Abstract
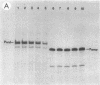
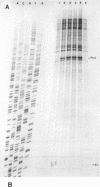
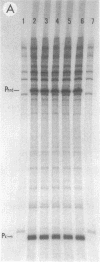
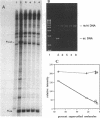
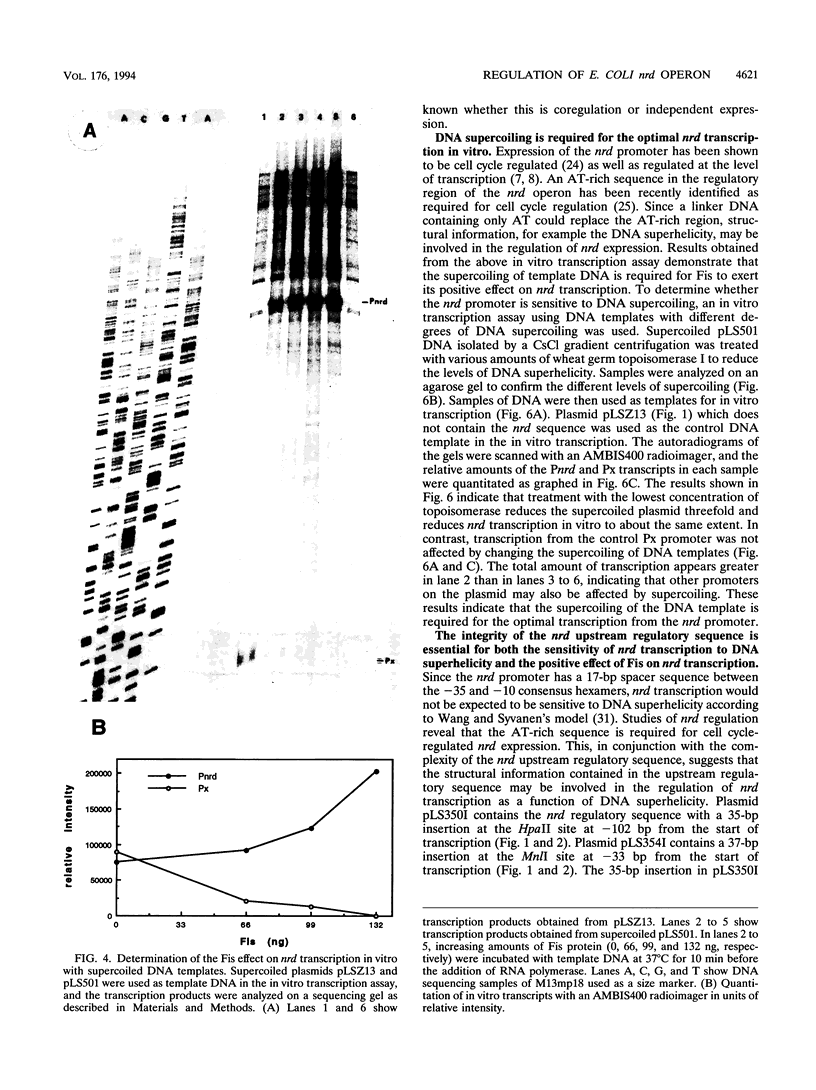
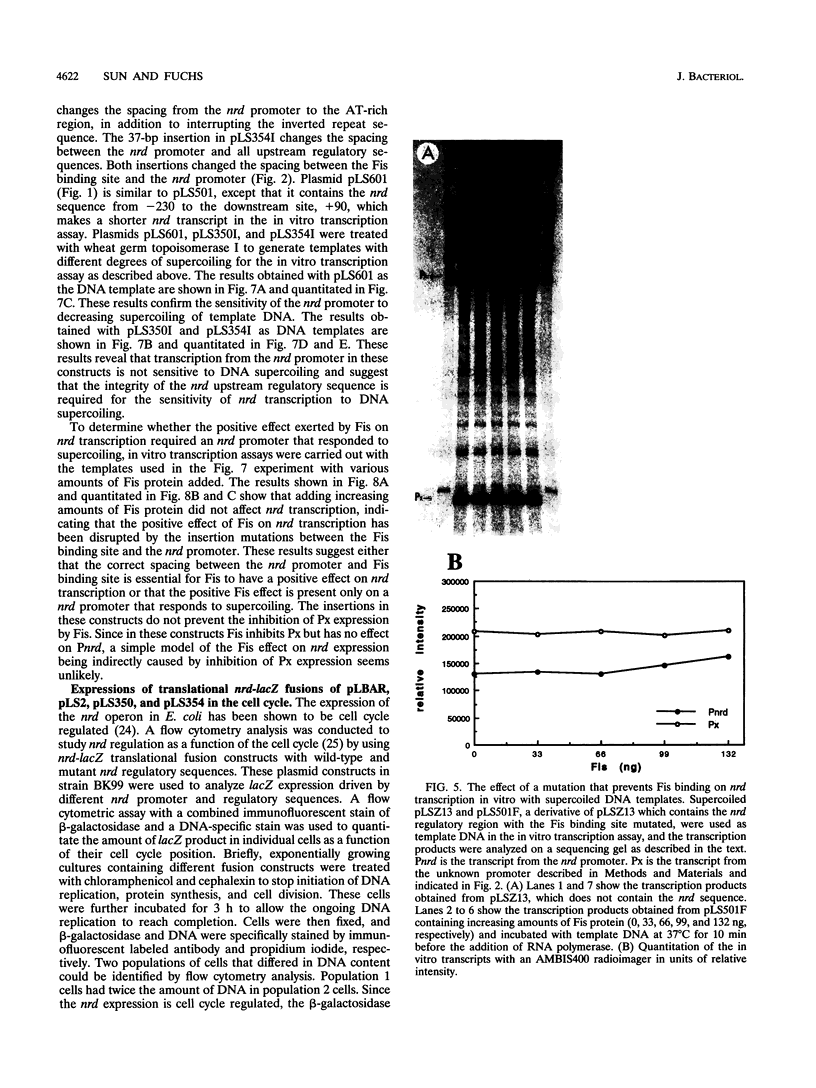
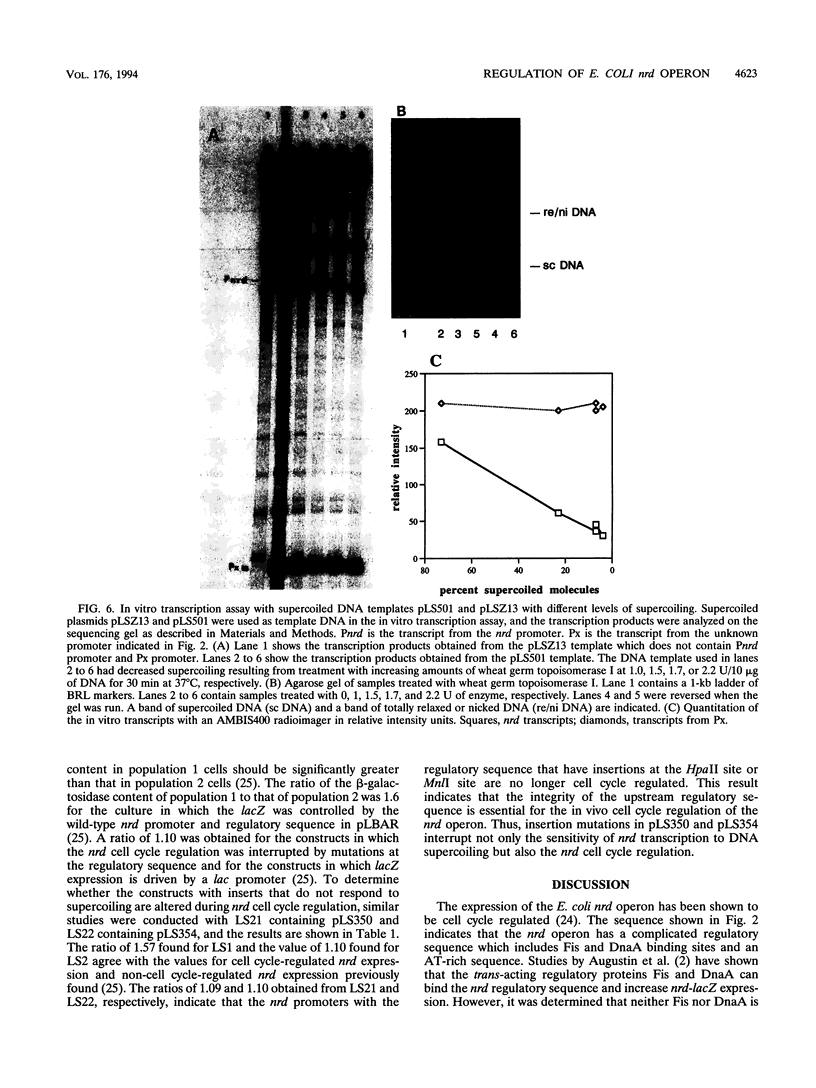
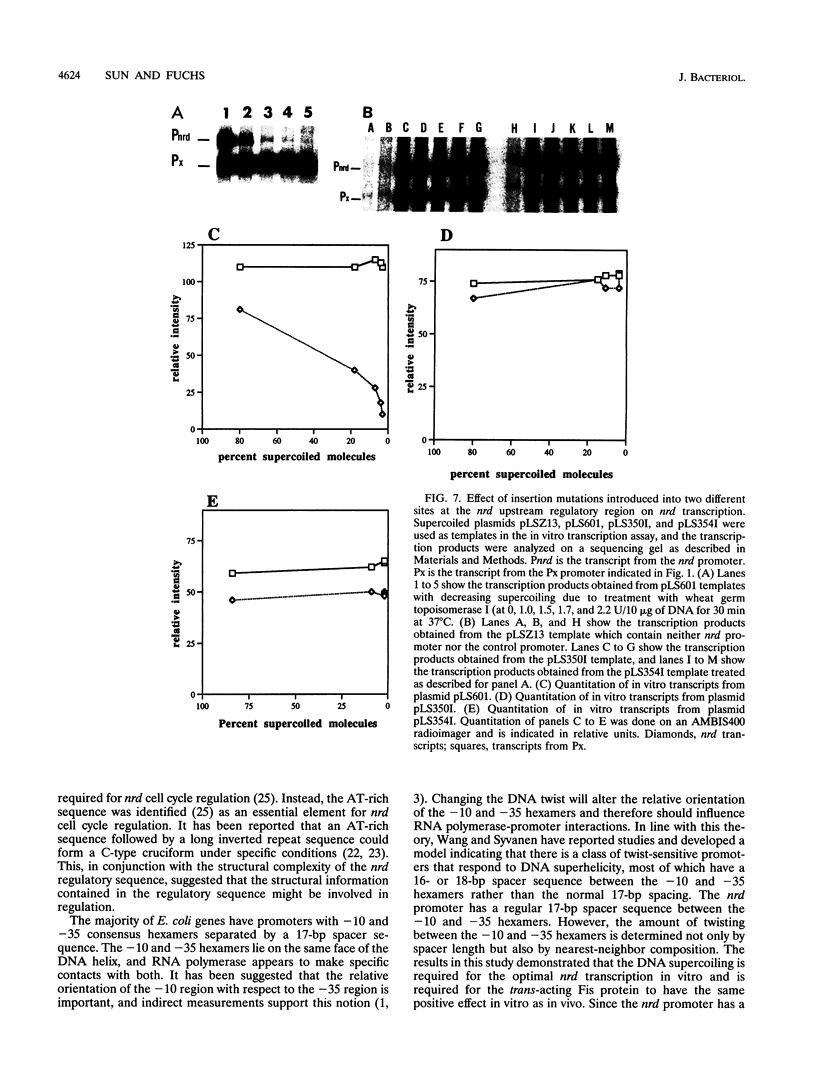
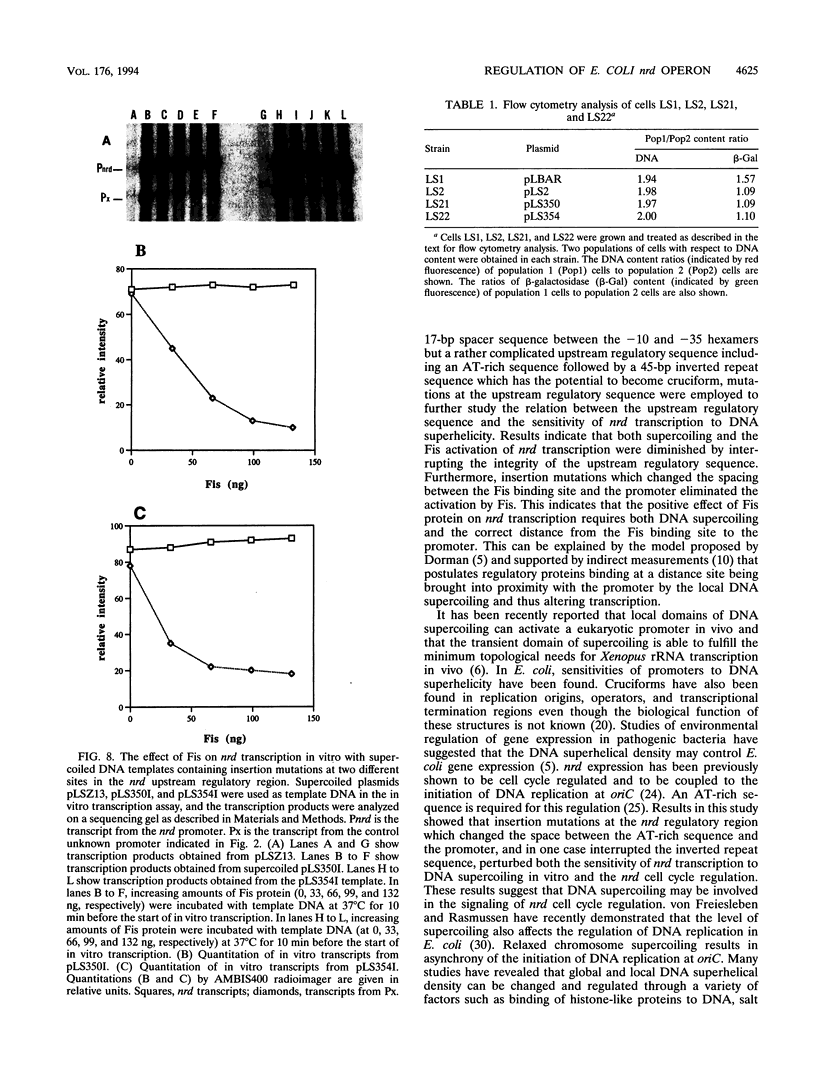
An in vitro RNA transcription assay was used to investigate the regulation of the expression of the nrd promoter. Using a linear DNA template, we found that Fis protein, which has a positive effect on expression of the nrd promoter in an nrd-lacZ fusion in vivo, had a moderate negative effect in vitro. However, with a supercoiled DNA template as substrate, we found that Fis had a concentration-dependent positive effect on nrd transcription in vitro. This positive effect was not present on two templates that had 35- or 37-bp insertions between the Fis binding site and the nrd promoter. In the absence of Fis protein, a dramatic decrease in transcription was observed in templates with reduced supercoiling generated by the treatment with wheat germ topoisomerase I. Templates with insertions of 35 bp into an HpaII site at -102 or 37 bp into the MnlI site at -33 bp from the start of transcription failed to exhibit the DNA supercoiling sensitivity of the nrd promoter. Analysis of cells containing either of these two nrd-lacZ fusion constructs that has an insertion at the regulatory region by flow cytometry indicated that these two constructs, unlike the parental construct, were not cell cycle regulated.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auble D. T., deHaseth P. L. Promoter recognition by Escherichia coli RNA polymerase. Influence of DNA structure in the spacer separating the -10 and -35 regions. J Mol Biol. 1988 Aug 5;202(3):471–482. doi: 10.1016/0022-2836(88)90279-3. [DOI] [PubMed] [Google Scholar]
- Augustin L. B., Jacobson B. A., Fuchs J. A. Escherichia coli Fis and DnaA proteins bind specifically to the nrd promoter region and affect expression of an nrd-lac fusion. J Bacteriol. 1994 Jan;176(2):378–387. doi: 10.1128/jb.176.2.378-387.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiec J. A., Gralla J. D. All three elements of the lac ps promoter mediate its transcriptional response to DNA supercoiling. J Mol Biol. 1987 May 5;195(1):89–97. doi: 10.1016/0022-2836(87)90329-9. [DOI] [PubMed] [Google Scholar]
- Buratowski S., Hahn S., Sharp P. A., Guarente L. Function of a yeast TATA element-binding protein in a mammalian transcription system. Nature. 1988 Jul 7;334(6177):37–42. doi: 10.1038/334037a0. [DOI] [PubMed] [Google Scholar]
- Dorman C. J. DNA supercoiling and environmental regulation of gene expression in pathogenic bacteria. Infect Immun. 1991 Mar;59(3):745–749. doi: 10.1128/iai.59.3.745-749.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filpula D., Fuchs J. A. Regulation of ribonucleoside diphosphate reductase synthesis in Escherichia coli: increased enzyme synthesis as a result of inhibition of deoxyribonucleic acid synthesis. J Bacteriol. 1977 Apr;130(1):107–113. doi: 10.1128/jb.130.1.107-113.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filpula D., Fuchs J. A. Regulation of the synthesis of ribonucleoside diphosphate reductase in Escherichia coli: specific activity of the enzyme in relationship to perturbations of DNA replication. J Bacteriol. 1978 Aug;135(2):429–435. doi: 10.1128/jb.135.2.429-435.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filutowicz M., Ross W., Wild J., Gourse R. L. Involvement of Fis protein in replication of the Escherichia coli chromosome. J Bacteriol. 1992 Jan;174(2):398–407. doi: 10.1128/jb.174.2.398-407.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Curtiss R., 3rd Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect Immun. 1990 Jun;58(6):1879–1885. doi: 10.1128/iai.58.6.1879-1885.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke P. D., Fuchs J. A. Regulation of ribonucleoside diphosphate reductase mRNA synthesis in Escherichia coli. J Bacteriol. 1983 Jun;154(3):1040–1045. doi: 10.1128/jb.154.3.1040-1045.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Dorman C. J., Stirling D. A., Waddell L., Booth I. R., May G., Bremer E. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988 Feb 26;52(4):569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- Hulton C. S., Seirafi A., Hinton J. C., Sidebotham J. M., Waddell L., Pavitt G. D., Owen-Hughes T., Spassky A., Buc H., Higgins C. F. Histone-like protein H1 (H-NS), DNA supercoiling, and gene expression in bacteria. Cell. 1990 Nov 2;63(3):631–642. doi: 10.1016/0092-8674(90)90458-q. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Bruist M. F., Simon M. I. Host protein requirements for in vitro site-specific DNA inversion. Cell. 1986 Aug 15;46(4):531–539. doi: 10.1016/0092-8674(86)90878-0. [DOI] [PubMed] [Google Scholar]
- Kajitani M., Ishihama A. Determination of the promoter strength in the mixed transcription system: promoters of lactose, tryptophan and ribosomal protein L10 operons from Escherichia coli. Nucleic Acids Res. 1983 Feb 11;11(3):671–686. doi: 10.1093/nar/11.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C., Kahmann R. Purification and properties of the Escherichia coli host factor required for inversion of the G segment in bacteriophage Mu. J Biol Chem. 1986 Nov 25;261(33):15673–15678. [PubMed] [Google Scholar]
- Larsen J. E., Albrechtsen B., Valentin-Hansen P. Analysis of the terminator region after the deoCABD operon of Escherichia coli K-12 using a new class of single copy number operon-fusion vectors. Nucleic Acids Res. 1987 Jul 10;15(13):5125–5140. doi: 10.1093/nar/15.13.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsen K. L., Gralla J. D. Interrelated effects of DNA supercoiling, ppGpp, and low salt on melting within the Escherichia coli ribosomal RNA rrnB P1 promoter. Mol Microbiol. 1992 Aug;6(16):2243–2251. doi: 10.1111/j.1365-2958.1992.tb01400.x. [DOI] [PubMed] [Google Scholar]
- Okamoto K., Freundlich M. Mechanism for the autogenous control of the crp operon: transcriptional inhibition by a divergent RNA transcript. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5000–5004. doi: 10.1073/pnas.83.14.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squires C., Krainer A., Barry G., Shen W. F., Squires C. L. Nucleotide sequence at the end of the gene for the RNA polymerase beta' subunit (rpoC). Nucleic Acids Res. 1981 Dec 21;9(24):6827–6840. doi: 10.1093/nar/9.24.6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K. M., Lilley D. M. A dominant influence of flanking sequences on a local structural transition in DNA. Cell. 1986 Dec 5;47(5):817–827. doi: 10.1016/0092-8674(86)90524-6. [DOI] [PubMed] [Google Scholar]
- Sullivan K. M., Lilley D. M. Influence of cation size and charge on the extrusion of a salt-dependent cruciform. J Mol Biol. 1987 Jan 20;193(2):397–404. doi: 10.1016/0022-2836(87)90227-0. [DOI] [PubMed] [Google Scholar]
- Sun L., Fuchs J. A. Escherichia coli ribonucleotide reductase expression is cell cycle regulated. Mol Biol Cell. 1992 Oct;3(10):1095–1105. doi: 10.1091/mbc.3.10.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Jacobson B. A., Dien B. S., Srienc F., Fuchs J. A. Cell cycle regulation of the Escherichia coli nrd operon: requirement for a cis-acting upstream AT-rich sequence. J Bacteriol. 1994 Apr;176(8):2415–2426. doi: 10.1128/jb.176.8.2415-2426.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A. Why bend DNA? Cell. 1990 Jan 26;60(2):177–180. doi: 10.1016/0092-8674(90)90729-x. [DOI] [PubMed] [Google Scholar]
- Tuggle C. K., Fuchs J. A. Regulation of the operon encoding ribonucleotide reductase in Escherichia coli: evidence for both positive and negative control. EMBO J. 1986 May;5(5):1077–1085. doi: 10.1002/j.1460-2075.1986.tb04325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuggle C. K., Fuchs J. A. Regulation of the operon encoding ribonucleotide reductase: role of the negative sites in nrd repression. J Bacteriol. 1990 Apr;172(4):1711–1718. doi: 10.1128/jb.172.4.1711-1718.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek H., Nilsson L., Bosch L. FIS-induced bending of a region upstream of the promoter activates transcription of the E coli thrU(tufB) operon. Biochimie. 1991 Jun;73(6):713–718. doi: 10.1016/0300-9084(91)90051-2. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Syvanen M. DNA twist as a transcriptional sensor for environmental changes. Mol Microbiol. 1992 Jul;6(14):1861–1866. doi: 10.1111/j.1365-2958.1992.tb01358.x. [DOI] [PubMed] [Google Scholar]
- Yuan H. S., Finkel S. E., Feng J. A., Kaczor-Grzeskowiak M., Johnson R. C., Dickerson R. E. The molecular structure of wild-type and a mutant Fis protein: relationship between mutational changes and recombinational enhancer function or DNA binding. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9558–9562. doi: 10.1073/pnas.88.21.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Olmo M., Pérez-Ortín J. E. A natural A/T-rich sequence from the yeast FBP1 gene exists as a cruciform in Escherichia coli cells. Plasmid. 1993 May;29(3):222–232. doi: 10.1006/plas.1993.1024. [DOI] [PubMed] [Google Scholar]
- von Freiesleben U., Rasmussen K. V. The level of supercoiling affects the regulation of DNA replication in Escherichia coli. Res Microbiol. 1992 Sep;143(7):655–663. doi: 10.1016/0923-2508(92)90060-2. [DOI] [PubMed] [Google Scholar]