Abstract
Mammalian CLOCK and BMAL1 are two members of bHLH-PAS-containing family of transcription factors that represent the positive elements of circadian autoregulatory feedback loop. In the form of a heterodimer, they drive transcription from E-box enhancer elements in the promoters of responsive genes. We have examined abundance, posttranslational modifications, cellular localization of endogenous and ectopically expressed CLOCK and BMAL1 proteins. Nuclear/cytoplasm distribution of CLOCK was found to be under circadian regulation. Analysis of subcellular localization of CLOCK in embryo fibroblasts of mice carrying different germ-line circadian mutations showed that circadian regulation of nuclear accumulation of CLOCK is BMAL1-dependent. Formation of CLOCK/BMAL1 complex following ectopic coexpression of both proteins is followed by their codependent phosphorylation, which is tightly coupled to CLOCK nuclear translocation and degradation. This binding-dependent coregulation is specific for CLOCK/BMAL1 interaction, as no other PAS domain protein that can form a complex with either CLOCK or BMAL1 was able to induce similar effects. Importantly, all posttranslational events described in our study are coupled with active transactivation complex formation, which argues for their significant functional role. Altogether, these results provide evidence for an additional level of circadian system control, which is based on regulation of transcriptional activity or/and availability of CLOCK/BMAL1 complex.
Keywords: Circadian rhythm, CLOCK/BMAL1 complex, transcriptional activation, phosphorylation, nuclear entry
Almost all living organisms, from cyanobacteria to mammals, display circadian rhythms (i.e., oscillations with 24-h periodicities) in multiple physiological and behavioral processes. These rhythms are generated endogenously and function under genetic control (King and Takahashi 2000; Young and Kay 2001). In mammals, the pacemaker regulating circadian rhythms is located in the hypothalamic suprachiasmatic nucleus (SCN; Klein et al. 1991). The SCN neurons contain autonomous self-sustained circadian oscillators capable of generating rhythmicity in electrical activity (Welsh et al. 1995). The master clock in the SCN synchronizes multiple peripheral clocks, which function in the variety of tissues, presumably through combination of neural and humoral signaling (Schibler and Sassone-Corsi 2002).
Within cells, the circadian oscillator is composed of autoregulatory transcriptional/translational feedback loops. Positive and negative elements of these loops function to coordinate transcriptional events at appropriate times to provide 24-h periodicities (King and Takahashi 2000; Reppert and Weaver 2001). Two basic helix-loop-helix (bHLH) PAS-domain transcription factors, CLOCK and BMAL1, form the positive elements of the central oscillatory loop (King et al. 1997; Gekakis et al. 1998; Hogenesch et al. 1998). The CLOCK:BMAL1 heterodimer binds to E-box elements in the promoters of target genes and drives rhythmic transcription of three Period (mPer1, mPer2, and mPer3) and two Cryptochrome genes (mCry1 and mCry2). When PER and CRY proteins are translated, they are translocated to the nucleus, where CRY proteins act as potent inhibitors of CLOCK/BMAL1-induced transcription (Griffin et al. 1999; Kume et al. 1999; van der Horst et al. 1999; Vitaterna et al. 1999; Shearman et al. 2000a), serving as a determinant of negative regulation, whereas one of the PERIOD proteins, mPER2, functions as a stimulator of Bmal1 transcription, forming the positive feedback loop (Zheng et al. 1999; Shearman et al. 2000b). These two loops are coupled through the orphan nuclear receptor REV-ERBα, which also provides a molecular basis for a negative feedback within the positive limb (Preitner et al. 2002). This basic mechanism of circadian machinery is formed by a set of conserved genes, although species-dependent functional variations were described for some core circadian system components (Young and Kay 2001).
In vitro transcriptional reporter assays, yeast two-hybrid screens, and coimmunoprecipitation experiments have been used successfully to identify molecular interactions of core clock components at the protein level (Griffin et al. 1999; Kume et al. 1999). It has been postulated that posttranslational modifications, translocation, and regulation of turnover of clock proteins may provide additional regulatory mechanisms by generating time delays necessary for establishing 24-h periodicities. The important role of clock proteins' posttranslational modifications and turnover has been demonstrated first for Drosophila (Kloss et al. 1998; Price and Kalderon 2002) and Neurospora clocks (Liu et al. 2000). In mammals, studies of circadian mutation tau revealed a link between PER and casein kinase Iε (Lowrey and Takahashi 2000), placing CKIε within the core clock machinery. The important role of phosphorylation was further confirmed by uncovering the molecular basis of FASPS— inherited circadian rhythm abnormality due to a mutation in hPER2 phosphorylation site (Toh et al. 2001).
The majority of the studies on posttranslational regulation performed so far were focused on negative components of the circadian transcriptional loop, which are responsible for periodic transcriptional repression and relief from repression normally occurring during the circadian cycle. Much less is known about the role of posttranslational events involved in the functional activity of the positive regulators of the mammalian circadian system—CLOCK and BMAL1. Recently, it was demonstrated that both CLOCK and BMAL1 can undergo phosphorylation in vivo, and that this process is regulated during the circadian cycle (Lee et al. 2001). However, it is still not clear how these modifications correlate with the functional activity of the transactivation complex. In this study, we analyzed CLOCK/BMAL1 interaction in an attempt to link structural and functional aspects of their joint activity. The obtained results demonstrate robust circadian oscillation in nuclear/cytoplasm distribution of CLOCK protein detectable both in serum-shock synchronized cultured fibroblasts and in vivo, in the mouse SCN—the site of mammalian master clock. Circadian oscillation of nuclear accumulation of CLOCK requires functional BMAL1 as it was completely blocked in BMAL1-deficient mice. Hence, the formation of CLOCK/BMAL1 heterodimer is essential for CLOCK and BMAL1 phosphorylation as well as for CLOCK nuclear translocation and degradation. Importantly, all of these events are tightly coupled with CLOCK/BMAL1-dependent transcriptional activation, indicating their functional role in the regulation of the circadian clock.
Results
Circadian oscillation of nuclear/cytoplasm distribution of CLOCK in mouse SCN
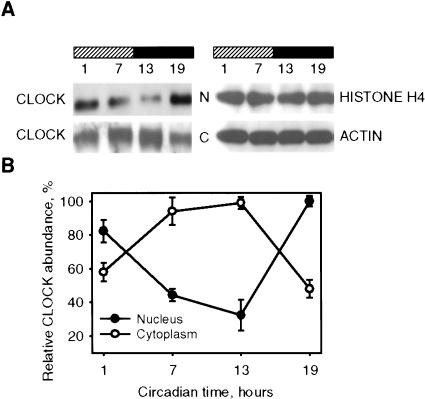
Among the major components of the circadian system, CLOCK has been unusual in showing no obvious changes in its expression throughout the circadian cycle at transcriptional or translational levels (Lee et al. 2001). Because the transactivation function of CLOCK requires its presence in the nucleus, and because many nuclear factors involved in signal transduction are regulated at the level of nuclear translocation, we decided to analyze variations in nuclear-cytoplasmic distribution of this protein in the mouse SCN—the site of mammalian master clock. As seen in Figure 1A, under constant darkness (DD) conditions, both nuclear and cytoplasmic CLOCK demonstrate circadian oscillation in abundance that occurs in anti-phase in two cell compartments. In the nucleus, the lowest levels of CLOCK protein were detected between circadian time (CT) 7 and 13, whereas in the cytoplasm, these time points were characterized by the peak of CLOCK abundance (quantitative analysis presented in Fig. 1B). Reverse phases of CLOCK protein nuclear and cytoplasmic oscillations provide the reason why circadian changes in CLOCK abundance could not be detected when analyzed in total cell lysates.
Figure 1.
Circadian oscillation of CLOCK nuclear/cytoplasm distribution in the SCN. (A) SCN extracts obtained from C57BL/6J mice at designated circadian times were fractionated and analyzed by Western blotting using CLOCK antibodies. Histone H4 and β-actin were used for normalization. N, nuclear fraction; C, cytoplasm fraction. (B) Quantitative analysis of CLOCK protein expression in nuclear and cytoplasm fraction of the SCN. Data represent the average of three independent experiments. Each value represents a percentage of maximal level of CLOCK in nucleus or cytoplasm.
Although in vivo CLOCK oscillation in the nuclear fraction of the SCN cells in DD strongly suggested its involvement in the basic clock mechanism, this was a puzzling observation, as the lowest levels of CLOCK were detected in the nucleus at the known time of maximal transcriptional activity of CLOCK/BMAL1 with respect to target clock gene (mPer1 and mPer2) activation (Shearman et al. 1997; Sun et al. 1997). We hypothesized that the regulation of CLOCK/BMAL1 transcriptional activity may be more complex than just oscillation in abundance and might involve posttranslational modifications or interactions with other proteins. To address the structural and functional aspects of the observed phenomenon and its potential connection with the regulation of CLOCK/BMAL1 transactivation, we used an experimental system based on the analysis of ectopically expressed proteins in a tissue culture model.
Posttranslational modifications of CLOCK and BMAL1 are induced by their dimerization
The cell culture transient transfection system provides an attractive model for the simultaneous monitoring of protein interactions, modifications, and functional activity. To discriminate between endogenously and exogenously expressed CLOCK and BMAl1, we generated HA- and Flag-tagged expression constructs of CLOCK and BMAL1 and functionally tested corresponding proteins for the ability to bind DNA (using EMSA) and to activate the reporter gene transcription (using luciferase reporter assay). As expected, both the mobility shift and transactivation of target reporter construct were detected only when CLOCK and BMAL1 were coexpressed (Supplementary Fig. 1A,B). Both functional activities were not affected by the presence of either HA or Flag tags, demonstrating that the expression system based on ectopic expression of both proteins provides an adequate model for biochemical studies.
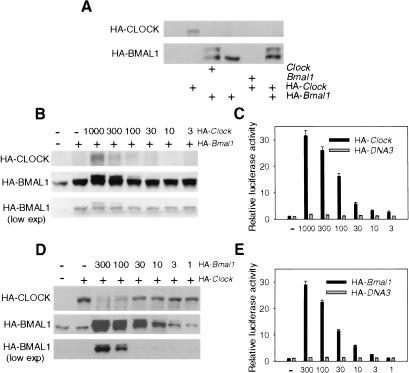
To examine CLOCK and BMAL1 proteins biochemically, HEK-293 cells were transiently transfected with corresponding expression constructs (pcHA-Clock and/or pcHA-Bmal1) and total cell lysates were analyzed by Western blotting. Individually expressed HA-CLOCK and HA-BMAL1 are detected as single bands of expected sizes (Fig. 2A). Interestingly, coexpression of both proteins resulted in changes of their expression pattern and total protein abundance. Thus, an additional HABMAL1-specific band with slower electrophoretic mobility was detected only in the cells cotransfected with pc-Clock or pcHA-Clock expression constructs. This new band represents a BMAL1 phosphorylated form that has been described recently in vivo (Lee et al. 2001) and was also confirmed in our experiments (see below). Quite unexpectedly, coexpression with either BMAL1 or HABMAL1 resulted in a dramatic decrease in the amounts of CLOCK protein in transfected cells. These effects depend neither on the cell type used nor on the presence of HA tag; the same results were obtained when NIH-3T3 cells or Flag-tagged expression constructs were used for transfection (data not shown). These results indicate that changes in the protein level in both partners of the CLOCK/BMAL1 complex are induced by their interaction.
Figure 2.
Effects of CLOCK and BMAL1 coexpression on their posttranslational modifications. (A) Western blot analysis of HEK-293 cells transfected with different combinations of Clock and Bmal1 expression plasmids. Dose dependence of HA-BMAL1 posttranslational modification (B), and transactivation of mPer1-Luc reporter (C) on HA-CLOCK amount. HEK-293 cells were transfected with constant amounts of pcHA-Bmal1 (100 ng), mPer1-Luc (50 ng), and pcDNA3-lacZ and indicated amount of pcHA-Clock. Dose dependence of HA-CLOCK abundance (D) and transactivation of mPer1-Luc reporter (E) on HA-BMAL1 amount. HEK-293 cells were transfected with constant amounts of pcHA-Clock (300 ng), mPer1-Luc (50 ng), and pcDNA3-lacZ and indicated amounts of pcHA-Bmal1. Expression of HA-CLOCK and HA-BMAL1 proteins in transfected cells were detected by Western blotting with anti-HA antibodies. Typical result from three independent experiments is presented. Note that in cells transfected by pcHA-Clock only, anti-HA antibodies at longer exposures recognize nonspecific band with electrophoretic mobility close to HA-BMAL1.
To characterize the effect of complex formation on each partner in more detail, we performed a series of cotransfection experiments, keeping one component at a constant concentration and varying the amount of the other one, and monitoring the changes in proteins profiles and abundance. Simultaneously, we monitored the functionality of the CLOCK/BMAL1 complex by testing its transactivation ability.
Western blot analysis of BMAL1 in transfected cells demonstrated that the abundance of the phosphorylated form of BMAL1 correlated with the amount of CLOCK protein expressed (Fig. 2B), indicating that the presence of CLOCK is critical for inducing BMAL1 modification. The effect of BMAL1 on CLOCK expression was quite different; the highest levels of CLOCK were detected in the cells with low BMAL1 levels. Increasing the amount of pcHA-Bmal1 plasmid from 1 to 300 ng resulted in a significant decrease in CLOCK abundance (Fig. 2D), presumably explained by its proteolytic degradation (BMAL1 had no effect on the activity of CMV promoter; data not shown). Interestingly, the level of activation of CLOCK/BMAL1-responsive promoter correlated directly with the amounts of pcHA-Clock (Fig. 2C) or pcHA-Bmal1 (Fig. 2E) in both experimental setups. Such correlation of low CLOCK abundance with high-transcriptional activity of circadian complex is consistent with our observation of nuclear CLOCK oscillation in the SCN, when low nuclear CLOCK abundance occurred at the time of CLOCK/BMAL1 maximal transcriptional activity (Fig. 1A). Thus, CLOCK degradation most likely occurs after or simultaneously with target-gene activation. Taken together, these results suggest that in addition to BMAL1 phosphorylation, CLOCK/BMAL1 heterodimerization induces CLOCK proteolytic degradation, and both modifications are tightly coupled with transactivation of responsive promoters.
Effects of CLOCK and BMAL1 coexpression on their intracellular distribution
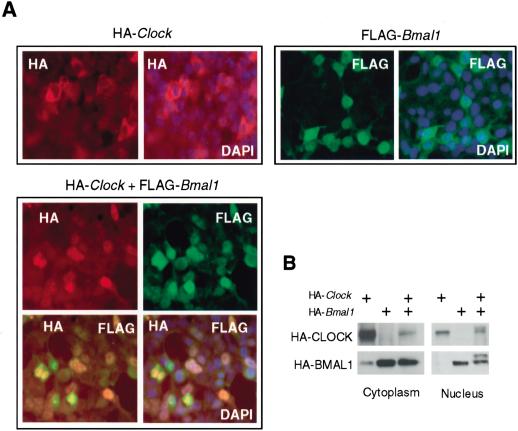
Because our in vivo data demonstrated differential distribution of CLOCK in nuclear and cytoplasm fraction in the SCN, we decided to examine whether this effect depends on CLOCK interaction with BMAL1. To analyze cellular distribution of both proteins, we performed in situ staining of HEK-293 cells transfected with HA-CLOCK and Flag-BMAL1 with fluorescently labeled anti-HA and anti-Flag antibodies (Fig. 3A). According to this assay, BMAL1 (green fluorescence), when ectopically expressed alone, is concentrated in the nuclei and gives a very weak diffuse signal in the cytoplasm of transfected cells. On the contrary, ectopically expressed HA-CLOCK is not detected in the nuclei of transfected cells and shows exclusively cytoplasmic localization. In contrast to BMAL1, CLOCK signal is not diffuse, but concentrates mostly around the nuclei. Interestingly, coexpression of both proteins has no effect on BMAL1 localization, but dramatically changes CLOCK intracellular distribution, which is now detected predominantly in the nuclei with practically no staining in the cytoplasm (Fig. 3A, bottom). The overlay of fluorescent signals corresponding to both proteins confirmed their colocalization in the nucleus. Thus, coexpression of BMAL1 promotes CLOCK nuclear accumulation.
Figure 3.
Nuclear/cytoplasm distribution of ectopically expressed CLOCK and BMAL1. (A) In situ detection of HA-CLOCK and Flag-BMAL1. HEK-293 cells were either individually transfected with pcHA-Clock and pcFlag-Bmal1 or cotransfected with both constructs. Secondary antibodies were conjugated with Texas Red (red fluorescence) for HA-CLOCK detection and FITC (green fluorescence) for Flag-BMAL1 detection. Orange color represents costaining of CLOCK and BMAL1 in cotransfected cells. DAPI (blue fluorescence) staining was used to visualize nuclei. (B) Western blot analysis of nuclear and cytoplasm fractions of HEK-293 cells transfected with pcHA-Clock and pcHA-Bmal1 in different combinations and probed with anti-HA antibodies.
BMAL1-dependent accumulation of nuclear CLOCK was confirmed by subcellular fractionation of HEK-293 cells transfected with either individual pcHA-Clock or pcHA-Bmal1 expression constructs, or cotransfected with both, followed by Western blot analysis of distribution of both proteins between nuclear and cytoplasmic fractions (Fig. 3B). Whereas coexpression of CLOCK and BMAL1 does not change BMAL1 expression level or sub-cellular distribution, it induces significant reduction in cytoplasmic CLOCK abundance. On the basis of our in situ staining data, such a decrease in cytoplasmic CLOCK may at least be partially explained by its BMAL1-dependent nuclear translocation. However, detected lower levels of nuclear CLOCK coexpressed with BMAL1 compared with high levels of individually expressed cytoplasmic CLOCK suggest that coexpression with BMAL1 results also in CLOCK degradation that may occur both in the nucleus and the cytoplasm of transfected cells. Interestingly, in contrast to our in situ data, CLOCK was detected in the nuclear fraction of cells individually transfected with pcHA-Clock (Fig. 3B). On the basis of the pattern of CLOCK in situ staining (spots concentrated around the nucleus as opposed to the diffuse signal across the cytoplasm), we believe that this band may represent the fraction of CLOCK associated with either endoplasmic reticulum or some other nuclear membrane structure, coisolating with the nuclear fraction. Alternatively, it may represent cosedimenting with the nuclei aggregates of cytoplasmic CLOCK, which are formed under the conditions of its overexpression. Subcellular fractionation of HEK-293 cells transfected with different combinations of expression constructs also facilitated the detection of slow-mobility bands, presumably corresponding to previously described phosphorylated forms of CLOCK and BMAL1 (Lee et al. 2001). Modified forms of both proteins were detected only in the nuclear fraction and only when both proteins were coexpressed (Fig. 3B). Slow-migrating bands disappeared if immunoprecipitated complexes were treated with alkaline phosphatase, and this effect was blocked by vanadate (data not shown), indicating that the detected additional bands correspond to phosphorylated forms of the proteins. Taken together, our observations on cellular localization of ectopically expressed proteins demonstrate that their coexpression triggers the set of tightly coupled posttranslational events, CLOCK and BMAL1 codependent phosphorylation and BMAL1-induced CLOCK nuclear translocation and degradation.
Proteasome-dependent and -independent degradation of CLOCK induced by interaction with BMAL1
BMAL1-induced decrease in total CLOCK protein abundance observed in our experiments may be explained by the increasing rate of its degradation triggered by CLOCK/BMAL1 complex formation. In an attempt to test whether the ubiquitin/proteasome pathway is involved in CLOCK protein turnover regulation, we treated cells transfected with pcHA-Clock, alone or in combination with pcHA-Bmal1, with different inhibitors of protein degradation, and analyzed CLOCK protein abundance in nuclear and cytoplasmic fractions by Western blot. None of the inhibitors tested affected CLOCK abundance when it was individually expressed. When coexpressed with BMAL1, only treatment with MG132, a potent inhibitor of 26S proteasome, resulted in CLOCK accumulation in the nucleus (Suppementary Fig. 2). All inhibitors had a very minor effect on a cytoplasmic CLOCK abundance. These data are consistent with our model, according to which CLOCK translocation to the nucleus in the complex with BMAL1 is followed by its subsequent degradation through proteasome-dependent mechanism. It cannot, however, explain the overall dramatic reduction in cytoplasmic CLOCK abundance triggered by BMAL1 expression, suggesting that CLOCK degradation may occur in the cytoplasm, where it goes through the proteasome-independent pathway.
The effect of BMAL1 expression on CLOCK turnover was confirmed by treating the cells with the protein synthesis inhibitor cycloheximide. Whereas no effect of cycloheximide on CLOCK abundance was detected in the cells transfected with pcHA-Clock only, rapid elimination of CLOCK from the nuclear fraction was observed in the cells transfected with the combination of constructs (Supplementary Fig. 2).
Direct CLOCK/BMAL1 interaction through PAS domain is necessary for BMAL1 phosphorylation and CLOCK nuclear translocation and degradation
Alterations in proteins' modifications, translocation, and degradation observed in our experiments always required coexpression of CLOCK and BMAL1, suggesting that dimerization of two transcription factors is the critical step in these processes. Like other members of PAS-domain family of proteins, CLOCK and BMAL1 form a complex through PAS domain interaction (Gekakis et al. 1998; Hogenesch et al. 1998). To estimate the role of PAS domain in posttranslational modifications of circadian transcription factors, we constructed two HA-tagged Clock deletion mutants deficient in bHLH and either one (Δ1-299) or both (Δ1-334) PAS domains and express them in HEK-293 cells individually or in combination with pcHA-Bmal1. Both mutants were detected on Western blots as bands with predicted molecular weights when probed with anti-HA antibodies (Fig. 4A). As expected, they lost their ability to activate mPer1 promoter in transactivation assay (data not shown). In contrast to effects observed with full-length CLOCK coexpression, BMAL1 expression had no effect on phosphorylation or total CLOCK protein abundance of truncated CLOCK proteins (Fig. 4A). Consistently, none of the mutants exerted any effect on BMAL1 phosphorylation and showed mostly cytoplasmic localization. Taken together, these results suggest that direct interaction between CLOCK and BMAL1 through the PAS domain is required for BMAL1 and CLOCK phosphorylation and CLOCK nuclear translocation and degradation.
Figure 4.
Posttranslational modifications of CLOCK and BMAl1 are induced only by specific complex formation through the PAS domain. (A) Western blot analysis of nuclear and cytoplasm fractions of HEK-293 cells cotransfected by pcFlag-Bmal1 and HA-tagged CLOCK expression constructs (either full-length or deletion mutants). Anti-HA and anti-Flag antibodies were used for CLOCK and BMAL1 detection. Black asterisks designate bands corresponding to BMAL1 and full-length CLOCK; white asterisks designate bands corresponding to CLOCK truncated products of expected sizes. N, nucleus; C, cytoplasm. (B) Western blot analysis of HA-CLOCK and HA-BMAL1 ectopically expressed in HEK-293 cells in different combinations with Arnt, Ahr, and Hif1α expression plasmids and detected with anti-HA antibodies.
It has been demonstrated previously that the members of PAS-domain family of proteins can form complexes in various combinations (Swanson et al. 1995; Hogenesch et al. 1997, 1998; Takahata et al. 1998). We therefore checked whether other members of the PAS domain proteins family could dimerize with either CLOCK or BMAL1 and induce similar posttranslational events.
PAS domain-containing proteins HIF1α, AHR, and ARNT were ectopically expressed in different combinations with HA-CLOCK and HA-BMAL1 followed by Western blot analysis of total cell lysates. Results presented in Figure 4B showed that BMAL1 phosphorylation, as well as CLOCK degradation, was detected only in the cells expressing both proteins, and none of the PAS proteins coexpressed with either CLOCK or BMAL1 could induce a similar effect. Nuclear/cytoplasm distribution of CLOCK and BMAL1 proteins was not affected either (data not shown). These results suggest that posttranslational modifications of circadian transcription factors CLOCK and BMAL1 are induced specifically by their interaction, and cannot be substituted by other PAS domain-containing proteins.
BMAL1-dependent circadian oscillation of nuclear CLOCK is part of basic circadian mechanism
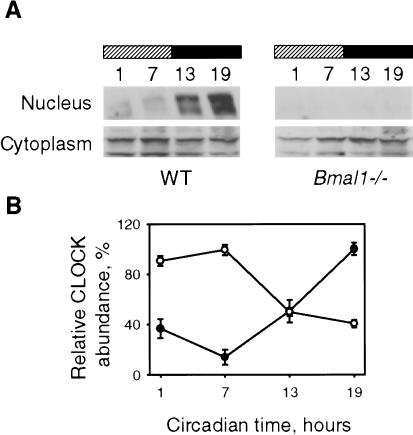
BMAL1-dependent subcellular localization, phosphorylation, and degradation of CLOCK have been determined under experimental conditions of ectopic overexpression of these proteins. To test whether this mechanism exists and is involved in regulation of the circadian complex under natural conditions, we analyzed CLOCK nuclear/cytoplasm distribution in mouse embryo fibroblasts (MEFs) isolated from mice with genetic defects in different components of circadian system. The disruption of circadian oscillator due to the Clock mutation or targeted disruption of both Cryptochrome genes (Cry1-/-Cry2-/-) had no effect on protein localization; CLOCK-specific immunoreactivity was detected both in the nucleus and cytoplasm, indistinguishable from control fibroblasts isolated from wild-type embryos. At the same time, in Bmal1-/- fibroblasts, CLOCK protein was detected exclusively in the cytoplasm, where its expression level was comparable with that of the control, wild-type fibroblasts (Fig. 5A). This fact indicates that BMAL1 has no effect on CLOCK expression level, but it is critical for CLOCK nuclear accumulation.
Figure 5.
CLOCK nuclear localization is BMAL1-dependent. (A) Western blot analysis of CLOCK in primary fibroblasts derived from mouse embryos of different circadian genotypes. No CLOCK-specific immunoreactivity could be detected in Bmal1-/- MEFs. Histone H4 and β-actin were used for normalization of nuclear and cytoplasm fractions. (B) Western blot analysis of CLOCK temporal expression profile in wild-type and Bmal1-/- MEFs stimulated by serum shock. Serum was added at time point 0 and removed after 2 h. Two distinct peaks of CLOCK abundance in the nuclear fraction of wild-type fibroblasts are detected. An arrow indicates the position of CLOCK band. No CLOCK immunoreactivity is detected in the nuclear fraction of Bmal1-/- MEFs at all times tested (note that different exposure times used for detection of nuclear CLOCK in wild-type and Bmal1-/- fibroblasts). (C) Quantitative analysis of CLOCK nuclear/cytoplasm distribution in wild-type MEFs. •, nuclear CLOCK; ○, cytoplasmic CLOCK. Each value represents a percentage of the maximal level of CLOCK in the nucleus or the cytoplasm.
To further investigate the involvement of CLOCK nuclear/cytoplasm localization control in the basic clock mechanism, we monitored CLOCK subcellular distribution in cultured fibroblasts at different time points after artificially induced synchronous circadian oscillation of transcription. As described previously, serum shock stimulates oscillation in major clock genes mRNA abundance in cultured fibroblasts with the period close to 24 h, which sustains in culture for few circadian cycles (Balsalobre et al. 1998, 2000). In our experiments, we monitored the induction of circadian oscillation at the transcriptional level by estimating mPer1 and mBmal1 mRNA abundance in cells collected every 4 h during the period of 48 h after the serum shock. Both genes demonstrated rhythmicity in their expression profiles similar to ones described previously (data not shown). Induction of circadian oscillation at the transcriptional level was accompanied by circadian cycling in CLOCK accumulation, both in the nucleus and the cytoplasm (Fig. 5B); during the 48-h period, nuclear CLOCK demonstrates two peaks in abundance that occurred at 12 and 36 h after the treatment, whereas peaks of the cytoplasmic CLOCK occurred at 24 and 48 h after the treatment. The 24-h periodicity argues for the circadian nature of the process. Western blot data quantitation (Fig. 5C) shows that, similar to the results obtained for the SCN, the oscillation of nuclear/cytoplasm distribution of CLOCK occurs in anti-phase. In contrast to wild-type fibroblasts, cells derived from Bmal1-deficient mice demonstrated exclusively cytoplasmic CLOCK localization, and serum shock failed to stimulate its nuclear accumulation (Fig. 5B). Taken together, these data demonstrate that the regulation of CLOCK subcellular distribution is involved in basic clock mechanism and is BMAL1-dependent.
To test whether observations made in tissue culture reflect the in vivo situation, we compared nuclear/cytoplasm distribution of CLOCK in the livers of wild-type and Bmal1-/- mice. Similar to CLOCK temporal profile in the SCN (Fig. 1A), in livers of control mice, both nuclear and cytoplasmic levels of CLOCK protein exhibit circadian oscillation, which is 180 degrees out-of-phase (Fig. 6A,B) with nuclear CLOCK lowest levels occurring at the time corresponding to predicted maximal transcriptional activity. In the livers of Bmal1-deficient mice, no CLOCK immunoreactivity was detected in nuclear fraction, supporting our previous observations made in cultured fibroblast that BMAL1 is required for CLOCK nuclear translocation.
Figure 6.
BMAL1 is required for circadian oscillation of nuclear CLOCK in mouse liver. (A) Western blot analysis of nuclear and cytoplasm CLOCK expression in livers of control wild-type and Bmal1-/- mice isolated at indicated circadian times. Note that longer exposure time was used for the nuclear fraction. (B) Quantitative analysis of CLOCK nuclear/cytoplasm distribution in livers of control mice. •, nuclear CLOCK; ○, cytoplasmic CLOCK. Mean data of three independent experiments are shown. Each value represents a percentage of the maximal level of CLOCK in the nucleus or the cytoplasm.
Discussion
CLOCK and BMAL1 are the key components of the mammalian circadian clock that form a positive limb of the major transcriptional autoregulatory feedback loop. In addition to core clock genes (Periods and Crypto- chromes), CLOCK/BMAL1 may activate a set of clock-controlled genes, thus linking clocks to output physiology under circadian control (Reppert and Weaver 2001). Similar to other members of PAS-domain family transcription factors, they have to dimerize in order to activate the responsive promoters. Studies of temporal profiling at the mRNA and the protein level suggested the critical role of BMAL1 for promoting circadian periodicity of transcriptional activation. Thus, it was demonstrated that mBmal1 is rhythmically expressed both in the SCN and in multiple body tissues, driving a BMAL1 protein rhythms (Lee et al. 2001).
Most studies demonstrate that, in contrast to mBmal1 expression, mClock mRNA does not show a circadian rhythm in the mouse SCN (Sun et al. 1997; Tei et al. 1997; Gekakis et al. 1998), and shows very low-amplitude oscillations in some peripheral tissues (Lee et al. 2001; Preitner et al. 2002; M. Antoch, unpubl.). No consistent oscillation pattern has been reported for CLOCK protein abundance either. In this study, we present evidence that circadian control of CLOCK occurs at the level of its BMAL1-dependent nuclear translocation. Such an accumulation may occur either as a result of increase in CLOCK nuclear import [similar to TIM-dependent PER nuclear entry described for Drosophila clock (Vosshall et al. 1994)] or decrease in nuclear CLOCK export [similar to CRY-dependent retarding of PER2 nuclear export implicated in mammalian system (Yagita et al. 2002)]. In both cases, this BMAL1-mediated translocation provides an additional regulatory level of circadian timekeeping machinery based on control of periodic availability and/or activity of transactivation complex. They also suggest the novel function for one of the core clock components—BMAL1—as a mediator of CLOCK nuclear accumulation.
The hypothetical model of such regulation is outlined schematically in Figure 7. Newly synthesized CLOCK protein is located predominantly in the cytoplasm, where it interacts with BMAL1 through the PAS-domain. This interaction by itself induces the cascade of posttranslational events potentially essential for regulating CLOCK/BMAL1 functional activities according to several possible scenarios (Fig. 7). According to one of the options, the complex formation may generate a signal for phosphorylation of both partners, which occurs in the cytoplasm. This modification, in turn, triggers heterodimer nuclear translocation similar to previously described Shaggy-regulated PER/TIM nuclear translocation (Martinek et al. 2001; Fig. 7A). After transactivation of responsive genes, one of the partners (CLOCK) is degraded, thus reducing nuclear concentration of active complex and preventing multiple rounds of activation.
Figure 7.
Schematic representation of posttranslational events induced by CLOCK/BMAL1 interaction. There are three possible scenarios: CLOCK/BMAL1 phosphorylation occurs in the cytoplasm and is required for BMAL1-dependent nuclear translocation of the complex (A); unphosphorylated CLOCK/BMAL1 are translocated to the nucleus (B,C), where they get phosphorylated either before (B) or after (C) initiation of transcription (see text for details).
Alternatively, the dimerization itself may serve as a signal for CLOCK/BMAL1 nuclear translocation followed by phosphorylation of both proteins in the nucleus, where it may be coupled to promoter transactivation and CLOCK proteolytic degradation (Fig. 7C). This mechanism is consistent with the recently proposed universal model of transcriptional regulation (a “black widow” model; Tansey 2001). According to this model, transcription factors interact with components of the basal transcriptional apparatus and recruit these components to start transcription. In turn, kinases in the basal transcriptional machinery [such as TFIIH (Lu et al. 1992) or TAFII250 (Dikstein et al. 1996)] mark transcription factors by phosphorylation, triggering their degradation. Several examples of ubiquitin-mediated proteasomal degradation of transcription factors coupled to phosphorylation and transactivation have been reported (Molinari et al. 1999; Salghetti et al. 1999; Wu et al. 2000). According to the third scenario, nuclear phosphorylation may not be coupled to promoter transactivation, but may rather work as a signal for CLOCK protein activation and/or degradation, thus limiting the availability of the functional complex for further rounds of transcriptional activation (Fig. 7B). Although we cannot at present choose among the above-described options, in any scenario, the proposed regulatory mechanism introduces a limiting step, preventing multiple rounds of activation, so when the critical concentration of circadian transcriptional repressors (i.e., CRYPTOCHROMES) is achieved, it is possible to effectively block transcriptional activation.
To fully decipher the CLOCK/BMAL1 interaction-based mechanisms proposed here, it would be important to localize the sites of CLOCK and BMAL1 phosphorylation as well as to define the kinases responsible for complex phosphorylation. Studies performed up to date in flies and mammals implied few kinases as essential for circadian function. Thus, in both systems, the essential role of casein kinase 1ε in PER phosphorylation, nuclear entry, and turnover has been demonstrated (Keesler et al. 2000; Lowrey and Takahashi 2000; Vielhaber et al. 2000). A second mammalian clock relevant kinase, casein kinase Iδ, phosphorylates mPER1 and mPER2 in vitro (Vielhaber et al. 2000; Camacho et al. 2001) and coimmunoprecipitates with major clock proteins in a time-dependent manner in vivo (Lee et al. 2001). Another kinase, Shaggy, regulates nuclear translocation of PER/TIM complexes in Drosophila (Martinek et al. 2001). Recently, it was demonstrated that CK1ε, implicated in PER phosphorylation, also phosphorylates BMAL1 in vitro and in cell culture (Eide et al. 2002). At the same time, CLOCK was not a direct substrate for CKIε according to this study as well as in our experiments; neither overexpression nor suppression of CKIε and GSK3δ with antisense and chemical inhibitors had significant effect on reporter construct transactivation or CLOCK/BMAL1 phosphorylation (data not shown). Thus, the kinase that phosphorylates CLOCK remains to be identified. The strong shift in electrophoretic mobility that we observe in SDS-PAGE suggests that CLOCK and BMAL1 are phosphorylated on multiple sites. It is possible that both proteins could be phosphorylated by different kinases. It has already been shown to be true for BMAL1, which can be the target for both CKIε and mitogen-activated protein kinase (Sanada et al. 2002). Mapping of the phosphorylation sites and characterization of biological properties of mutants deficient in certain phosphorylation sites should open new aspects of molecular regulation of CLOCK/BMAL1 functional activity.
Another finding reported here is the proteolytic degradation of CLOCK coupled to functional transactivation complex formation. Our data on inhibitory analysis suggest that transcriptional activation is closely coupled to CLOCK degradation, which, at least in part, goes through ubiquitin-mediated proteasomal degradation. It is known that the majority of transcription factors degrade through ubiquitin/proteasome-dependent mechanism, which is often preceded by protein phosphorylation. This mechanism has been also implicated for other components of the circadian system, Drosophila TIM (Naidoo et al. 1999; Grima et al. 2002) as well as Drosophila and mammalian PERs (Price et al. 1998; Keesler et al. 2000; Vielhaber et al. 2000). It has been also described previously for other members of PAS-domain transcription factors. For example, AHR, which under normal conditions is located in the cytoplasm, upon ligand binding gets transported to the nucleus, where it interacts with its partner ARNT. After binding to the specific dioxin response element and activating transcription from adjacent promoter, it is subject to rapid degradation through proteasome pathway. Another example is HIF1α, which under normoxic conditions, undergoes rapid degradation via the ubiquitin-proteasome pathway, but under hypoxic conditions, gets stabilized and in complex with ARNT, drives transcription of responsive promoters (for review, see Gu et al. 2000).
Is CLOCK proteolytic degradation observed in transient transfection experiments implicated in circadian function in vivo? If BMAL1 induces CLOCK nuclear degradation, one would predict that Bmal1 deficiency should result in the increased levels of the cytoplasmic CLOCK. However, no significant changes in total CLOCK levels were found in Bmal1-deficient fibroblasts as well as in the livers of Bmal1-/- mice as compared with wild-type controls (Figs. 5, 6). There are several possible explanations for the lack of a dramatic reduction in the levels of total CLOCK protein during the circadian cycle in vivo. First, in transient transfection experiments, both CLOCK and BMAL1 are greatly overexpressed, thus strengthening the natural effect of endogenous proteins, with HA-BMAL1 always being expressed at higher levels than HA-CLOCK (Fig. 2A), which is opposite to estimations made for in vivo expressed proteins (Lee et al. 2001). Alternatively, there may be some additional not yet identified factor(s) involved in CLOCK/BMAL1 complex stabilization in vivo that were not expressed at the adequately high levels in experiments with transient transfection. In any case, on the basis of correlation between low CLOCK nuclear abundance and high level of complex transcriptional activity, we believe that BMAL1-induced degradation of CLOCK occurs in normal condition in vivo, but it functions mostly for regulation of CLOCK/BMAL1 transcriptional complex activity, rather then total protein accumulation.
Interestingly, all observed posttranslational events leading to circadian transcriptional complex nuclear translocation and subsequent activation of responsive promoters are induced by CLOCK/BMAL1-specific dimerization and cannot be imitated by the interaction of either CLOCK or BMAL1 with other members of PAS-domain protein superfamily. To our knowledge, this is the first documented example of the dimerization of two transcription factors serving as a signal for inducing a cascade of functionally significant posttranslational events, and may represent a novel mechanism of transcriptional regulation that could be implicated beyond the circadian system.
Materials and methods
Plasmids
Luciferase reporter plasmid with mPer1 promoter region is described in Wilsbacher et al. (2002). Plasmids for expression HIF1α (PL611), ARNT (PL87), and AHR (PL65) were described in Dolwick et al. (1993) and Hogenesch et al. (1997). Full-length mClock, mBmal1, and mClock deletion mutants were amplified by PCR and cloned in pcDNA3-HA (see details in Supplemental Material).
Cell culture, transient transfection, and serum-shock procedures
HEK-293 and NIH-3T3 cells were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal calf serum and 100 U/mL of penicillin and streptomycin. Typically, cells were transfected in 6-well plates with the indicated amount of plasmids (final DNA amount was adjusted to 1000 ng with pcDNA3 vector) using LipofectAMINE PLUS reagents (Invitrogene) according to the manufacturer's protocol. A total of 1 ng of pcDNA3-βGal was added to normalize transfection efficiency. Cells were collected for analysis 24 h after transfection. Serum shock on primary MEFs was performed as described previously (Balsalobre et al. 1998). Briefly, cells were maintained in DMEM with 5% serum for 7 d. At time = 0, medium was replaced by 50% horse serum, and after 2 h, exchanged to serum-free medium. Cells were collected at indicated time points and used for total RNA isolation and nuclear/cytoplasm protein fractionation.
Preparation of nuclear/cytoplasm extracts of mouse SCN and livers
Wild-type C57BL/6J mice were synchronized to 12:12 light:dark cycle (LD 12:12) for a period of 2 wk before being placed in DD. Animals were sacrificed during the first cycle of DD with 6-h intervals (four time-points). Brains were removed, and SCN dissected under the microscope. SCN from 10 individual animals were pooled, homogenized in buffer A (20 mM HEPES at pH 7.9, 20 mM NaF, 1 mM Na3VO4, 1 mM Na4P2O7, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, and proteinase inhibitors cocktail) and used for nuclear/cytoplasm total protein fractionation as described below. Livers were cut into pieces, washed five times with cold PBS, homogenized in buffer A, and processed similarly to the SCN.
Total cell lysates and nuclear/cytoplasm fractionation
To obtain total cell lysates, cells were incubated on ice-cold RIPA [150 mM NaCl; 50 mM Tris-HCl at pH 8.0, 0.5% Na-Dox, 0.1% SDS, and proteinase cocktail (Sigma)]. For nuclear extract preparation, cells were suspended in buffer A and incubated on ice for 15 min. After adding NP40 to 0.2%, the cells were incubated on ice for another 15 min, and resuspended by vortexing for 15 sec. The nuclei were pelleted in a microfuge at full speed for 20 sec, and the supernatant was used as the cytoplasm fraction.
Western blotting procedure and antibodies
Cell lysates were normalized either by transfection efficiency (for in vitro expressed proteins) or by measuring total protein concentration (for primary MEFs, SCN, and liver extracts) using the Bio-Rad Dc Protein Assay kit according to the manufacturer's protocol. After normalization, proteins were separated in 6% SDS-PAGE and transferred to Immobilon-P membrane (Millipore). HA-tagged proteins were detected with Y11 antibodies (Santa Cruz Biotechnologies). Endogenous CLOCK was detected with guinea pig anti-CLOCK antibodies. Anti-H4 histone (N-18) and anti-actin (C-11) antibodies from Santa Cruz Biotechnologies were used to control for nuclear and cytoplasmic protein separation. HRP-conjugated anti-rabbit (Santa Cruz Biotechnologies) or anti-guinea pig (Jackson Laboratory) were used as secondary antibodies. Proteins were visualized with an ECL detection kit (Amersham).
EMSA
The following 43-mers were used for binding assays: 5′-TT TAGTGAAAAGCCGCCGCTCACGTGGCGAACTGCGTGA CTTG-3′ and 5′-TTTCAAGTCACGCAGTTCGCCACGTG AGCGGCGGCTTTTCACT-3′ as described in Lee et al. (1999; see Supplemental Material).
In situ staining for HA-CLOCK and Flag-BMAL1
Transfected cells were fixed with 4% paraformaldehyde/PBS for 5 min, washed twice with PBS, treated with 1% Triton X-100 PBS for 10 min, and incubated with either anti-HA rabbit polyclonal [Sc-805 (Y-11), 1:50 dilution, Santa-Cruz Biotechnology] or anti-Flag mouse monoclonal (1:250 dilution, Sigma) antibodies. After a 40-min incubation, cells were washed three times with PBS and incubated with corresponding secondary antibodies for another 40 min. As secondary antibodies, 1:300 dilutions of 115-096-068 FITC-conjugated goat anti-mouse IgG + IgM or 111-085-144 LRSC-conjugated goat anti-rabbit IgG (H + L) were used (Jackson ImmunoResearch Laboratories). Images were captured by Leica DMIRB inverted microscope equipped with MicroMax digital camera (Princeton Instruments) using ImagePro Plus software.
Reporter assays
For β-Galactosidase detection, cell lysates were incubated with 1 mg/mL ONPG solution as described in Sambrook et al. (1989). After incubation, OD were measured at 420 nm. Luciferase activity detection was performed with Luciferase Assay System (Promega) according to the manufacturer's protocol. Luminescence intensity was measured using Victor2 Wallac multichannel reader (Perkin Elmer).
Acknowledgments
This work was supported in part by grants from Ohio Cancer Research Associates to M.P.A. and NIH grants CA60730 and CA75179 to A.V.G. We thank Dr. J. Takahashi for providing expression clones for mClock and mBmal1 and Dr. Bradfield for expression clones for Hif1α (PL611), Arnt (PL87), Ahr (PL65), and Bmal1-/- knockout mice. We thank Dr. S. Reppert for the generous gift of anti-CLOCK antibodies. We thank Dmitry Gudkov for technical assistance with manuscript preparation.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Corresponding author.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1099503.
Footnotes
Supplemental material is available at www.genesdev.org.
References
- Balsalobre A., Damiola, F., and Schibler, U. 1998. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 93: 929-937. [DOI] [PubMed] [Google Scholar]
- Balsalobre A., Marcacci, L., and Schibler, U. 2000. Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr. Biol. 10: 1291-1294. [DOI] [PubMed] [Google Scholar]
- Camacho F., Cilio, M., Guo, Y., Virshup, D.M., Patel, K., Khorkova, O., Styren, S., Morse, B., Yao, Z., and Keesler, G.A. 2001. Human casein kinase Iδ phosphorylation of human circadian clock proteins period 1 and 2. FEBS Lett. 489: 159-165. [DOI] [PubMed] [Google Scholar]
- Dikstein R., Ruppert, S., and Tjian, R. 1996. TAFII250 is a bipartite protein kinase that phosphorylates the base transcription factor RAP74. Cell 84: 781-790. [DOI] [PubMed] [Google Scholar]
- Dolwick K.M., Swanson, H.I., and Bradfield, C.A. 1993. In vitro analysis of Ah receptor domains involved in ligand-activated DNA recognition. Proc. Natl. Acad. Sci. 90: 8566-8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eide E.J., Vielhaber, E.L., Hinz, W.A., and Virshup, D.M. 2002. The circadian regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase ε. J. Biol. Chem. 277: 17248-17254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekakis N., Staknis, D., Nguyen, H.B., Davis, F.C., Wilsbacher, L.D., King, D.P., Takahashi, J.S., and Weitz, C.J. 1998. Role of the CLOCK protein in the mammalian circadian mechanism. Science 280: 1564-1569. [DOI] [PubMed] [Google Scholar]
- Griffin E.A., Staknis, D., and Weitz, C.J. 1999. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science 286: 768-771. [DOI] [PubMed] [Google Scholar]
- Grima B., Lamouroux, A., Chelot, E., Papin, C., Limbourg-Bouchon, B., and Rouyer, F. 2002. The F-box protein Slimb controls the levels of clock proteins Period and Timeless. Nature 420: 178-182. [DOI] [PubMed] [Google Scholar]
- Gu Y.Z., Hogenesch, J.B., and Bradfield, C.A. 2000. The PAS superfamily: Sensors of environmental and developmental signals. Annu. Rev. Pharmacol. Toxicol. 40: 519-561. [DOI] [PubMed] [Google Scholar]
- Hogenesch J.B., Chan, W.K., Jackiw, V.H., Brown, R.C., Gu, Y.Z., Pray-Grant, M., Perdew, G.H., and Bradfield, C.A. 1997. Characterization of a subset of the basic-helix-loop-helix-PAS superfamily that interacts with components of the dioxin signaling pathway. J. Biol. Chem. 272: 8581-8593. [DOI] [PubMed] [Google Scholar]
- Hogenesch J.B., Gu, Y.Z., Jain, S., and Bradfield, C.A. 1998. The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc. Natl. Acad. Sci. 95: 5474-5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keesler G.A., Camacho, F., Guo, Y., Virshup, D., Mondadori, C., and Yao, Z. 2000. Phosphorylation and destabilization of human period I clock protein by human casein kinase I ε. Neuroreport 11: 951-955. [DOI] [PubMed] [Google Scholar]
- King D.P. and Takahashi, J.S. 2000. Molecular genetics of circadian rhythms in mammals. Annu. Rev. Neurosci. 23: 713-742. [DOI] [PubMed] [Google Scholar]
- King D.P., Zhao, Y., Sangoram, A.M., Wilsbacher, L.D., Tanaka, M., Antoch, M.P., Steeves, T.D., Vitaterna, M.H., Kornhauser, J.M., Lowrey, P.L., et al. 1997. Positional cloning of the mouse circadian clock gene. Cell 89: 641-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein D.C., Moore, R.Y., and Reppert, S.M. 1991. The suprachiasmatic nucleus: The mind's clock. Oxford University Press, New York.
- Kloss B., Price, J.L., Saez, L., Blau, J., Rothenfluh, A., Wesley, C.S., and Young, M.W. 1998. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase I ε. Cell 94: 97-107. [DOI] [PubMed] [Google Scholar]
- Kume K., Zylka, M.J., Sriram, S., Shearman, L.P., Weaver, D.R., Jin, X., Maywood, E.S., Hastings, M.H., and Reppert, S.M. 1999. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 98: 193-205. [DOI] [PubMed] [Google Scholar]
- Lee C., Bae, K., and Edery, I. 1999. PER and TIM inhibit the DNA binding activity of a Drosophila CLOCK-CYC/dBMAL1 heterodimer without disrupting formation of the heterodimer: A basis for circadian transcription. Mol. Cell. Biol. 19: 5316-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C., Etchegaray, J.P., Cagampang, F.R., Loudon, A.S., and Reppert, S.M. 2001. Posttranslational mechanisms regulate the mammalian circadian clock. Cell 107: 855-867. [DOI] [PubMed] [Google Scholar]
- Liu Y., Loros, J., and Dunlap, J.C. 2000. Phosphorylation of the Neurospora clock protein FREQUENCY determines its degradation rate and strongly influences the period length of the circadian clock. Proc. Natl. Acad. Sci. 97: 234-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrey P.L. and Takahashi, J.S. 2000. Genetics of the mammalian circadian system: Photic entrainment, circadian pacemaker mechanisms, and post-translational regulation. Annu. Rev. Genet. 34: 533-562. [DOI] [PubMed] [Google Scholar]
- Lu H., Zawel, L., Fisher, L., Egly, J.M., and Reinberg, D. 1992. Human general transcription factor IIH phosphorylates the C-terminal domain of RNA polymerase II. Nature 358: 641-645. [DOI] [PubMed] [Google Scholar]
- Martinek S., Inonog, S., Manoukian, A.S., and Young, M.W. 2001. A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell 105: 769-779. [DOI] [PubMed] [Google Scholar]
- Molinari E., Gilman, M., and Natesan, S. 1999. Proteasome-mediated degradation of transcriptional activators correlates with activation domain potency in vivo. EMBOJ. 18: 6439-6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo N., Song, W., Hunter-Ensor, M., and Sehgal, A. 1999. A role for the proteasome in the light response of the timeless clock protein. Science 285: 1737-1741. [DOI] [PubMed] [Google Scholar]
- Preitner N., Damiola, F., Lopez-Molina, L., Zakany, J., Duboule, D., Albrecht, U., and Schibler, U. 2002. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110: 251-260. [DOI] [PubMed] [Google Scholar]
- Price J.L., Blau, J., Rothenfluh, A., Abodeely, M., Kloss, B., and Young, M.W. 1998. double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell 94: 83-95. [DOI] [PubMed] [Google Scholar]
- Price M.A. and Kalderon, D. 2002. Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by Glycogen Synthase Kinase 3 and Casein Kinase 1. Cell 108: 823-835. [DOI] [PubMed] [Google Scholar]
- Reppert S.M. and Weaver, D.R. 2001. Molecular analysis of mammalian circadian rhythms. Annu. Rev. Physiol. 63: 647-676. [DOI] [PubMed] [Google Scholar]
- Salghetti S.E., Kim, S.Y., and Tansey, W.P. 1999. Destruction of Myc by ubiquitin-mediated proteolysis: Cancer-associated and transforming mutations stabilize Myc. EMBOJ. 18: 717-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch, E.F., and Maniatis, T. 1989. Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- Sanada K., Okano, T., and Fukada, Y. 2002. Mitogen-activated protein kinase phosphorylates and negatively regulates basic helix-loop-helix-PAS transcription factor BMAL1. J. Biol. Chem. 277: 267-271. [DOI] [PubMed] [Google Scholar]
- Schibler U. and Sassone-Corsi, P. 2002. A web of circadian pacemakers. Cell 111: 919-922. [DOI] [PubMed] [Google Scholar]
- Shearman L.P., Zylka, M.J., Weaver, D.R., Kolakowski Jr., L.F., and Reppert, S.M. 1997. Two period homologs: Circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron 19: 1261-1269. [DOI] [PubMed] [Google Scholar]
- Shearman L.P., Jin, X., Lee, C., Reppert, S.M., and Weaver, D.R. 2000a. Targeted disruption of the mPer3 gene: Subtle effects on circadian clock function. Mol. Cell. Biol. 20: 6269-6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman L.P., Sriram, S., Weaver, D.R., Maywood, E.S., Chaves, I., Zheng, B., Kume, K., Lee, C.C., van der Horst, G.T., Hastings, M.H., et al. 2000b. Interacting molecular loops in the mammalian circadian clock. Science 288: 1013-1019. [DOI] [PubMed] [Google Scholar]
- Sun Z.S., Albrecht, U., Zhuchenko, O., Bailey, J., Eichele, G., and Lee, C.C. 1997. RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell 90: 1003-1011. [DOI] [PubMed] [Google Scholar]
- Swanson H.I., Chan, W.K., and Bradfield, C.A. 1995. DNA binding specificities and pairing rules of the Ah receptor, ARNT, and SIM proteins. J. Biol. Chem. 270: 26292-26302. [DOI] [PubMed] [Google Scholar]
- Takahata S., Sogawa, K., Kobayashi, A., Ema, M., Mimura, J., Ozaki, N., and Fujii-Kuriyama, Y. 1998. Transcriptionally active heterodimer formation of an Arnt-like PAS protein, Arnt3, with HIF-1a, HLF, and clock. Biochem. Biophys. Res. Commun. 248: 789-794. [DOI] [PubMed] [Google Scholar]
- Tansey W.P. 2001. Transcriptional activation: Risky business. Genes & Dev. 15: 1045-1050. [DOI] [PubMed] [Google Scholar]
- Tei H., Okamura, H., Shigeyoshi, Y., Fukuhara, C., Ozawa, R., Hirose, M., and Sakaki, Y. 1997. Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature 389: 512-516. [DOI] [PubMed] [Google Scholar]
- Toh K.L., Jones, C.R., He, Y., Eide, E.J., Hinz, W.A., Virshup, D.M., Ptacek, L.J., and Fu, Y.H. 2001. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 291: 1040-1043. [DOI] [PubMed] [Google Scholar]
- van der Horst G.T., Muijtjens, M., Kobayashi, K., Takano, R., Kanno, S., Takao, M., de Wit, J., Verkerk, A., Eker, A.P., van Leenen, D., et al. 1999. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature 398: 627-630. [DOI] [PubMed] [Google Scholar]
- Vielhaber E., Eide, E., Rivers, A., Gao, Z.H., and Virshup, D.M. 2000. Nuclear entry of the circadian regulator mPER1 is controlled by mammalian casein kinase I ε. Mol. Cell. Biol. 20: 4888-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaterna M.H., Selby, C.P., Todo, T., Niwa, H., Thompson, C., Fruechte, E.M., Hitomi, K., Thresher, R.J., Ishikawa, T., Miyazaki, J., et al. 1999. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc. Natl. Acad. Sci. 96: 12114-12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall L.B., Price, J.L., Sehgal, A., Saez, L., and Young, M.W. 1994. Block in nuclear localization of period protein by a second clock mutation, timeless. Science 263: 1606-1609. [DOI] [PubMed] [Google Scholar]
- Welsh D., Logothetis, D., Meister, M., and Reppert, S. 1995. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron 14: 697-706. [DOI] [PubMed] [Google Scholar]
- Wilsbacher L.D., Yamazaki, S., Herzog, E.D., Song, E.J., Radcliffe, L.A., Abe, M., Block, G., Spitznagel, E., Menaker, M., and Takahashi, J.S. 2002. Photic and circadian expression of luciferase in mPeriod1-luc transgenic mice invivo. Proc. Natl. Acad. Sci. 99: 489-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Hemesath, T.J., Takemoto, C.M., Horstmann, M.A., Wells, A.G., Price, E.R., Fisher, D.Z., and Fisher, D.E. 2000. c-Kit triggers dual phosphorylations, which couple activation and degradation of the essential melanocyte factor Mi. Genes & Dev. 14: 301-312. [PMC free article] [PubMed] [Google Scholar]
- Yagita K., Tamanini, F., Yasuda, M., Hoeijmakers, J.H., van der Horst, G.T., and Okamura, H. 2002. Nucleocytoplasmic shuttling and mCRY-dependent inhibition of ubiquitylation of the mPER2 clock protein. EMBOJ. 21: 1301-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M.W. and Kay, S.A. 2001. Time zones: A comparative genetics of circadian clocks. Nat. Rev. Gene.t 2: 702-715. [DOI] [PubMed] [Google Scholar]
- Zheng B., Larkin, D.W., Albrecht, U., Sun, Z.S., Sage, M., Eichele, G., Lee, C.C., and Bradley, A. 1999. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature 400: 169-173. [DOI] [PubMed] [Google Scholar]