Abstract
Neurotransmitter release requires the specific docking of synaptic vesicles to the presynaptic plasma membrane followed by a calcium-triggered fusion event. Herein we report a previously unsuspected interaction of the synaptic vesicle protein and likely calcium sensor synaptotagmin with the plasma membrane t-SNARE SNAP-25. This interaction appears to resolve the apparent paradox that synaptic vesicles are capable of docking even when VAMP (vesicle-associated membrane protein) or syntaxin is cleaved or deleted and suggests that two species of v-SNAREs (VAMP and synaptotagmin) and two species of t-SNAREs (SNAP-25 and syntaxin) interact to functionally dock synaptic vesicles.
Keywords: neurotransmission, exocytosis, fusion
The assembly of vesicle (v-SNARE) and target-localized (t-SNARE) SNARE proteins is critical for the docking of transport vesicles to target membranes to enable fusion to occur (1–3). For example, in the endoplasmic reticulum and Golgi transport pathways, mutation of SNARE (SNAP receptor) proteins or addition of neutralizing antibodies in cell-free systems prevents SNARE complex assembly and blocks vesicle docking (4–6). By contrast, when corresponding SNARE proteins are inactivated in neuronal synapses, docking still appears to occur and unfused vesicles accumulate at the plasma membrane whether transmission is blocked by proteolytic neurotoxins that cleave the synaptic v-SNARE VAMP (vesicle-associated membrane protein)/synaptobrevin or the plasma membrane t-SNARE syntaxin (7–9) or whether the gene for syntaxin is deleted (10). This apparent contradiction to the SNARE hypothesis for vesicle docking (1) has led some to conclude that SNARE proteins are exclusively involved in vesicle fusion and not in docking (7, 11).
However, another possibility is that, in the case of the synapse and other instances of regulated exocytosis, additional proteins are used in docking (in connection with regulation) that are not used in the constitutively operating systems like the endoplasmic reticulum and Golgi, perhaps involving additional SNARE proteins that, for example, are not cleaved by toxins. Recently, it was found that the likely calcium sensor for exocytosis, the synaptic vesicle membrane protein synaptotagmin I (tagmin) (11, 12), is also a specialized v-SNARE capable of binding the brain-specific form of SNAP (soluble NSF attachment protein) (β-SNAP) but not the ubiquitous SNAP (α-SNAP) and entering complexes containing VAMP, syntaxin, and SNAP-25 (synaptosome-associated protein of 25 kDa), another t-SNARE (13). This suggested a means by which a calcium sensor could be incorporated into docking and fusion complexes and led us to suspect that tagmin could be part of an additional docking mechanism that could resolve the apparent paradox mentioned above.
Herein we provide biochemical evidence for a direct, high- affinity interaction of tagmin with the plasma membrane t-SNARE SNAP-25. This interaction helps to explain why vesicles can dock, though not fuse, when either syntaxin or VAMP are inactivated. Tagmin and SNAP-25 form a stoichiometric complex both in the absence and in the presence of calcium. In the absence of calcium, this binary complex binds the other two synaptic SNAREs, syntaxin and VAMP, with high efficiency, thus connecting the likely calcium sensor tagmin with the core complex of the docking and fusion machinery (2, 14).
MATERIALS AND METHODS
Immunoprecipitation.
Native tagmin was immunoprecipitated from octyl β-d-glucopyranoside extract of bovine brain cortex (13) with an anti-tagmin mAb (M48) (15) covalently coupled to protein G-Sepharose Fast Flow (Pharmacia) (1) in the presence of 2 mM EGTA. After incubation for 3 h at 4°C, the beads were washed extensively with buffer A (25 mM Hepes·KOH, pH 7.6/100 mM KCl/0.8% octyl β-d-glucopyranoside/0.2 mM dithiothreitol) containing 2 mM EGTA. Bound proteins were eluted with 600 μl of 200 mM glycine·HCl, pH 2.5/1% N,N-bis[3-(d-gluconamido)propyl]cholamide and precipitated with 6.5% trichloroacetic acid. Samples were then analyzed by SDS/PAGE, and the proteins were transferred to nitrocellulose or alternatively stained with Coomassie blue R. Western blots were immunodecorated with rabbit polyclonal antibodies against VAMP 2 (amino acids 1–20; Wako Biochemicals, Osaka), SNAP-25 (amino acids 195–206), syntaxin (amino acids 4–260), and synaptotagmin (amino acids 79–421).
Binding of SNAP-25, Syntaxin, and VAMP to Tagmin and Its Fragments.
Native tagmin (5 μg/sample) was immunoprecipitated as above, and then the beads were washed extensively with buffer A containing 1.0 M NaCl and finally were rinsed in buffer A alone. Purified glutathione S-transferase (GST) fusion proteins containing the cytoplasmic domain of tagmin I (amino acids 79–421; 5 μg) or GST alone (3 μg) were immobilized on 20 μl of 50% glutathione–agarose beads (Sigma). Native or recombinant tagmin was incubated for 1 h at 4°C with His6–SNAP-25 (0.5–32 μg) in buffer A containing 1 mg/ml ovalbumin in the presence or absence of 200 μM calcium buffered with 2 mM EGTA. Substitution of octyl β-d-glucopyranoside for Triton X-100 (0.5%) did not alter significantly the SNAP-25/tagmin interaction. For Fig. 3, GST–tagmin was incubated for 1 h at 4°C with His6–SNAP-25 (16) (2.5 μg) and/or full-length syntaxin 1B (17) (3 μg) and/or VAMP–His6 cytoplasmic domain (amino acids 1–96; 2 μg) in the presence or absence of 200 μM calcium buffered with 2 mM EGTA. For Fig. 4B, GST–tagmin was incubated for 1 h at 4°C with His6–SNAP-25 (5 μg) which had been pretreated for 30 min at 37°C with 100 nM activated botulinum neurotoxin serotype A or E (BoNT/A or BoNT/E) (18). Under these conditions, the cleavage of His6–SNAP-25 was complete. Alternatively, the GST–tagmin/SNAP-25 complex, formed by incubating 12 μg/sample His6–SNAP-25 with GST–tagmin beads for 1 h at 4°C, was treated for 5 min at 37°C with 150 nM of activated botulinum neurotoxin A or E (18) (see Fig. 4A). Subsequently, the immobilized proteins were washed three times with the same buffer without ovalbumin. Beads containing the recombinant tagmin or its fragments were prepared for SDS/PAGE. In contrast, beads containing native tagmin immobilized on anti-tagmin antibody were treated with 200 mM glycine·HCl, pH 2.5/1% N,N-bis[3-(d-gluconamido)propyl]cholamide and the eluate was precipitated with trichloroacetic acid as described before. Proteins were analyzed by SDS/PAGE and stained with Coomassie blue R. The GST–tagmin/His6–SNAP-25 molar ratio at saturation was determined in the presence and absence of calcium by comparing scans of Coomassie blue-stained gel, as in Fig. 2B with GST–tagmin and His6–SNAP-25 titration curves.
Figure 3.
SNAP-25 promotes the assembly of the SNARE complex on tagmin. GST–tagmin was incubated alone (lane 1) or with His6–SNAP-25 (lanes 3, 6–8, 10, and 13–15) and/or full-length syntaxin 1B (lanes 2, 5, 6, 8, 9, 12, 13, and 15) and/or VAMP–His6 (lanes 4, 5, 7, 8, 11, 12, 14, and 15) in the presence of 2 mM EGTA (A) or 200 μM free calcium (B). The empty arrow indicates a contamination of GST from the recombinant syntaxin 1B preparation that interacts with glutathione–agarose beads. No nonspecific binding of SNARE proteins was observed by incubating them with GST-containing beads under the same conditions.
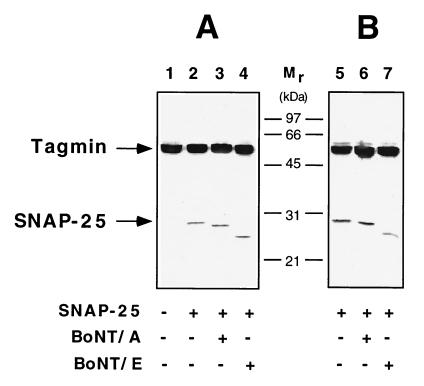
Figure 4.
SNAP-25 in complex with tagmin is cleaved by botulinum neurotoxins. (A) His6–SNAP-25 prebound to GST–tagmin was incubated with buffer only (lane 2) or with activated BoNT/A (lane 3) or activated BoNT/E (lane 4). Under these conditions, the BoNT-generated amino-terminal fragments of SNAP-25 remain bound to tagmin. Botulinum neurotoxin treatment does not abolish the de novo formation of the tagmin/SNAP-25 complex (B). GST–tagmin was incubated with His6–SNAP-25 (lane 5) or His6–SNAP-25 pretreated with activated BoNT/A (lane 6) or activated BoNT/E (lane 7) in the presence of 200 μM calcium.
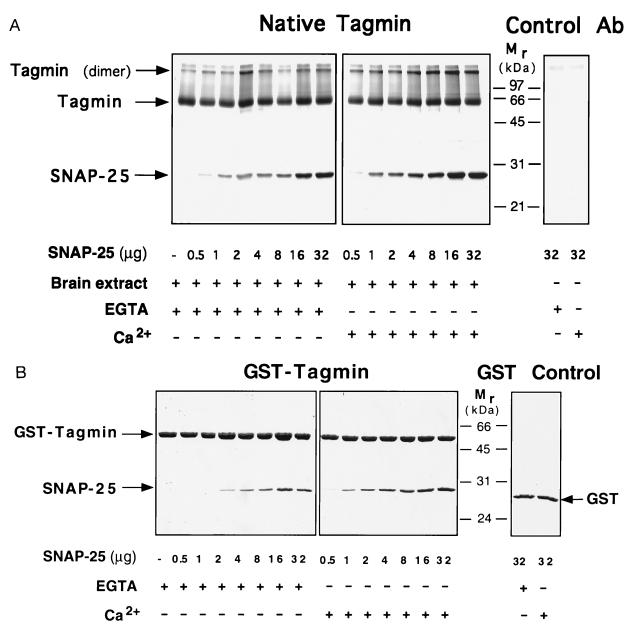
Figure 2.
Native as well as recombinant tagmin binds SNAP-25 in a saturable and stoichiometric manner. Native tagmin immunopurified from brain cortex (A) and the recombinant cytoplasmic domain of tagmin (B) were incubated with increasing amounts of His6–SNAP-25 in the presence or absence of 200 μM free calcium as indicated. As control, beads containing the anti-tagmin antibody used for immunoprecipitation (15) (A, Control Ab) or GST (B, GST Control) were incubated with the maximal amount of His6–SNAP-25 (32 μg) in the presence or absence of calcium.
RESULTS AND DISCUSSION
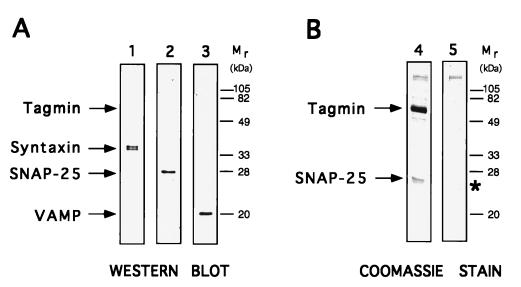
Although the binding of tagmin to many ligands has been described (11, 13, 17, 19–28), potential additional partners in cell extracts might have been obscured by the major bands of the light and heavy chains of IgG present in standard immunoprecipitates. To minimize such technical problems, we used antibodies covalently coupled to beads. As expected, when tagmin is immunoprecipitated from crude detergent extracts of brain cortex, VAMP, syntaxin, and SNAP-25 all coprecipitate, as revealed in a qualitative manner by Western blot analysis (16) (Fig. 1A, lanes 1–3). Unexpectedly, however, Coomassie blue-stained SDS gels (Fig. 1B, lane 4) revealed that SNAP-25 was present in considerable molar excess over both VAMP and syntaxin, suggesting that tagmin and SNAP-25 may form a complex that is even more abundant than that of tagmin with the previously described equimolar SNARE complex of SNAP-25, syntaxin, and VAMP.
Figure 1.
Native tagmin is associated with SNARE proteins in brain extract. Tagmin was immunopurified from brain cortex detergent extract in association with SNARE proteins, as revealed by Western blot analysis (A) using specific antibodies against syntaxin (lane 1), SNAP-25 (lane 2), and VAMP (lane 3). The analysis of the Coomassie blue-stained gels (B) reveals that an excess of SNAP-25 over syntaxin and VAMP is associated with tagmin (lane 4). For comparison, a sample containing only anti-tagmin antibody incubated in the absence of brain extract is shown in lane 5 (the asterisk indicates the position of the antibody L chain).
Indeed, saturable and high-affinity binding of SNAP-25 to tagmin could be easily demonstrated. Soluble (nonacylated) recombinant His6–SNAP-25 bound to both native tagmin (Fig. 2A) and to GST fusion protein containing the entire cytoplasmic domain of tagmin (GST–tagmin; Fig. 2B), forming a complex with ≈1:1 stoichiometry, as determined by scanning of Coomassie blue-stained gels and comparison with standard GST–tagmin and His6–SNAP-25 titration curves (not shown). Saturable binding was achieved when a 5- to 8-fold molar excess of the free protein over the GST-bound protein was used. Calcium slightly promoted the binding of SNAP-25 to tagmin (Fig. 2B), and, at saturation, an additional 30% (in mol/mol) of His6–SNAP-25 bound to GST–tagmin. The reason for this additional binding is unclear. The recombinant cytoplasmic domain of tagmin (cleaved from the GST fusion protein by thrombin) also bound to GST–SNAP-25 (not shown). In all cases, the tagmin–SNAP-25 complexes were resistant to extraction with KCl up to at least 250 mM (not shown). The specificity of the binding of tagmin to SNAP-25 was underscored by the fact that binding occurred exclusively to the C2B portion of the cytoplasmic domain of tagmin (not shown).
The ability of the tagmin–SNAP-25 complex to interact with the other SNAREs was tested (Fig. 3). No significant interaction of tagmin with VAMP (in the absence or the presence of either SNAP-25 or syntaxin) was detectable, regardless of whether calcium was present (Fig. 3, lanes 11, 12, and 14) or not (Fig. 3, lanes 4, 5, and 7). Syntaxin has been reported to bind tagmin in a calcium-dependent manner (26, 27). While we could repeat this observation (Fig. 3, lane 9 vs. lane 2), we also found that, even in the absence of calcium, syntaxin will form a complex with tagmin provided that SNAP-25 is also added (Fig. 3, lane 6 vs. lane 2). This complex, now containing tagmin, SNAP-25, and syntaxin, constitutes a scaffold for VAMP binding (Fig. 3, lane 8), thus illustrating the synergistic nature of interactions among the two t-SNAREs and the two v-SNAREs. The formation of a complex between SNAP-25 and tagmin at resting calcium concentrations could be a key event in linking a calcium sensor to the docking and fusion machinery (13), containing NSF (N-ethylmaleimide-sensitive fusion protein), SNAREs, and SNAPS. Recent findings indicate that SNAP-25 and syntaxin directly interact with the N-type Ca2+ channel in a calcium-dependent way (29, 30) and that this interaction profoundly modifies the gating properties of the channel (31). These evidences, together with our results, further support the idea of a strict link among the components of the SNARE complex, the Ca2+ channel, and the likely calcium sensor synaptotagmin at the active zone of the nerve terminal.
SNAP-25 is also known to be the target of proteolytic neurotoxins (BoNT/A and BoNT/E) that block neurotransmission in vivo (9). Cleavage with BoNT/A or BoNT/E releases 9 and 26 amino acids, respectively, from the carboxyl terminus of SNAP-25. In contrast to SNAP-25 in a complex with VAMP and syntaxin 1 (32), SNAP-25 bound to tagmin was accessible to both BoNT/A and BoNT/E. The resulting cleaved amino-terminal fragments of SNAP-25 remained associated with tagmin (Fig. 4A, lanes 3 and 4). In addition, the SNAP-25 fragments missing 9 or 26 amino acids at the carboxyl terminus still bound to tagmin (Fig. 4B, lanes 5–7). These results are consistent with the reports that show that the inhibitory effects of BoNT/A and BoNT/E, of carboxyl-terminal SNAP-25 peptides, and of anti-SNAP-25 antibodies on regulated exocytosis are at a postdocking stage after the ATP-dependent priming step (33, 34). Likewise, the inability of synaptic vesicles that accumulate when syntaxin or VAMP are cleaved and/or deleted to fuse with the plasma membrane could mean that the integrity of these SNARE proteins is necessary for NSF to disrupt the proposed synaptic docking and fusion particle (containing tagmin, VAMP, syntaxin, and SNAP-25, α-SNAP, β-SNAP, and NSF) (13), or this inability could mean that these SNARE proteins are needed for bilayer fusion after particle disruption; or, both of these possibilities might apply.
Our finding that tagmin and SNAP-25 form a high-affinity, stoichiometric complex can explain why the inactivation or deletion of individual SNAREs can accumulate unfused, docked vesicles in synapses. If VAMP was the only tether of a synaptic vesicle to the synaptic plasma membrane, then cleavage of VAMP by toxins would be expected to prevent docking (11), but it does not do so (7). Similarly, syntaxin is required to hold SNAP-25 and VAMP in a ternary complex (32), so synaptic vesicles would not be expected to dock efficiently when syntaxin is deleted or cleaved by a neurotoxin. However, with tagmin as an additional tether, these expectations no longer hold because the interaction of tagmin with SNAP-25 that we uncovered was independent of both syntaxin and VAMP. Furthermore, SNAP-25 proteolytically cleaved by BoNT/A and BoNT/E still interacted efficiently with tagmin, and thus vesicle fusion should be abolished by these toxins, but membrane attachment should not be. In such cases, the vesicle would be bound to the target membrane but unable to proceed to fuse. In other words, it would be nonfunctionally docked. Given the network of interactions documented among all four of these SNARE proteins, it now seems apparent that no single alteration of a SNARE could prevent membrane attachment of the synaptic vesicle, but any alteration could prevent functional docking. The interactions known to form stoichiometric subcomplexes are as follows: SNAP-25–syntaxin (35); syntaxin–SNAP-25–VAMP (1, 35); syntaxin–tagmin (in the presence of calcium) (26, 27); and now SNAP-25–tagmin.
The binding of SNAP-25 to tagmin also raises the possibility of a multistep mechanism needed to achieve functional docking because the bulk of syntaxin in membranes is known to be complexed with unc-18/N-Sec 1 (36), in which state syntaxin cannot bind either SNAP-25 or VAMP (37). With syntaxin thus inactive, the specialized v-SNARE tagmin could potentially dock the synaptic vesicle by binding the t-SNARE SNAP-25. Blockade at this step would result in nonfunctional docking. Functional docking would result when unc-18/N-Sec 1 is released (by a process still unknown but perhaps involving a rab protein) (2), allowing syntaxin to join SNAP-25–tagmin to form a ternary complex that can recruit the other v-SNARE, VAMP (as shown in Fig. 3A).
Tagmin I is known to form stoichiometric complexes with (using mainly its C2B domain) PInsP2 (phosphatidylinositol bisphosphate) in the presence of calcium above 5–20 μM (28), PInsP3 (phosphatidylinositol trisphosphate) in the absence of calcium (28), phosphatidylserine above 5 μM calcium (C2A domain) (22, 24, 38), syntaxin in the presence of calcium above 200 μM (C2A domain) (26, 27), β-SNAP in the presence or absence of calcium (C2B domain) (13), and now SNAP-25 in the presence or absence of calcium. Tagmin also has been shown to bind N-type calcium channels (17, 20, 21), neurexins (19), and the clathrin AP-2 adaptor complex (25, 39) although these interactions have not been shown to be direct or only have been reported using extremely sensitive immunological methods, making it unclear if they are efficient high-affinity interactions like those cited above, which are likely to be physiologically relevant.
What emerges is a picture of a protein machinery with specific calcium-dependent subreactions in which tagmin plays a central role in adapting a general NSF–SNAP–SNARE docking and fusion mechanism to calcium regulation. Deeper understanding of the various subcomplexes and partial (binding) reactions to calcium-regulated docking and fusion awaits a functional reconstitution using defined components.
Acknowledgments
We thank R. H. Scheller for the kind gift of tagmin constructs and the tagmin-specific mAb and N. Arango for technical assistance. This work was supported by National Institute of Health grants to J.E.R. and by the Mathers Charitable Foundation. Fellowship support came from the European Molecular Biology Organization (G. Schiavo).
Footnotes
Abbreviations: SNAP-25, synaptosome-associated protein of 25 kDa; SNARE, SNAP receptor; v-SNARE, vesicle SNARE; t-SNARE, target-localized SNARE; tagmin, synaptotagmin I; VAMP, vesicle-associated membrane protein; GST, glutathione S-transferase; NSF, N-ethylmaleimide-sensitive fusion protein; SNAP, soluble NSF attachment protein; BoNT/A, botulinum neurotoxin serotype A; BoNT/E, botulinum neurotoxin serotype E.
References
- 1.Söllner T, Whiteheart S W, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman J E. Nature (London) 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 2.Rothman J E. Nature (London) 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 3.Rothman J E, Wieland F T. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- 4.Dascher C, Ossig R, Gallwitz D, Schmitt H D. Mol Cell Biol. 1991;11:872–885. doi: 10.1128/mcb.11.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shim J, Newman A P, Ferro-Novick S. J Cell Biol. 1991;113:55–64. doi: 10.1083/jcb.113.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newman A P, Shim J, Ferro-Novick S. Mol Cell Biol. 1990;10:3405–3414. doi: 10.1128/mcb.10.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt J M, Bommert K, Charlton M P, Kistner A, Habermann E, Augustine G J, Betz H. Neuron. 1994;12:1269–1279. doi: 10.1016/0896-6273(94)90443-x. [DOI] [PubMed] [Google Scholar]
- 8.Sweeney S T, Broadie K, Keane J, Niemann H, O’Kane C J. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 9.Montecucco C, Schiavo G. Q Rev Biophys. 1995;28:423–472. doi: 10.1017/s0033583500003292. [DOI] [PubMed] [Google Scholar]
- 10.Broadie K, Prokop A, Bellen H J, O’Kane C J, Schulze K L, Sweeney S T. Neuron. 1995;15:663–673. doi: 10.1016/0896-6273(95)90154-x. [DOI] [PubMed] [Google Scholar]
- 11.Südhof T C. Nature (London) 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 12.Kelly R B. Curr Biol. 1995;5:257–259. doi: 10.1016/s0960-9822(95)00054-6. [DOI] [PubMed] [Google Scholar]
- 13.Schiavo G, Gmachl M J, Stenbeck G, Söllner T H, Rothman J E. Nature (London) 1995;378:733–736. doi: 10.1038/378733a0. [DOI] [PubMed] [Google Scholar]
- 14.Söllner T. FEBS Lett. 1995;369:80–83. doi: 10.1016/0014-5793(95)00594-y. [DOI] [PubMed] [Google Scholar]
- 15.Matthew W D, Tsavaler L, Reichardt L F. J Cell Biol. 1981;91:257–269. doi: 10.1083/jcb.91.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Söllner T, Bennett M K, Whiteheart S W, Scheller R H, Rothman J E. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 17.Bennett M K, Calakos N, Scheller R H. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- 18.Schiavo G, Montecucco C. Methods Enzymol. 1995;248:643–652. doi: 10.1016/0076-6879(95)48041-2. [DOI] [PubMed] [Google Scholar]
- 19.Petrenko A G, Perin M S, Davletov B A, Ushkaryov Y A, Geppert M, Südhof T C. Nature (London) 1991;353:65–68. doi: 10.1038/353065a0. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida A, Oho C, Omori A, Kuwahara R, Ito T, Takahashi M. J Biol Chem. 1992;267:24925–24928. [PubMed] [Google Scholar]
- 21.Leveque C, Hoshino T, David P, Shoji-Kasai Y, Leys K, Omori A, Lang B, el Far O, Sato K, Martin-Moutot N, Newson-Davis J, Takahashi M, Seagar M J. Proc Natl Acad Sci USA. 1992;89:3625–3629. doi: 10.1073/pnas.89.8.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davletov B A, Südhof T C. J Biol Chem. 1993;268:26386–26390. [PubMed] [Google Scholar]
- 23.Hata Y, Davletov B, Petrenko A G, Jahn R, Südhof T C. Neuron. 1993;10:307–315. doi: 10.1016/0896-6273(93)90320-q. [DOI] [PubMed] [Google Scholar]
- 24.Chapman E R, Jahn R. J Biol Chem. 1994;269:5735–5741. [PubMed] [Google Scholar]
- 25.Zhang J Z, Davletov B A, Südhof T C, Anderson R G. Cell. 1994;78:751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]
- 26.Chapman E R, Hanson P I, An S, Jahn R. J Biol Chem. 1995;270:23667–23671. doi: 10.1074/jbc.270.40.23667. [DOI] [PubMed] [Google Scholar]
- 27.Li C, Ullrich B, Zhang J Z, Anderson R G, Brose N, Südhof T C. Nature (London) 1995;375:594–599. doi: 10.1038/375594a0. [DOI] [PubMed] [Google Scholar]
- 28.Schiavo G, Gu Q-M, Prestwich G D, Söllner T H, Rothman J E. Proc Natl Acad Sci USA. 1996;93:13327–13332. doi: 10.1073/pnas.93.23.13327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rettig J, Sheng Z-H, Kim D K, Hodson C D, Snutch T, Catterall W A. Proc Natl Acad Sci USA. 1996;93:7363–7368. doi: 10.1073/pnas.93.14.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheng Z H, Rettig J, Cook T, Catterall W A. Nature (London) 1996;379:451–454. doi: 10.1038/379451a0. [DOI] [PubMed] [Google Scholar]
- 31.Wiser O, Bennett M K, Atlas D. EMBO J. 1996;15:4100–4110. [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Südhof T C, Niemann H. EMBO J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee A, Kowalchyk J A, DasGupta B R, Martin T F. J Biol Chem. 1996;271:20227–20230. doi: 10.1074/jbc.271.34.20227. [DOI] [PubMed] [Google Scholar]
- 34.Metha P, Battenberg E, Wilson M C. Proc Natl Acad Sci USA. 1996;93:10471–10476. doi: 10.1073/pnas.93.19.10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chapman E R, An S, Barton N, Jahn R. J Biol Chem. 1994;269:27427–27432. [PubMed] [Google Scholar]
- 36.Hata Y, Slaughter C A, Südhof T C. Nature (London) 1993;366:347–351. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- 37.Pevsner J, Hsu S C, Braun J E, Calakos N, Ting A E, Bennett M K, Scheller R H. Neuron. 1994;13:353–361. doi: 10.1016/0896-6273(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 38.Brose N, Petrenko A G, Südhof T C, Jahn R. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- 39.Ullrich B, Li C, Zhang J Z, McMahon H, Anderson R G, Geppert M, Südhof T C. Neuron. 1994;13:1281–1291. doi: 10.1016/0896-6273(94)90415-4. [DOI] [PubMed] [Google Scholar]