Abstract
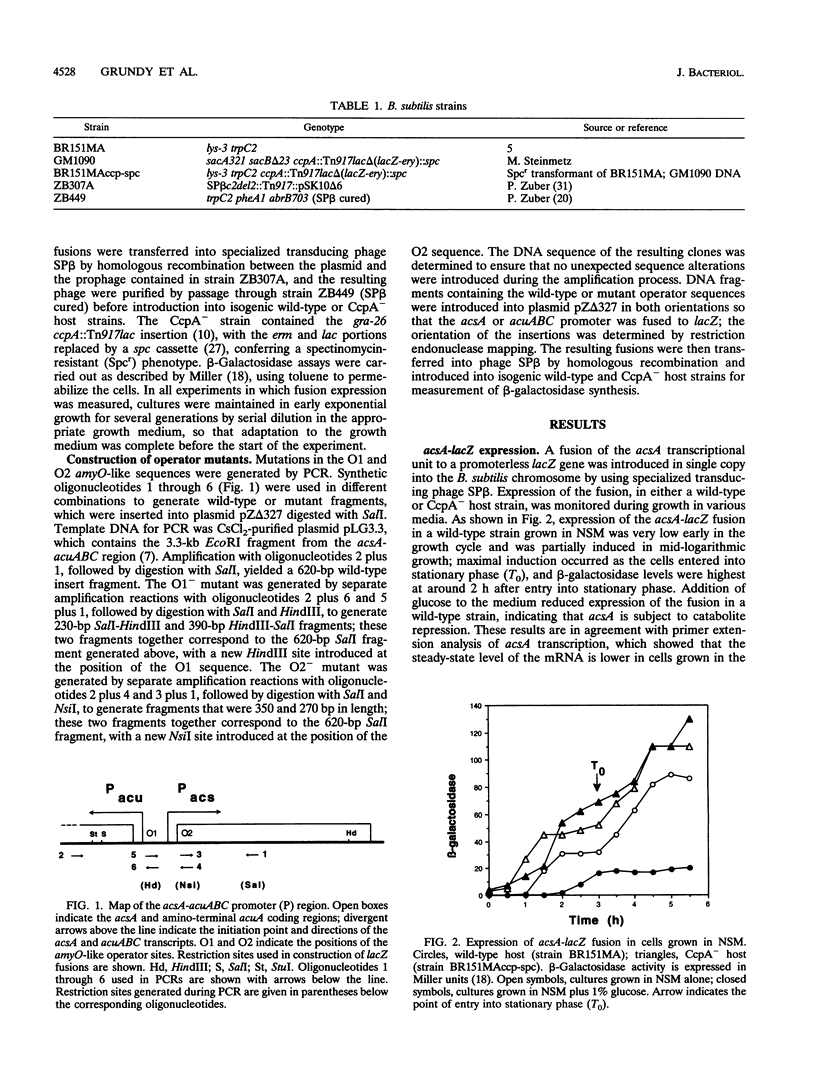
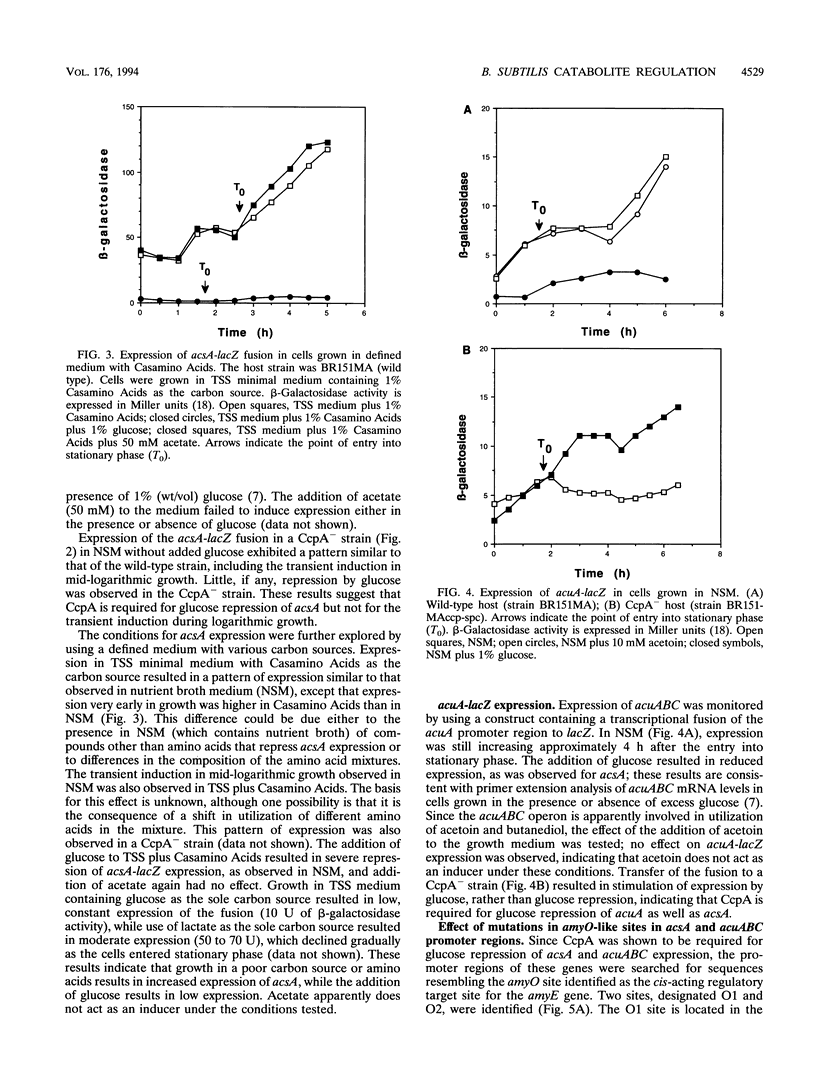
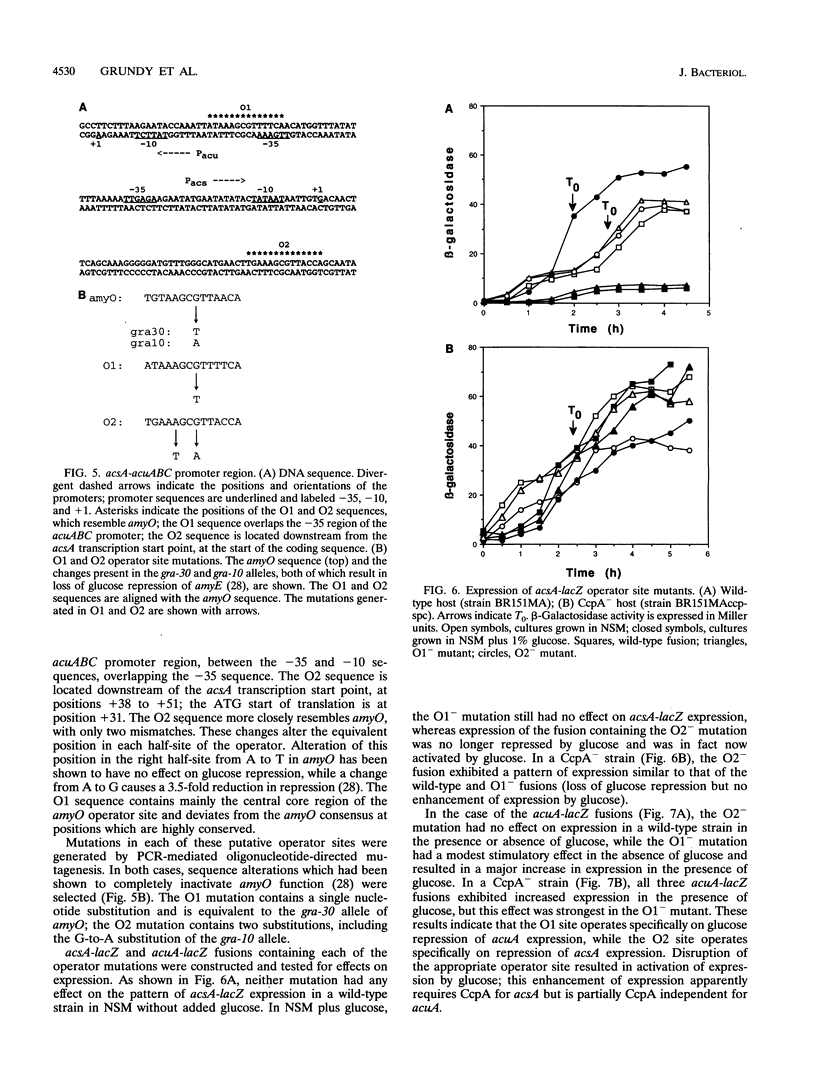
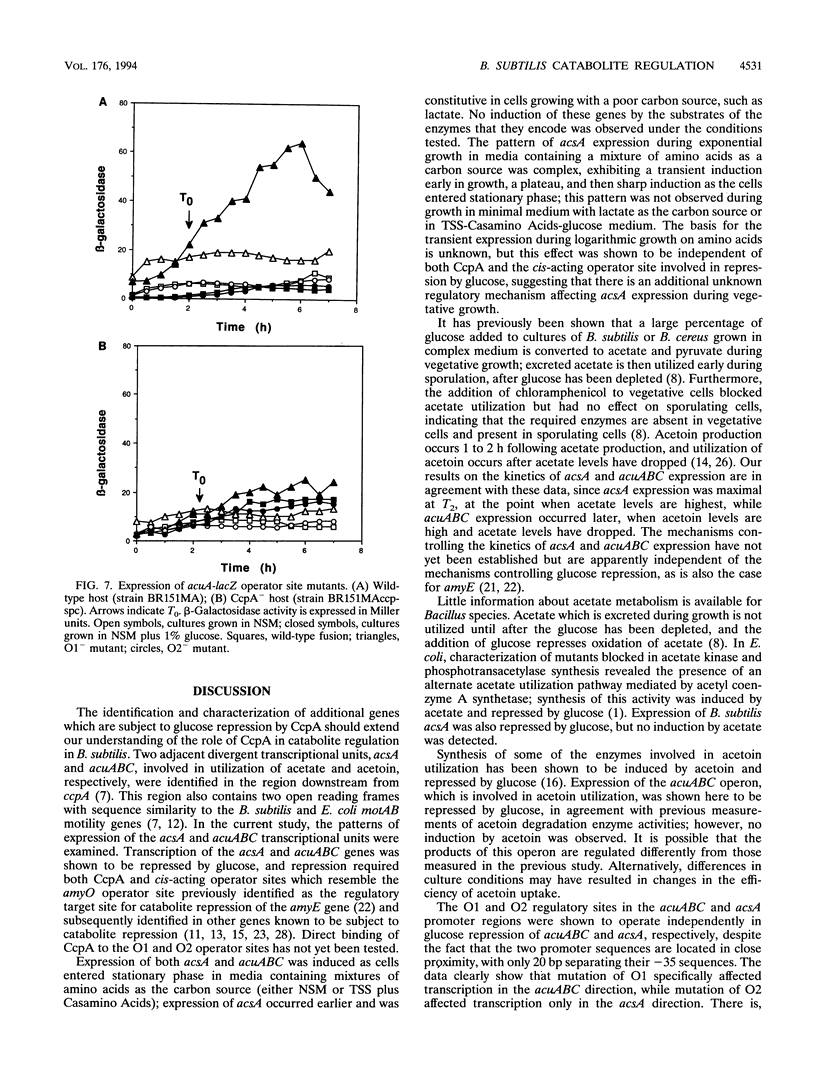
The Bacillus subtilis acsA (acetyl coenzyme A synthetase) and acuABC (acetoin utilization) genes were previously identified in the region downstream from the ccpA gene, which encodes a protein required for catabolite repression of the amyE (alpha-amylase) gene. The acsA and acuABC genes are divergently transcribed, with only 20 bp separating the -35 sequences of their promoters. Expression of these genes was maximal in stationary phase and was repressed by the addition of glucose to the growth medium. Two sites resembling amyO, the cis-acting regulatory target site for amyE, were identified in the acsA and acuABC promoter regions. Glucose repression of acsA and acuABC transcription was dependent on both CcpA and the amyO-like sequences.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown T. D., Jones-Mortimer M. C., Kornberg H. L. The enzymic interconversion of acetate and acetyl-coenzyme A in Escherichia coli. J Gen Microbiol. 1977 Oct;102(2):327–336. doi: 10.1099/00221287-102-2-327. [DOI] [PubMed] [Google Scholar]
- Fisher S. H., Rosenkrantz M. S., Sonenshein A. L. Glutamine synthetase gene of Bacillus subtilis. Gene. 1984 Dec;32(3):427–438. doi: 10.1016/0378-1119(84)90018-0. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Miwa Y. Catabolite repression of the Bacillus subtilis gnt operon mediated by the CcpA protein. J Bacteriol. 1994 Jan;176(2):511–513. doi: 10.1128/jb.176.2.511-513.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy F. J., Henkin T. M. The rpsD gene, encoding ribosomal protein S4, is autogenously regulated in Bacillus subtilis. J Bacteriol. 1991 Aug;173(15):4595–4602. doi: 10.1128/jb.173.15.4595-4602.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy F. J., Waters D. A., Allen S. H., Henkin T. M. Regulation of the Bacillus subtilis acetate kinase gene by CcpA. J Bacteriol. 1993 Nov;175(22):7348–7355. doi: 10.1128/jb.175.22.7348-7355.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy F. J., Waters D. A., Takova T. Y., Henkin T. M. Identification of genes involved in utilization of acetate and acetoin in Bacillus subtilis. Mol Microbiol. 1993 Oct;10(2):259–271. doi: 10.1111/j.1365-2958.1993.tb01952.x. [DOI] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. Biochemistry of sporulation. I. Metabolism of acetate by vegetative and sporulating cells. J Bacteriol. 1963 Feb;85:451–460. doi: 10.1128/jb.85.2.451-460.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin T. M., Chambliss G. H. Genetic mapping of a mutation causing an alteration in Bacillus subtilis ribosomal protein S4. Mol Gen Genet. 1984;193(2):364–369. doi: 10.1007/BF00330694. [DOI] [PubMed] [Google Scholar]
- Henkin T. M., Grundy F. J., Nicholson W. L., Chambliss G. H. Catabolite repression of alpha-amylase gene expression in Bacillus subtilis involves a trans-acting gene product homologous to the Escherichia coli lacl and galR repressors. Mol Microbiol. 1991 Mar;5(3):575–584. doi: 10.1111/j.1365-2958.1991.tb00728.x. [DOI] [PubMed] [Google Scholar]
- Jacob S., Allmansberger R., Gärtner D., Hillen W. Catabolite repression of the operon for xylose utilization from Bacillus subtilis W23 is mediated at the level of transcription and depends on a cis site in the xylA reading frame. Mol Gen Genet. 1991 Oct;229(2):189–196. doi: 10.1007/BF00272155. [DOI] [PubMed] [Google Scholar]
- Kominek L. A., Halvorson H. O. Metabolism of poly-beta-hydroxybutyrate and acetoin in Bacillus cereus. J Bacteriol. 1965 Nov;90(5):1251–1259. doi: 10.1128/jb.90.5.1251-1259.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus A., Hueck C., Gärtner D., Hillen W. Catabolite repression of the Bacillus subtilis xyl operon involves a cis element functional in the context of an unrelated sequence, and glucose exerts additional xylR-dependent repression. J Bacteriol. 1994 Mar;176(6):1738–1745. doi: 10.1128/jb.176.6.1738-1745.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López J., Fortinagel P. The regulation of the butanediol cycle in Bacillus subtilis. Biochim Biophys Acta. 1972 Oct 25;279(3):554–560. doi: 10.1016/0304-4165(72)90177-8. [DOI] [PubMed] [Google Scholar]
- Martin I., Debarbouille M., Klier A., Rapoport G. Induction and metabolite regulation of levanase synthesis in Bacillus subtilis. J Bacteriol. 1989 Apr;171(4):1885–1892. doi: 10.1128/jb.171.4.1885-1892.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa Y., Fujita Y. Promoter-independent catabolite repression of the Bacillus subtilis gnt operon. J Biochem. 1993 Jun;113(6):665–671. doi: 10.1093/oxfordjournals.jbchem.a124100. [DOI] [PubMed] [Google Scholar]
- Nakano M. M., Zuber P. Cloning and characterization of srfB, a regulatory gene involved in surfactin production and competence in Bacillus subtilis. J Bacteriol. 1989 Oct;171(10):5347–5353. doi: 10.1128/jb.171.10.5347-5353.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson W. L., Chambliss G. H. Isolation and characterization of a cis-acting mutation conferring catabolite repression resistance to alpha-amylase synthesis in Bacillus subtilis. J Bacteriol. 1985 Mar;161(3):875–881. doi: 10.1128/jb.161.3.875-881.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson W. L., Park Y. K., Henkin T. M., Won M., Weickert M. J., Gaskell J. A., Chambliss G. H. Catabolite repression-resistant mutations of the Bacillus subtilis alpha-amylase promoter affect transcription levels and are in an operator-like sequence. J Mol Biol. 1987 Dec 20;198(4):609–618. doi: 10.1016/0022-2836(87)90204-x. [DOI] [PubMed] [Google Scholar]
- Oda M., Katagai T., Tomura D., Shoun H., Hoshino T., Furukawa K. Analysis of the transcriptional activity of the hut promoter in Bacillus subtilis and identification of a cis-acting regulatory region associated with catabolite repression downstream from the site of transcription. Mol Microbiol. 1992 Sep;6(18):2573–2582. doi: 10.1111/j.1365-2958.1992.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Renna M. C., Najimudin N., Winik L. R., Zahler S. A. Regulation of the Bacillus subtilis alsS, alsD, and alsR genes involved in post-exponential-phase production of acetoin. J Bacteriol. 1993 Jun;175(12):3863–3875. doi: 10.1128/jb.175.12.3863-3875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck E. L., Freese E. Control of metabolite secretion in Bacillus subtilis. J Gen Microbiol. 1973 Oct;78(2):261–275. doi: 10.1099/00221287-78-2-261. [DOI] [PubMed] [Google Scholar]
- Weickert M. J., Chambliss G. H. Site-directed mutagenesis of a catabolite repression operator sequence in Bacillus subtilis. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6238–6242. doi: 10.1073/pnas.87.16.6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber P., Losick R. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J Bacteriol. 1987 May;169(5):2223–2230. doi: 10.1128/jb.169.5.2223-2230.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



