Abstract
Equine arteritis virus (EAV) is a positive-strand RNA virus that uses a discontinuous transcription mechanism to generate a nested set of six subgenomic mRNAs from which its structural genes are expressed. A stable bacterial plasmid (pEAV030) containing a full-length cDNA copy of the 12.7-kb EAV genome was constructed. After removal of a single point mutation in the replicase gene, RNA transcripts generated in vitro from pEAV030 were shown to be infectious upon electroporation into BHK-21 cells. A genetic marker mutation was introduced at the cDNA level and recovered from the genome of the progeny virus. The potential of pEAV030 as a tool to express foreign genes was demonstrated by the efficient expression of the chloramphenicol acetyltransferase (CAT) reporter gene from two different subgenomic mRNAs. The point mutation that initially rendered the full-length clone noninfectious was found to result in a particularly intriguing phenotype: RNA carrying this mutation can replicate efficiently but does not produce the subgenomic mRNAs required for structural protein expression. To our knowledge, this mutant provides the first evidence that the requirements for arterivirus genome replication and discontinuous mRNA synthesis are, at least partially, different and that these processes may be separated experimentally.
Keywords: equine arteritis virus, nidoviruses, coronaviruses, replication, heterologous gene expression
The genetic modification of RNA virus genomes depends on the availability of full-length cDNA clones from which functional RNA transcripts can be generated. In the case of positive-strand RNA viruses, the naked (recombinant) genome is infectious: it functions as the mRNA for viral replicase translation, and an infection can simply be initiated by transfection of a permissive host cell. On the basis of virion structure, genome organization and replication strategy, four major groups of animal positive-strand RNA viruses have been distinguished. For three of these groups, the picornaviruses, togaviruses, and flaviviruses, the construction of infectious cDNA clones has been described (1–7). Their availability has increased our understanding of many aspects of the replication and pathogenesis of these viruses, and has created new possibilities for vaccine development and heterologous protein expression (8, 9).
The fourth major group of animal positive-strand RNA viruses is comprised of the coronavirus and arterivirus families, which have recently been united in the new order of the Nidovirales (Tenth International Congress of Virology, Jerusalem, August 1996). Despite the remarkable differences in virion architecture and genome size (13–15 kb for arteriviruses, 27–32 kb for coronaviruses), an evolutionary link between these virus groups has been postulated (10, 11). The genomes of both virus groups are polycistronic (Fig. 1), and comparative sequence analysis strongly suggested that their replicase genes, but not their structural genes, are related by common ancestry. The nidovirus replicase is encoded by two large ORFs, 1a and 1b, of which the latter is expressed by ribosomal frameshifting (10, 12). The ORF1a and ORF1ab replicase polyproteins are processed extensively by a number of ORF1a-encoded proteinases (for reviews, see refs. 13 and 14). A key feature of nidovirus replication is the expression of the downstream structural genes (Fig. 1) from a nested set of subgenomic mRNAs that is generated by discontinuous transcription (for reviews, see refs. 15–17). In addition to being 3′-coterminal, the subgenomic mRNAs also contain a common 5′ “leader” sequence, which is derived from the 5′ end of the genomic RNA (Fig. 1). Despite numerous reports, the details of coronavirus discontinuous transcription are poorly understood. The 3′ end of the common leader is complementary to the promoter sequence for subgenomic mRNA transcription, of which multiple copies are present in the genome-length negative strand. This complementarity suggests a base pairing step between these two elements during discontinuous transcription and led to the early proposal of the so-called “leader-primed transcription model” (18, 19). Renewed discussion was incited by the more recent detection in infected cells of a set of subgenomic minus strands, complementary to the subgenomic mRNAs (20–23). Several transcription models, including polymerase jumping during minus-strand RNA synthesis, have now been put forward and are not necessarily mutually exclusive (21, 24–26). Although studied in less detail, the mechanism of arterivirus subgenomic RNA transcription appears to be, in essence, identical to that of coronaviruses (23, 27–29).
Figure 1.
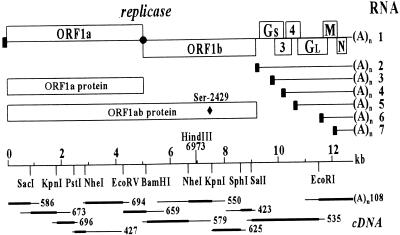
Genome organization and expression of the arterivirus prototype equine arteritis virus (EAV). (Upper) EAV genome and its ORFs, the replicase polyproteins, and the nested set of subgenomic mRNAs (RNAs 2–7). The black box represents the common leader sequence. The structural proteins GS, GL, M, and N are encoded by the ORFs 2, 5, 6, and 7, respectively. (Lower) The cDNA clones (10) and restriction sites used for the construction of pEAV030 are shown. The HindIII site at nt 6973, which was created in pEAV030H by the introduction of a marker mutation, and the position of Ser-2429 in the replicase polyprotein, are indicated.
Obviously, an infectious cDNA clone would be an important tool to study the unique features of nidovirus replication and transcription. Compared with coronaviruses, the considerably smaller genome size of arteriviruses offers important advantages. The 12.7-kb genome of EAV, the arterivirus prototype (10, 30), is infectious upon transfection (31) and during replication six subgenomic mRNAs are generated (Fig. 1). This report describes the construction of pEAV030, the first infectious nidovirus cDNA clone. The potential for heterologous gene expression from EAV subgenomic mRNAs was demonstrated by using a reporter gene. Fortuitously, we also obtained a noninfectious cDNA clone that carries an intriguing replicase point mutation. RNA containing this mutation replicated efficiently, but no virus particles were produced due to the fact that subgenomic mRNA synthesis, and therefore also the expression of viral structural proteins, was blocked.
METHODS
Cells and Virus.
Baby hamster kidney cells (BHK-21; ATCC CCL10) and the EAV Bucyrus strain (32) were used for all experiments. As usual, infection experiments with EAV were carried out at 39.5°C, because this elevated temperature substantially reduces the replication time of the virus (27).
Determination and Reconstruction of the 5′ End of the EAV Genome.
Direct RNA sequencing was performed according to the method of Fichot and Girard (33). The most 5′ EAV nucleotide was determined using the 5′-Amplifinder RACE kit (CLONTECH) to perform the single-strand ligation to single-stranded cDNA procedure (34). Using a PCR, cDNA clone 586 (Fig. 1) was extended with the newly determined 5′ sequence. At the same time, an upstream NotI restriction site and T7 RNA polymerase promoter were introduced.
Construction of Full-Length EAV cDNA Clones.
The initial full-length clone, pEAV030F, was constructed in plasmid pUC18 following a multistep strategy based on the genomic cDNA library (Fig. 1) (10). The correct 5′-terminal sequence and upstream T7 RNA polymerase promoter were derived from the extended version of clone 586 described above. At the 3′ end, the short poly(A) tail present in cDNA clone 108 was extended to ≈140 nt and a unique downstream XhoI restriction site was introduced. Details of this procedure will be published elsewhere. Subsequently, a point mutation at nt 7508 was removed from pEAV030F using a newly generated reverse transcription–PCR (RT-PCR) fragment. RNA transcribed from this new clone, pEAV030, was infectious. Finally, pEAV030H was created by introducing a marker mutation into pEAV030. Using PCR mutagenesis (35), nt C-6973 was replaced by A, creating a HindIII restriction site in the ORF1b region.
pEAV030-Based Chloramphenicol Acetyltransferase (CAT) Expression Vectors.
A restriction fragment containing the CAT reporter gene was inserted upstream of ORF7 into a HindIII site (nt 12,303) in pEAV030H. Clones containing the CAT gene in the sense and antisense orientation were obtained and were named pEAVCAT7 and pEAVTAC7, respectively. An NcoI restriction site was engineered at the ORF2 initiation codon in pEAV030H (nt 9824) and pEAVCAT2 was obtained by inserting the CAT gene between this novel NcoI site and a downstream ORF2 PstI site (nt 10,046).
RNA Transcription and Transfection.
Full-length transcripts were generated in vitro from XhoI-linearized plasmid DNA. The reaction conditions have been described (23), but NTP concentrations were raised to 1 mM each and 1 mM of capping analog [m7G(5′)ppp(5′)G; GIBCO/BRL] was added. The RNA quality and yield were assessed by agarose gel electrophoresis and spectrophotometry. RNA transcripts were introduced into BHK cells by means of electroporation. Cells were grown to subconfluence, trypsinized, washed with PBS, and resuspended in PBS at a concentration of 107 cells per ml. RNA transcript (10–20 μg) was added to 600 μl of this cell suspension in an electroporation cuvette (0.4 cm diameter). Two pulses of 850 V, 2310 Ω, and 25 μF were given with an Equibo EasyJect Plus electroporator (Eurogentec, Liège, Belgium), after which the cells were seeded and incubated at 39.5°C. For plaque assays, electroporated cells were allowed to attach to the surface for 2 hr. Subsequently, fresh BHK cells were added to obtain a confluent cell layer. After another hour, superfluous cells were removed and an agar overlay was applied. Plaques were detected after 48–72 hr at 39.5°C.
Immunofluorescence Assays (IFAs).
Cells were formaldehyde-fixed as described (36). A rabbit antiserum directed against nonstructural protein 2 (nsp2) (37) was used to detect EAV replicase synthesis. Antisera directed against various structural EAV proteins have been described (38, 39) and were kindly provided by A. Glaser and A. A. F. de Vries (Utrecht University). A CAT-specific rabbit antiserum was purchased from 5 Prime → 3 Prime Inc.
RNA Analysis.
Procedures for metabolic RNA labeling, RNA isolation, and RNA electrophoresis have been described (29). Poly(A)-selection was carried out using the batch method described by Sambrook et al. (40). Metabolic RNA labeling was performed using 250 μCi (1 Ci = 37 GBq) of [3H]uridine per ml of medium, in the presence of 10 μg/ml of dactinomycin to inhibit host RNA synthesis.
RT-PCR to Detect the Marker Mutation.
RT reactions on intracellular (i.c.) RNA from transfected cells were carried out using Moloney murine leukemia virus reverse transcriptase (GIBCO/BRL). For the RNA1 RT-PCR, an RT primer complementary to nt 7534–7550 was used. Subsequently, a PCR was performed using the RT primer and a primer corresponding to nt 6335–6355. For the RNA7 RT-PCR, an RT primer complementary to nt 12,680–12,707 (the genomic 3′ end) was used. The RNA7 PCR was carried out using primers corresponding to nt 81–100 (in the leader sequence) and the complement of nt 12,692–12,708 (in the body sequence). As a control, the RNA1 and RNA7 PCR products were digested with HpaI (nt 7303) and BspHI (nt 12,533), respectively. The presence of the marker mutation (nt 6973) in the RNA1 RT-PCR fragment derived from pEAV030H RNA was established by digestion with HindIII.
RESULTS
Determination of the 5′ End of the EAV Genome.
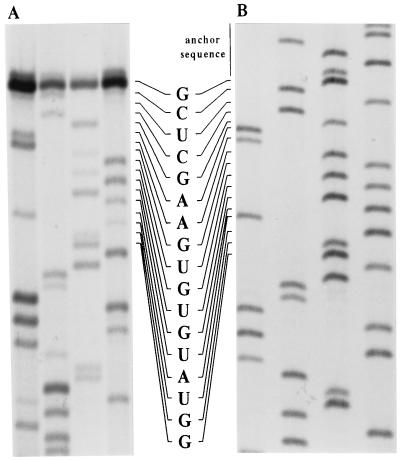
Previously, the EAV genomic sequence, with the exception of the 17 most 5′ nucleotides, had been determined (10). To obtain the missing sequence, direct RNA sequencing was carried out on i.c. RNA from EAV-infected cells (Fig. 2A). A novel sequence of 16 nt (nt 2–17, CUCGAAGUGUGUAUGG) was revealed. The first nucleotide could not be read and was obtained using the single-strand ligation to single-stranded cDNA method (34). Out of six RT-PCR clones, two had an A and four had a G as the most 5′ EAV nucleotide (Fig. 2B). Therefore, a G was used at position 1 of the full-length EAV cDNA clone.
Figure 2.
Sequence analysis of the EAV genomic 5′ end. Lane order (left to right): A, C, G, U/T. (A) Direct RNA sequence analysis of i.c. RNA from EAV-infected cells using a primer complementary to nt 81–100 of the leader sequence. (B) Sequence analysis of one of the RT-PCR clones containing the genomic 5′ end, which was obtained using the 5′-Amplifinder RACE kit.
Construction of a Full-Length EAV cDNA Clone.
A full-length cDNA copy of the EAV genome downstream of the T7 RNA polymerase promoter was engineered in plasmid pUC18 by joining restriction fragments from suitable cDNA clones (Fig. 1). Modified versions of clones 586 and 108 were used to introduce the proper 5′- and 3′-terminal sequences. However, in vitro-transcribed RNA from this clone, named pEAV030F, was not infectious.
The entire EAV sequence had previously been determined from two independent cDNA clones (10). Mismatches were resolved by sequencing a third clone to obtain a consensus sequence. However, for two differences a third clone was not available at that time: nt 5899 and 7508 were both found to be either C or T. When pEAV030F, containing T-5899 and C-7508, was found to be noninfectious, these two positions were redetermined. Analysis of newly generated RT-PCR products revealed that T is the correct nucleotide in both cases. Thus, construct pEAV030F contained a mutation at nt 7508, resulting in a Ser-2429 → Pro change in the replicase polyprotein. This mutation was removed and electroporation of transcripts from the new full-length clone, pEAV030, resulted in the cytopathogenic effects (c.p.e.) that are typical of EAV infection. A translationally silent marker mutation was introduced into the ORF1b region of pEAV030, thereby creating a novel HindIII restriction site. The clone carrying this tag, pEAV030H, was used for further studies.
pEAV030H Transcripts Are Infectious.
BHK cells were transfected with in vitro-generated EAV030H RNA and used for various assays. The following control transfections were included: i.c. RNA from EAV-infected cells (“i.c. EAV RNA”), EAV030 RNA, pEAV030H plasmid DNA, and a 3′-truncated pEAV030H transcript (EAV030HEco), obtained by linearization at a unique EcoRI restriction site (nt 11,488) and thus lacking the 3′-terminal 1.2 kb of the EAV genome.
Transfections with i.c. EAV RNA, EAV030 RNA, and EAV030H RNA all resulted in complete c.p.e. within 36 hr. Thus, the introduction of the ORF1b marker mutation had not affected the infectivity of the infectious clone. From various infectious centre assays and IFAs, the specific infectivity of EAV030 and EAV030H transcripts was calculated to vary between 103 and 104 plaques/μg. Plaques or c.p.e. were not observed when pEAV030H DNA or EAV030HEco RNA were transfected.
The viral RNA synthesized in electroporated cells was 3H-labeled in the presence of dactinomycin from 6 to 15 hr after transfection. Poly(A)-containing RNA was prepared and analyzed by denaturing agarose gel electrophoresis. In cells electroporated with EAV030H RNA a set of seven RNA species was detected, which were indistinguishable from the viral mRNAs in infected cells (Fig. 3A; lane H).
Figure 3.
Analysis of i.c. RNA from cells infected with EAV (lane V), or transfected with EAV030H (lane H), EAV030F (lane F), EAVCAT2 (lane C2), or EAVCAT7 (lane C7). (A) Agarose gel electrophoresis of poly(A)-selected, metabolically labeled RNA. Cells were labeled from 6 to 15 hr after transfection using [3H]uridine in the presence of dactinomycin. The black arrowhead points toward the extended RNA2 produced by EAVCAT2. The open arrowheads indicate the set of extended mRNAs produced by EAVCAT7. (B) RT-PCR analysis of the RNA1 region containing the EAV030H marker mutation. The RT-PCR products from infected cells (lane V) and EAV030H-transfected cells (lane H) are shown. The DNA in the lanes marked with ∗ was digested with HindIII. Lanes D and D* show a PCR control on pEAV030H plasmid DNA. Lane C shows a PCR control without template. Lambda DNA (lane λ) digested with BamHI, EcoRI, and HindIII was used as size marker. (C) RNA1- and RNA7-specific RT-PCR reactions on i.c. RNA isolated at 15 hr after electroporation from cells transfected with EAV030H (lane H) and EAV030F (lane F) RNA. The DNA in the lanes marked with ∗ was digested with HpaI (lanes RNA1) or BspHI (lanes RNA7), respectively.
An RT-PCR (Fig. 3B) was used to detect the ORF1b marker mutation in virus-specific i.c. RNA from EAV030H-transfected cells. Digestion with HindIII confirmed that the RT-PCR fragment derived from this sample indeed contained the novel restriction site created by the marker mutation. This excluded the possibility of a contamination with the wild-type virus used in our laboratory. The pEAV030H DNA and EAV030HEco RNA transfections were RT-PCR negative (data not shown), indicating that the input RNA/DNA was not detected in this assay. Virus was isolated from five EAV030H plaques and used for two undiluted passages, after which the RT-PCR assay was repeated. The progeny of all five plaques was found to contain the marker mutation (data not shown), confirming once more that these viruses were derived from the pEAV030H cDNA clone.
Finally, the synthesis of EAV replicase and structural proteins was analyzed by IFA (Fig. 4). At 10 hr after EAV030H RNA transfection, single cells were positive for the nsp2 replicase cleavage product (Fig. 4a) (37), and for the structural proteins GL, M, and N (data not shown), which are encoded by ORFs 5, 6, and 7, respectively (Fig. 1) (38). Ten hours later, clusters of cells that were positive for both nsp2 and GL were observed (Fig. 4 b and c), indicating that virus had spread from the initially transfected cells. Again no signal was observed upon pEAV030H DNA and EAV030HEco RNA transfection (data not shown), indicating that also the IFA required RNA replication to obtain a positive result.
Figure 4.
Immunofluorescence assays on EAV030H- and EAV030F-transfected BHK-21 cells at 10 and 20 hr after electroporation. (a and d) Cells were labeled using an EAV nsp2-specific polyclonal rabbit antiserum (37) and a Cy3-conjugated donkey-anti-rabbit-IgG second antibody. (b–c and e–f) Cells were double-labeled for EAV nsp2 as described above (b and e) and EAV GL (c and f) using mAb 93B (39) and a fluorescein isothiocyanate-conjugated goat-anti-mouse-IgG second antibody. (×110.)
EAV030F RNA Is Defective in Subgenomic RNA Synthesis.
A more detailed analysis of cells transfected with RNA from the initial, noninfectious pEAV030F clone revealed a very interesting defect in this mutant. At 10 hr after EAV030F RNA transfection, an IFA revealed single cells with a distinct EAV replicase signal (Fig. 4d), which was comparable to that after transfection of RNA from the infectious pEAV030H clone (Fig. 4a). However, in contrast to what was observed after EAV030H transfection, the signal in the EAV030F-transfected cell culture did not spread to surrounding cells (compare Fig. 4 b and e). This suggested EAV030F RNA replication without virus production.
Additional IFAs revealed that the structural proteins GL (Fig. 4f), M, and N were not synthesized in detectable amounts. Metabolic RNA labeling (Fig. 3A, lane F) during the first replication cycle (6–15 hr after transfection) confirmed the replication of EAV030F RNA and also revealed the absence of detectable subgenomic mRNA synthesis. As usual (23), metabolic labeling of EAV RNA revealed the generation of a number of minor RNA species of various sizes, which have remained unexplained thus far. However, these bands were clearly not unique for the EAV030F sample (Fig. 3A) and did not comigrate with the EAV mRNAs. To confirm the absence of subgenomic mRNA synthesis, a more sensitive assay was performed. Intracellular RNA samples from cells electroporated with EAV030H and EAV030F RNA were analyzed using RT-PCR assays aimed at the detection of the genomic RNA1 and the abundant subgenomic RNA7. Both samples yielded comparable amounts of RNA1 RT-PCR product. However, in contrast to the result for EAV030H, only a trace of RNA7 RT-PCR product was recovered from the EAV030F sample (Fig. 3C). Together, these data showed that EAV030F RNA is defective in subgenomic RNA synthesis. In agreement with this observation, cells transfected with EAV030F RNA did not develop c.p.e. or yield plaques, even after a 96-hr incubation period.
Heterologous Gene Expression from Subgenomic mRNAs.
To express the CAT gene from subgenomic EAV mRNAs, it was inserted into pEAV030H at two different positions. In pEAVCAT2, the CAT gene occupied the position of ORF2 (Fig. 1) to achieve its expression from subgenomic mRNA2. Due to this insertion, EAVCAT2 RNA was 572 nt longer than pEAV030H RNA. An IFA revealed strong CAT expression at 10–20 hr after EAVCAT2 RNA transfection (Fig. 5a). Double-labeling experiments confirmed that expression of the foreign gene was strictly coupled to the synthesis of EAV nonstructural and structural proteins (Fig. 5 b–d). A metabolic labeling of the RNA made in transfected cells revealed the synthesis of the expected seven RNAs that are known to form a 3′ coterminal nested set, including an RNA2 that was larger than usual (Fig. 3A, lane C2).
Figure 5.
Immunofluorescence assays on EAVCAT2-transfected BHK-21 cells at 20 hr after electroporation. Cells were double-labeled using an anti-CAT polyclonal rabbit antiserum (a) and an EAV GL-specific mAb (b) or using an EAV nsp2-specific polyclonal rabbit antiserum (c) and the EAV GL-specific mAb (d). See the legend to Fig. 4 for more details. (×225.)
In pEAVCAT7, a 799-bp restriction fragment containing the CAT gene was placed just upstream of the ORF7 initiation codon to achieve reporter gene expression from subgenomic mRNA7. Indeed, an IFA revealed strong CAT expression at 10 hr after EAVCAT7 RNA transfection (data not shown), whereas cells transfected with a construct containing the CAT gene in the antisense orientation were IFA-negative. A subsequent RNA analysis (Fig. 3A, lane C7) revealed the generation of the expected set of enlarged mRNAs by EAVCAT7.
DISCUSSION
In this paper we describe the construction of a full-length arterivirus cDNA clone from which infectious RNA can be produced in vitro using T7 RNA polymerase. The infectivity of pEAV030 transcripts was demonstrated using several biological and biochemical assays. Final proof was the generation and passaging of EAV030H virus containing a genetic marker mutation that had been introduced at the cDNA level (Fig. 3B). Thus far, no biological differences have been detected between pEAV030H-derived virus and the EAV Bucyrus strain used in our laboratory.
In contrast to what has been described for the infectious cDNA clones of several other viruses (9), we have not experienced any stability problems during the construction and propagation of pEAV030 in Escherichia coli. The 15.6-kb construct was based on pUC18, a high copy number plasmid, and its stability was not affected by the insertion of the CAT gene at two different positions. These properties obviously are important for the future development and exploitation of pEAV030 as an expression vector. The development of such a vector would be particularly interesting in view of the polycistronic genome organization of EAV. The generation of a set of subgenomic mRNAs will, in principle, allow the simultaneous expression of multiple foreign genes from different subgenomic mRNAs. The fact that the EAV structural genes overlap substantially (in different reading frames) (10), and that all subgenomic promoters are located in upstream genes (23), will certainly cause practical problems. Nevertheless, the first results obtained with pEAV030 as a tool to express a foreign gene were promising (Fig. 5): high CAT levels were detected in cells transfected with EAVCAT2 and EAVCAT7 RNA. However, it should be noted that, thus far, the specific infectivity of EAV030 RNA (i.e., the number of cells transfected per microgram of transcript) is relatively low compared with that of the alphavirus-based expression vectors, for example (41, 42), which we used for comparison. Additional experiments are required to determine whether this difference reflects an intrinsic property of EAV030 RNA, or suboptimal in vitro transcription and transfection conditions.
Thus far, no detailed information is available on the RNA and protein requirements for replication and packaging of EAV RNA and the assembly of progeny virus. The infectious clone will be an excellent tool to study these fundamental aspects of arterivirus replication, which are also important for the development of a pEAV030-based expression system. Virus was not produced after EAVCAT2 and EAVCAT7 RNA transfection, which was not surprising because these constructs each lack expression of one of the four known structural EAV proteins (38), the ORF2-encoded small glycoprotein GS and the ORF7-encoded nucleocapsid protein N, respectively. Experiments aimed at providing these proteins in trans are now in progress. Especially the 5′ end of ORF2 may be a convenient insertion site for foreign sequences because this region does not overlap with other genes (Fig. 1) and the RNA2 promoter is located in the upstream replicase gene (23). Furthermore, the ORF2-encoded GS glycoprotein is only a minor constituent of EAV particles (38, 43). Thus, efficient complementation of its disruption in pEAVCAT2 may be feasible—e.g., in a GS-expressing cell line or by using an additional expression vector. This kind of complementation could form the basis for the development of a “suicidal” EAV vector, a system generating virus particles that can infect target cells and replicate but cannot produce progeny virus due to the inactivation of an essential structural gene.
A field of research that will certainly benefit from the availability of the infectious cDNA clone is the analysis of the arterivirus replicase and the transcriptional processes directed by it. A detailed analysis of the complex proteolytic processing of the EAV replicase has been carried out in our laboratory (11, 36, 37, 44, 45). Three viral proteases and at least 13 processing end products have been identified, and the antisera to detect most of these proteins are available. The two elements required to functionally characterize the EAV replicase subunits are combined in pEAV030: an RNA replicon and a functional replicase gene. Although an obstacle during the development of the infectious clone, the T-7508 to C mutation in the initial pEAV030F construct resulted in the fortuitous detection of an interesting defect: the apparently complete inhibition of subgenomic RNA transcription (Figs. 3 and 4). Especially the results from the sensitive biological assays carried out with EAV030F-transfected cells (the absence of c.p.e. or plaques after a 4-day incubation) suggest a complete block of structural protein expression. In that case, the trace of RNA7 RT-PCR product that was detected in the EAV030F sample (Fig. 3C) should be explained as an artefact of the RT or PCR procedure.
Formally, we cannot yet exclude that the effect of the T-7508 to C mutation is exerted at the RNA level. However, we consider it more likely that the resulting replicase point mutation is involved. Residue Ser-2429 is conserved in the three available arterivirus replicase sequences. It is located between two well conserved domains in the nidovirus replicase (10): a region containing a number of conserved Cys and His residues (residues 2374–2426 in EAV) and the putative RNA helicase domain (residues 2500–2800 in EAV). The first was originally identified in coronaviruses (46) and has been proposed to be a metal-binding finger domain. Preliminary evidence supporting this hypothesis has been reported (47). The putative helicase domain was identified on the basis of convincing sequence similarity with many other positive-stranded RNA virus sequences (10, 48). In EAV, the two domains described above are both located in a 50-kDa replicase cleavage product (p50) (36). A more detailed analysis of the properties of the Ser-2429 → Pro mutant is now in progress. A recent time course experiment has not revealed any differences between the proteolytic processing of the wild-type and EAV030F replicase polyprotein.
At the RNA level, we are currently trying to establish whether subgenomic minus-strand RNAs are produced by EAV030F, a finding that would indicate that the joining of leader and body sequences occurs at the level of negative-strand RNA synthesis. To our knowledge, this mutant provides the first evidence that the requirements for nidovirus genome replication and discontinuous mRNA synthesis are, at least partially, different, and may be separated experimentally. We are convinced that future experiments using pEAV030 and its derivatives will increase our understanding of the unique features of nidovirus replication.
Acknowledgments
We thank Peter Bredenbeek, Willem Luytjes, and Guido van Marle for helpful discussions and suggestions; René Rijnbrand for providing the plasmid containing the CAT gene; and Yvonne van der Meer for assistance with IFA-related photographic work. We are grateful to Twan de Vries and Amy Glaser (Institute of Veterinary Virology, Utrecht University) for stimulating discussions and the kind exchange of oligonucleotides and antisera.
Footnotes
Abbreviations: BHK, baby hamster kidney; CAT, chloramphenicol acetyltransferase; c.p.e., cytopathogenic effects; EAV, equine arteritis virus; i.c., intracellular; IFA, immunofluorescence assay; nsp, nonstructural protein; RT-PCR, reverse transcription–PCR.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. Y07862Y07862).
References
- 1.Racaniello V R, Baltimore D. Science. 1981;214:916–919. doi: 10.1126/science.6272391. [DOI] [PubMed] [Google Scholar]
- 2.Rice C M, Levis R, Strauss J H, Huang H V. J Virol. 1987;61:3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rice C M, Grakoui A, Galler R, Chambers T J. New Biol. 1989;1:285–296. [PubMed] [Google Scholar]
- 4.Lai C J, Zhao B, Hori H, Bray M. Proc Natl Acad Sci USA. 1991;88:5139–5143. doi: 10.1073/pnas.88.12.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang C Y, Dominguez G, Frey T K. J Virol. 1994;68:3550–3557. doi: 10.1128/jvi.68.6.3550-3557.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moormann R J M, van Gennip H G P, Miedema G K W, Hulst M M, van Rijn P A. J Virol. 1996;70:763–770. doi: 10.1128/jvi.70.2.763-770.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyers G, Thiel H J, Rumenapf T. J Virol. 1996;70:1588–1595. doi: 10.1128/jvi.70.3.1588-1595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bredenbeek P J, Rice C M. Semin Virol. 1992;3:297–310. [Google Scholar]
- 9.Boyer J C, Haenni A L. Virology. 1994;198:415–426. doi: 10.1006/viro.1994.1053. [DOI] [PubMed] [Google Scholar]
- 10.den Boon J A, Snijder E J, Chirnside E D, de Vries A A F, Horzinek M C, Spaan W J M. J Virol. 1991;65:2910–2920. doi: 10.1128/jvi.65.6.2910-2920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snijder E J, Wassenaar A L M, Spaan W J M, Gorbalenya A E. J Biol Chem. 1995;270:16671–16676. doi: 10.1074/jbc.270.28.16671. [DOI] [PubMed] [Google Scholar]
- 12.Brierley I, Diggard P, Inglis S C. Cell. 1989;57:537–547. doi: 10.1016/0092-8674(89)90124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown T D K, Brierley I. In: The Coronaviridae. Siddell S G, editor. New York: Plenum; 1995. pp. 191–217. [Google Scholar]
- 14.Snijder E J, Spaan W J M. In: The Coronaviridae. Siddell S G, editor. New York: Plenum; 1995. pp. 239–255. [Google Scholar]
- 15.Spaan W J M, Cavanagh D, Horzinek M C. J Gen Virol. 1988;69:2939–2952. doi: 10.1099/0022-1317-69-12-2939. [DOI] [PubMed] [Google Scholar]
- 16.Lai M M C. Annu Rev Microbiol. 1990;44:303–333. doi: 10.1146/annurev.mi.44.100190.001511. [DOI] [PubMed] [Google Scholar]
- 17.van der Most R G, Spaan W J M. In: The Coronaviridae. Siddell S G, editor. New York: Plenum; 1995. pp. 11–31. [Google Scholar]
- 18.Baric R S, Stohlman S A, Lai M M C. J Virol. 1983;48:633–640. doi: 10.1128/jvi.48.3.633-640.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spaan W J M, Delius H, Skinner M, Armstrong J, Rottier P J M, Smeekens S, van der Zeijst B A M, Siddell S G. EMBO J. 1983;2:1839–1844. doi: 10.1002/j.1460-2075.1983.tb01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sethna P B, Hung S L, Brian D A. Proc Natl Acad Sci USA. 1989;86:5626–5630. doi: 10.1073/pnas.86.14.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawicki S G, Sawicki D L. J Virol. 1990;64:1050–1056. doi: 10.1128/jvi.64.3.1050-1056.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sethna P B, Hofmann M A, Brian D A. J Virol. 1991;65:320–325. doi: 10.1128/jvi.65.1.320-325.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.den Boon J A, Spaan W J M, Snijder E J. Virology. 1996;213:364–372. doi: 10.1006/viro.1995.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeong Y S, Makino S. J Virol. 1992;66:3339–3346. doi: 10.1128/jvi.66.6.3339-3346.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Most R G, de Groot R J, Spaan W J M. J Virol. 1994;68:3656–3666. doi: 10.1128/jvi.68.6.3656-3666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawicki S G, Sawicki D L. In: Corona and Related Viruses. Talbot P J, Levy G A, editors. New York: Plenum; 1995. pp. 499–505. [Google Scholar]
- 27.van Berlo M F, Horzinek M C, van der Zeijst B A M. Virology. 1982;118:345–352. doi: 10.1016/0042-6822(82)90354-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Vries A A F, Chirnside E D, Bredenbeek P J, Gravestein L A, Horzinek M C, Spaan W J M. Nucleic Acids Res. 1990;18:3241–3247. doi: 10.1093/nar/18.11.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.den Boon J A, Kleijnen M F, Spaan W J M, Snijder E J. J Virol. 1996;70:4291–4298. doi: 10.1128/jvi.70.7.4291-4298.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plagemann P G W. In: Fields Virology. Fields B N, Knipe D W, Howley P M, editors. Philadelphia: Raven; 1996. pp. 1105–1120. [Google Scholar]
- 31.van der Zeijst B A M, Horzinek M C. Virology. 1975;68:418–425. doi: 10.1016/0042-6822(75)90283-4. [DOI] [PubMed] [Google Scholar]
- 32.Doll E R, Bryans J T, McCollum W H M, Wallace M E. Cornell Vet. 1957;47:3–41. [PubMed] [Google Scholar]
- 33.Fichot O, Girard M. Nucleic Acids Res. 1990;18:6162. doi: 10.1093/nar/18.20.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dumas J B, Edwards M, Delort J, Mallet J. Nucleic Acids Res. 1991;19:5227–5232. doi: 10.1093/nar/19.19.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Landt O, Grunert H P, Hahn U. Gene. 1990;96:125–128. doi: 10.1016/0378-1119(90)90351-q. [DOI] [PubMed] [Google Scholar]
- 36.van Dinten L C, Wassenaar A L M, Gorbalenya A E, Spaan W J M, Snijder E J. J Virol. 1996;70:6625–6633. doi: 10.1128/jvi.70.10.6625-6633.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snijder E J, Wassenaar A L M, Spaan W J M. J Virol. 1994;68:5755–5764. doi: 10.1128/jvi.68.9.5755-5764.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Vries A A F, Chirnside E D, Horzinek M C, Rottier P J M. J Virol. 1992;66:6294–6303. doi: 10.1128/jvi.66.11.6294-6303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glaser A L, de Vries A A F, Dubovi E J. J Gen Virol. 1995;76:2223–2233. doi: 10.1099/0022-1317-76-9-2223. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 41.Liljeström P, Garoff H. Bio/Technology. 1991;9:1356–1361. doi: 10.1038/nbt1291-1356. [DOI] [PubMed] [Google Scholar]
- 42.Bredenbeek P J, Frolov I, Rice C M, Schlesinger S. J Virol. 1993;67:6439–6446. doi: 10.1128/jvi.67.11.6439-6446.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Vries A A F, Post S M, Raamsman M J, Horzinek M C, Rottier P J M. J Virol. 1995;69:4668–4674. doi: 10.1128/jvi.69.8.4668-4674.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snijder E J, Wassenaar A L M, Spaan W J M. J Virol. 1992;66:7040–7048. doi: 10.1128/jvi.66.12.7040-7048.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snijder E J, Wassenaar A L M, van Dinten L C, Spaan W J M, Gorbalenya A E. J Biol Chem. 1996;271:4864–4871. doi: 10.1074/jbc.271.9.4864. [DOI] [PubMed] [Google Scholar]
- 46.Gorbalenya A E, Koonin E V, Donchenko A P, Blinov V M. Nucleic Acids Res. 1989;17:4847–4861. doi: 10.1093/nar/17.12.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoo D, Parker M D, Cox G J, Babiuk L A. In: Corona and Related Viruses. Talbot P J, Levy G A, editors. New York: Plenum; 1995. pp. 437–442. [Google Scholar]
- 48.Gorbalenya A E, Koonin E V. Curr Opin Struct Biol. 1993;3:419–429. [Google Scholar]