase mutants pleiotropically altered in glucose catabolite repression. J Bacteriol. 1984 Feb;157(2):612–621. doi: 10.1128/jb.157.2.612-621.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. H., Sonenshein A. L. Control of carbon and nitrogen metabolism in Bacillus subtilis. Annu Rev Microbiol. 1991;45:107–135. doi: 10.1146/annurev.mi.45.100191.000543. [DOI] [PubMed] [Google Scholar]
- Fisher S. H., Sonenshein A. L. Glutamine-requiring mutants of Bacillus subtilis. Biochem Biophys Res Commun. 1977 Dec 7;79(3):987–995. doi: 10.1016/0006-291x(77)91207-4. [DOI] [PubMed] [Google Scholar]
- Fouet A., Sonenshein A. L. A target for carbon source-dependent negative regulation of the citB promoter of Bacillus subtilis. J Bacteriol. 1990 Feb;172(2):835–844. doi: 10.1128/jb.172.2.835-844.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutowski J. C., Schreier H. J. Interaction of the Bacillus subtilis glnRA repressor with operator and promoter sequences in vivo. J Bacteriol. 1992 Feb;174(3):671–681. doi: 10.1128/jb.174.3.671-681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin T. M., Sonenshein A. L. Mutations of the Escherichia coli lacUV5 promoter resulting in increased expression in Bacillus subtilis. Mol Gen Genet. 1987 Oct;209(3):467–474. doi: 10.1007/BF00331151. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Manning J. M., Moore S., Rowe W. B., Meister A. Identification of L-methionine S-sulfoximine as the diastereoisomer of L-methionine SR-sulfoximine that inhibits glutamine synthetase. Biochemistry. 1969 Jun;8(6):2681–2685. doi: 10.1021/bi00834a066. [DOI] [PubMed] [Google Scholar]
- Miller E. S., Brenchley J. E. L-Methionine SR-sulfoximine-resistant glutamine synthetase from mutants of Salmonella typhimurium. J Biol Chem. 1981 Nov 10;256(21):11307–11312. [PubMed] [Google Scholar]
- Nakano Y., Kimura K. Purification and characterization of a repressor for the Bacillus cereus glnRA operon. J Biochem. 1991 Feb;109(2):223–228. [PubMed] [Google Scholar]
- Nicholson W. L., Chambliss G. H. Isolation and characterization of a cis-acting mutation conferring catabolite repression resistance to alpha-amylase synthesis in Bacillus subtilis. J Bacteriol. 1985 Mar;161(3):875–881. doi: 10.1128/jb.161.3.875-881.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F. L., Coote J. G. Glutamine synthetase and glutamate synthase activities during growth and sporulation in Bacillus subtilis. J Gen Microbiol. 1979 Jun;112(2):373–377. doi: 10.1099/00221287-112-2-373. [DOI] [PubMed] [Google Scholar]
- Pesole G., Bozzetti M. P., Lanave C., Preparata G., Saccone C. Glutamine synthetase gene evolution: a good molecular clock. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):522–526. doi: 10.1073/pnas.88.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reysset G. New class of Bacillus subtilis glutamine-requiring mutants. J Bacteriol. 1981 Nov;148(2):653–658. doi: 10.1128/jb.148.2.653-658.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzio R. A., Rowe W. B., Meister A. Studies on the mechanism of inhibition of glutamine synthetase by methionine sulfoximine. Biochemistry. 1969 Mar;8(3):1066–1075. doi: 10.1021/bi00831a038. [DOI] [PubMed] [Google Scholar]
- Rowe W. B., Ronzio R. A., Meister A. Inhibition of glutamine synthetase by methionine sulfoximine. Studies on methionine sulfoximine phosphate. Biochemistry. 1969 Jun;8(6):2674–2680. doi: 10.1021/bi00834a065. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier H. J., Brown S. W., Hirschi K. D., Nomellini J. F., Sonenshein A. L. Regulation of Bacillus subtilis glutamine synthetase gene expression by the product of the glnR gene. J Mol Biol. 1989 Nov 5;210(1):51–63. doi: 10.1016/0022-2836(89)90290-8. [DOI] [PubMed] [Google Scholar]
- Schreier H. J., Fisher S. H., Sonenshein A. L. Regulation of expression from the glnA promoter of Bacillus subtilis requires the glnA gene product. Proc Natl Acad Sci U S A. 1985 May;82(10):3375–3379. doi: 10.1073/pnas.82.10.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier H. J., Rostkowski C. A., Nomellini J. F., Hirschi K. D. Identification of DNA sequences involved in regulating Bacillus subtilis glnRA expression by the nitrogen source. J Mol Biol. 1991 Jul 20;220(2):241–253. doi: 10.1016/0022-2836(91)90010-4. [DOI] [PubMed] [Google Scholar]
- Schreier H. J., Sonenshein A. L. Altered regulation of the glnA gene in glutamine synthetase mutants of Bacillus subtilis. J Bacteriol. 1986 Jul;167(1):35–43. doi: 10.1128/jb.167.1.35-43.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch M. A., Aronson A. I., Brown S. W., Schreier H. J., Sonenhein A. L. Sequence of the Bacillus subtilis glutamine synthetase gene region. Gene. 1988 Nov 30;71(2):257–265. doi: 10.1016/0378-1119(88)90042-x. [DOI] [PubMed] [Google Scholar]
- Tanaka E., Kimura K. Identification of amino acid residues modified by two ATP analogs in Bacillus subtilis glutamine synthetase. J Biochem. 1991 Nov;110(5):780–784. doi: 10.1093/oxfordjournals.jbchem.a123659. [DOI] [PubMed] [Google Scholar]
- Wedler F. C., Boyer P. D. Mechanisms of enzyme control as probed by equilibrium exchange rates: patterns of modifier effects with a two substrate, two product system. J Theor Biol. 1973 Mar;38(3):539–558. doi: 10.1016/0022-5193(73)90255-5. [DOI] [PubMed] [Google Scholar]
- Yamashita M. M., Almassy R. J., Janson C. A., Cascio D., Eisenberg D. Refined atomic model of glutamine synthetase at 3.5 A resolution. J Biol Chem. 1989 Oct 25;264(30):17681–17690. doi: 10.2210/pdb2gls/pdb. [DOI] [PubMed] [Google Scholar]
- Zhang J., Strauch M., Aronson A. I. Glutamine auxotrophs of Bacillus subtilis that overproduce glutamine synthetase antigen have altered conserved amino acids in or near the active site. J Bacteriol. 1989 Jun;171(6):3572–3574. doi: 10.1128/jb.171.6.3572-3574.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]




