Abstract
The neurotrophins are signaling factors that are essential for survival and differentiation of distinct neuronal populations during the development and regeneration of the nervous system. The long-term effects of neurotrophins have been studied in detail, but little is known about their acute effects on neuronal activity. Here we use permeabilized whole-cell patch clamp to demonstrate that neurotrophin-3 (NT-3) and nerve growth factor activate calcium-dependent, paxilline-sensitive potassium channels (BK channels) in cortical neurons. Application of NT-3 or nerve growth factor produced a rapid and gradual rise in BK current that was sustained for 30–50 min; brain-derived neurotrophic factor, ciliary neurotrophic factor, and insulin-like growth factor-1 had no significant effect. The response to NT-3 was blocked by inhibitors of protein kinases, phospholipase C, and serine/threonine protein phosphatase 1 and 2a. Omission of Ca2+ from the extracellular medium prevented the NT-3 effect. Our results indicate that NT-3 stimulates BK channel activity in cortical neurons through a signaling pathway that involves Trk tyrosine kinase, phospholipase C, and protein dephosphorylation and is calcium-dependent. Activation of BK channels may be a major mechanism by which neurotrophins acutely regulate neuronal activity.
The neurotrophins are a family of neurotrophic factors, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), and neurotrophin 4/5, that play an important role in the development and regeneration of the peripheral and central nervous system (1, 2). The functions of neurotrophins on distinct neuronal populations in developing and adult brain are mediated by the cellular expression of the Trk family of tyrosine kinase receptors (3). The expression of NGF, BDNF, and NT-3 in neurons is controlled by excitatory and inhibitory neurotransmitter systems (4–6), which suggests that neurotrophins may also participate in the regulation of neuronal activity in the nervous system. Acute exposure to BDNF and NT-3 rapidly enhance synaptic transmission at developing neuromuscular synapses from Xenopus in culture, hippocampal slices from adult rat brain, and fetal rat hippocampal cultures (7–10). Furthermore, the application of NT-3 potentiates neuronal activity in embryonic cortical neurons (11). Because Ca2+-activated K+ channels (BK channels) contribute to spike repolarization and after-hyperpolarization of neurons (12–13) and may modulate transmitter release in some neurons (14–15), we decided to investigate whether neurotrophins regulate the activity of BK channels in primary cultures of embryonic cortical neurons using whole-cell patch clamp. Previous studies have shown that agonists such as somatostatin, natriuretic peptide, prolactin, and secreted Alzheimer β-amyloid precursor protein (sAPP) stimulate BK channels in a diversity of cell types (16–19). These effects may involve protein phosphorylation or dephosphorylation as BK channels are modulated by protein kinases and phosphatases (20, 21). We report here that NT-3 and NGF produced a rapid rise in BK current that was sustained for 30–50 min after removal of the neurotrophin. The response involves activation of Trk tyrosine kinase, phospholipase C (PLC), protein phosphatase 1 or 2a, and the presence of Ca2+. These results indicate a previously unreported role of neurotrophins in modulation of BK channels that may be involved in regulating neuronal activity.
EXPERIMENTAL PROCEDURES
Cell Culture.
Neuronal cell cultures were prepared from brains of mouse embryo E17 as described by Drejer et al. (22). Forebrain and cortex were removed, freed from meninges, trituated, and trypsinized. Dissociated cells were cultured in high potassium DMEM with N2 supplement (GIBCO) on poly-d-lysine-coated coverslips and used for experiments after 5–8 days. Medium-sized bipolar or tripolar neurons were selected for the patch-clamp recordings.
Chemicals.
All neurotrophic factors were purchased from Alomone Labs (Jerusalem). Tetraethylammonium (TEA) and paxilline were from Sigma. K252a was from Calbiochem, U73122 and U73343 were from Biomol (Plymouth Meeting, PA), and okadaic acid was from Alexis (San Diego). All other reagents were from commercial dealers and of the purest grade available.
Electrophysiology.
All currents were recorded in whole-cell voltage-clamp mode with amphotericin B perforated patches using an EPC-9 patch-clamp amplifier (HEKA Electronics, Lambrecht, Germany). Pipettes were fabricated from borosilicate glasses pulled with a steep tapering and a final resistance of 3.5 MΩ (DMZ-Universal Puller; Zeitz Instrumente, Augsburg, Germany). The coverslips (diameter = 3.5 mm) with cultured neurons were placed in a costum-made perfusion chamber (vol = 15 μl) and continuously superfused at a rate of 1 ml/min with buffer composed of 140 mM NaCl, 4 mM KCl, 2 mM CaCl2, and 10 mM Hepes (pH 7.4). The pipette solution contained 140 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 2 mM EGTA, 10 mM Hepes (pH 7.2), and 1.2 mg/ml amphotericin B. After gigaseal formation, only neurons that developed a constant series resistance (Rs) below 50 MΩ (in most experiments below 20 MΩ) within less than 15 min were selected for recording. A 30–80% compensation of Rs could be obtained. Current traces were corrected for leak and residual capacitative artifacts (P/10 subtraction procedure). The holding potential was kept at 0 mV to permanently inactivate Kv, Na+, and Ca2+ currents. BK-channel currents were elicited by 100-msec voltage steps to 100 mV at a frequency of 0.2 Hz. The inclusion criteria for the experiments were constant Rs values throughout the experiment, recording of voltage activated currents lasting at least 15 min, and sensitivity to 3 mM TEA (or 1 μM paxilline). Neurotrophic factors were applied for 3 min followed by a wash-out period. All experiments were performed at room temperature.
Data Analysis.
Data acquisition was controlled by pulse software (HEKA Electronics). Analyses and illustrations were performed with igor software (WaveMetrics, Lake Oswego, OR) or sigmaplot. Data are expressed as mean ± SEM.
RESULTS
Activation of BK Currents by NT-3.
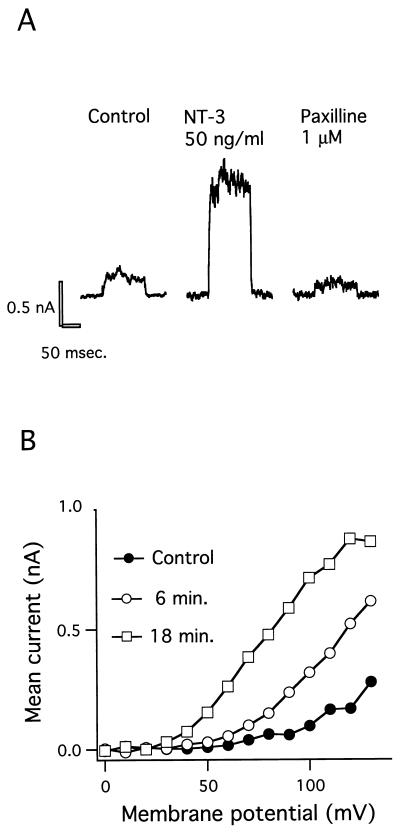
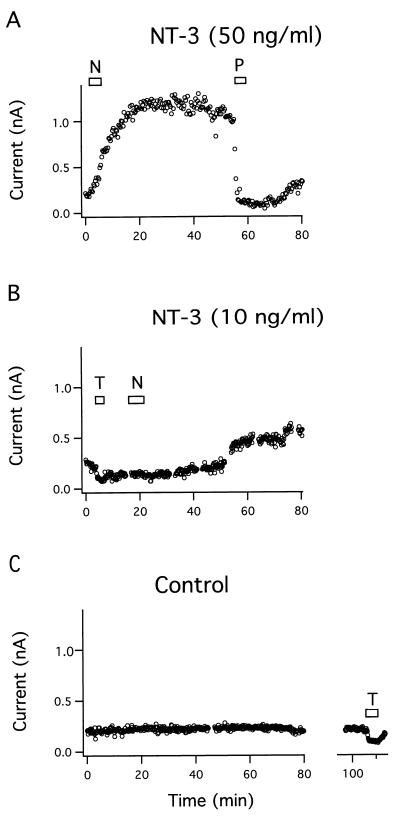
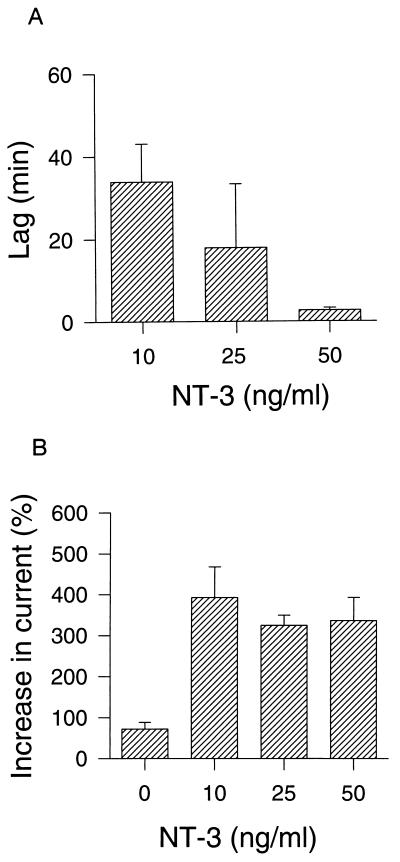
To investigate whether neurotrophins can modulate the activity of BK channels, we examined the effects of NT-3 on K+ currents in primary cultures of neurons from fetal mouse forebrain and cortex using permeabilized whole-cell voltage-clamp recordings. The outward current elicited by voltage steps from 0 to 100 mV increased by 4-fold following exposure to NT-3 (50 ng/ml) (Fig. 1A). Subsequent administration of the selective BK channel blocker paxilline (1 μM) (23) decreased the activated current below the level of the control current. The blocker TEA (3 mM) was often used to verify the potassium channel component of the whole-cell current in the beginning of the experiments, because paxilline washed out slowly and only partly. The enhancement of the voltage-activated BK current by NT-3 was recorded by steps to potentials ranging from 0 to 130 mV, and the current-voltage curve shifted leftward with time by the neurotrophin (Fig. 1B). After application of 50 ng/ml NT-3 for 3 min the BK current increased gradually and reached a maximum after 20 min that was sustained for the following 30 min until the BK channel was blocked with 1 μM paxilline (Fig. 2A). The effect of 50 ng/ml NT-3 was observed in five out of six cells examined. At a NT-3 concentration of 10 ng/ml the BK current was activated in three out of seven cells examined, with a time lag of about 30 min (Fig. 2B). The time lag is defined as the time period from the start of the NT-3 application to the onset of the increase in BK current. Control recordings showed that the BK current in cells bathed in saline was constant for up to 2 hr or decreased slightly in five out of five cells examined, indicating that no spontaneous activation of the BK channel occurred (Fig. 2C). The time lag of BK channel activation by NT-3 was dose-dependent, and the mean value decreased from 34 to 18 to 2.7 min when NT-3 concentrations were increased from 10 to 25 to 50 ng/ml, respectively (Fig. 3A). In contrast, the maximal effect of NT-3 was concentration-independent within the same concentration range (Fig. 3B).
Figure 1.
Enhancement of BK current by NT-3 in cortical neuron. (A) Effect of NT-3 on the voltage-activated whole-cell current. The pulse protocol used in all experiments was a holding potential of 0 mV, voltage steps to 100 mV for 100 msec, and then a return to 0 mV for 5 sec. The current traces were obtained in buffer solution (Control) 20 min after exposure to NT-3 (50 ng/ml) and 2 min after administration of the BK channel blocker paxilline (1 μM). The leak current was small and constant during the experiment and has been subtracted. (B) Current vs. voltage relationship (I–V curve) under control conditions as well as 6 and 18 min after exposure to 50 ng/ml NT-3.
Figure 2.
Time course of NT-3 effect on BK current. (A) The BK current was increased by 50 ng/ml NT-3 (N) (five out of six cells). The effect was completely blocked by 1 μM paxilline (P). (B) BK current was increased by 10 ng/ml NT-3 (three out of seven cells). (C) In buffer alone (Control), the BK current was constant for 2 hr in five out of five cells examined. (T = 3 mM TEA.)
Figure 3.
Dose-response relationships of NT-3-activated BK current. (A) The time lag (i.e., the time from the addition of NT-3 to the onset of the increase in BK current) was determined at different NT-3 concentrations. (B) The maximal NT-3 effect was measured at different NT-3 concentrations and expressed as a percentage of the control current. In the control experiments the BK current was measured 60 min after start of the recording, at which time it had decreased to 75% of the initial value. Data are expressed as mean ± SEM (n = 3–5).
Effect of Other Neurotrophic Factors.
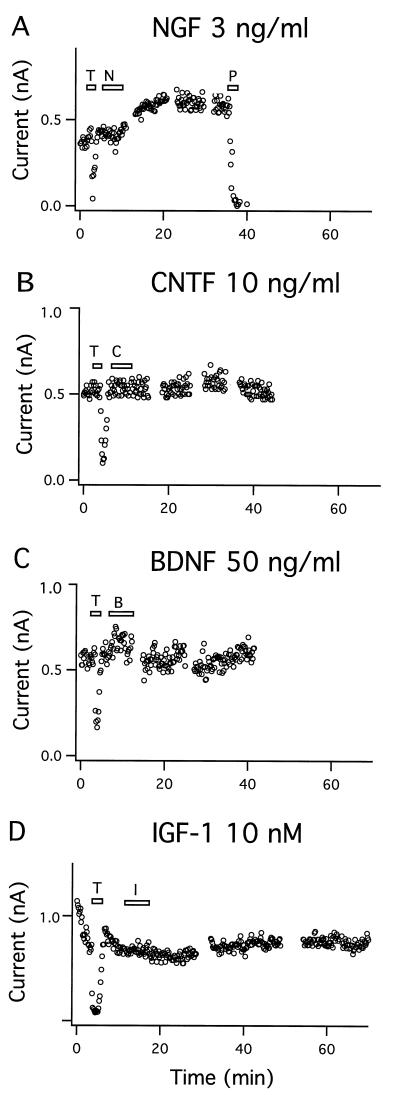
NGF (3 ng/ml) activated the BK current in two out of six cells examined as shown in Fig. 4A. The time course was comparable to that of the NT-3-induced response. The increase in BK current obtained with a maximal concentration of NGF was only 0.5-fold of the initial current compared with 4-fold with NT-3. The proportion of NGF-responsive neurons was ≈30% compared with 85% that responded to NT-3. In accordance, the NGF-receptor TrkA is limited to neurons in the basal forebrain, whereas the NT-3-receptor TrkC is widespread in the cortex of developing rat brain (3). The NT-3 and NGF concentrations used are similar to those observed to elicit developmentally significant effects (24). The neurotrophic factors BDNF (50 ng/ml), ciliary neurotrophic factor (CNTF) (10 ng/ml), and insulin-like growth factor (IGF-I) (10 nM) did not elicit any significant effect on the BK channel activity for periods of 40–70 min (Fig. 4 B–D). Receptors for these three neurotrophic factors have all been demonstrated on cortical neurons (3, 25, 26).
Figure 4.
Specificity of modulation of BK current by neurotrophic factors. (A) The BK current was augmented by 3 ng/ml NGF (N) (two out of six cells). The BK channel was blocked by 1 μM paxilline (P). (B-D) Neither 10 ng/ml CNTF (C), 50 ng/ml BDNF (B), nor 10 nM IGF-I (I) elicited any significant effect on the BK current in 3–5 experiments. (T = 3 mM TEA.)
Signaling Pathway.
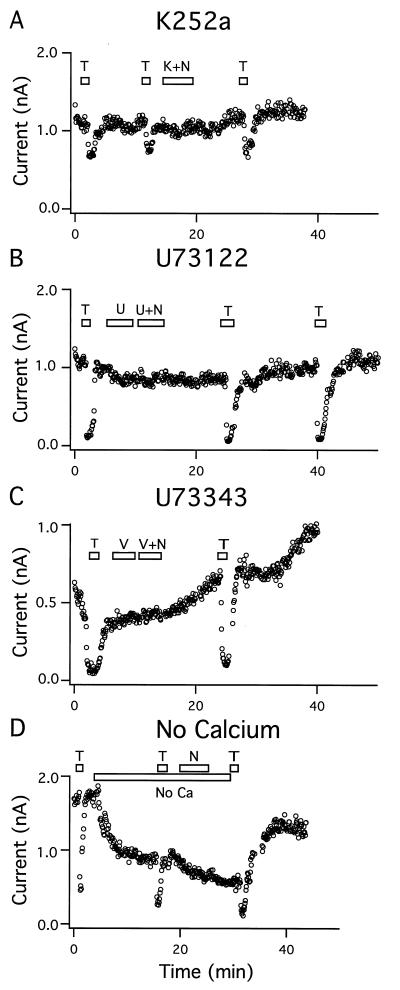
To identify potential downstream effectors of the neurotrophins, we examined whether the NT-3-induced activation of BK channels could be modulated by the application of inhibitors or activators of the Trk signaling pathway (27). Application of K252a (200 nM), a potent but nonspecific kinase inhibitor (28), during the introduction of NT-3 (50 ng/ml) blocked the enhancement of BK current in four out of four cells (Fig. 5A). Next, we examined the effect of a specific PLC inhibitor, U73122 (29), on the NT-3 activation of BK channels. Neurons exposed to U73122 (1 μM) showed no response to NT-3 (50 ng/ml) in five out of five cells examined (Fig. 5B). In contrast, NT-3 still produced a significant increase in BK current in the presence of an inactive analogue of the PLC inhibitor, U73343, as observed in two out of two cells (Fig. 5C). The role of Ca2+ in the NT-3-induced BK current was studied by the omission of Ca2+ from the extracellular medium. The BK current was partly reduced after the removal of extracellular Ca2+, an effect that could be restored by the addition of Ca2+. In the absence of Ca2+, the application of NT-3 had no effect on the BK current in 12 out of 12 cells examined, suggesting that external Ca2+ is necessary for the NT-3 effect (Fig. 5D). However, treatment with NT-3 (50 ng/ml) did not induce Ca2+ transients in cortical cells as studied by FURA2/fluorescent imaging of Ca2+ in single cells (N.R.H. and S. Dissing, unpublished observations). This seems to be consistent with our observation that NT-3 did not induce transient BK currents.
Figure 5.
BK channel activation by NT-3 was mediated by protein kinases, involved PLC, and was Ca2+-dependent. (A) Addition of 200 nM K252a (K) inhibited the stimulation of BK current by 50 ng/ml NT-3 (N) (four out of four cells). (B) Pretreatment with 1 μM U73122 (U) blocked the effect of NT-3 on BK current. (C) Addition of 1 μM U73343 (V) was devoid of effect (two out of two cells). (D) In the absence of extracellular Ca2+ (No Ca) the BK current decreased and NT-3 had no stimulatory effect (12 out of 12 cells). (T = 3 mM TEA.)
Protein Dephosphorylation.
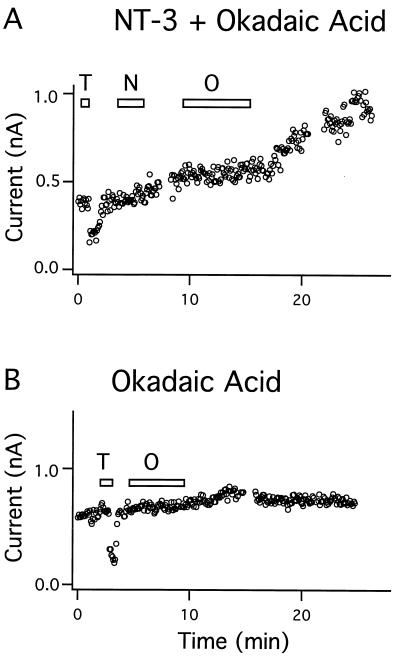
Okadaic acid is a potent and highly specific inhibitor of serine/threonine protein phosphatases 1 and 2a (30). Application of okadaic acid (30 nM) inhibited the effect of NT-3 when it was applied after the neurotrophin in 6 out 6 cells examined (Fig. 6A); the inhibition was reversible as the NT-3-induced rise in BK current continued after removal of okadaic acid. Okadaic acid by itself did not reduce the steady-state BK current in 6 out 6 cells examined (Fig. 6B). These findings suggest that NT-3 stimulates BK channels through dephosphorylation of a channel protein or a regulatory protein.
Figure 6.
Regulation of BK channel activity by protein dephosphorylation. (A) Addition of 30 nM okadaic acid (O) specifically blocked the stimulation of BK current by 50 ng/ml NT-3 (N) (six out of six cells). (B) Okadaic acid (30 nM) had no effect on the basal current (six out of six cells). (T = 3 mM TEA.)
DISCUSSION
The present findings demonstrate that the neurotrophins NT-3 and NGF strongly increase the activity of BK channels, a K+ channel subtype expressed in most neuronal cells. The neurotrophic factors BDNF, CNTF, and IGF-I do not share this ability to acutely modulate the BK channel activity. The time course of the channel activation is concentration-dependent, and at the highest concentrations a maximal effect is obtained within less than 20 min following exposure. This rapid effect suggests a direct modulation of channel activity, which is independent of gene expression.
The signal transduction pathway activated by NT-3 in these cells involves activation of protein kinases as well as stimulation of PLC, because K252a and U73122 fully blocked the change in BK current. In accordance, rapid phosphorylation and activation of PLC-γ1 by NT-3 has been reported in cultures of embryonal rat cortical neurons (31), suggesting that activation of PLC is involved in the NT-3 effect on BK channels. At the level of the BK channel protein, the modulation in activity could be accomplished by, for example, an increase in the internal Ca2+ concentration or by a change in the phosphorylation state of the channel. Although the presence of external Ca2+ is important for the response and NT-3 may induce a small influx of Ca2+, we have not been able to demonstrate an increase in the intracellular Ca2+ level to sufficiently explain the increased channel activity.
The inhibition by okadaic acid of the NT-3 response in these cells indicates that dephosphorylation of the channel or of another key protein in the signaling pathway is a necessary component of the NT-3-induced BK channel activation. In contrast, activation of protein kinase C with phorbol ester resulted in a significant decrease in the steady-state and NT-3-activated BK currents, indicating that phosphorylation deactivates the BK channel (unpublished observations). Previous studies have shown that BK channels are regulated by protein kinases and phosphatases (20, 21). Stimulation of BK channels by agonists that induce protein dephosphorylation such as somatostatin, natriuretic peptide, and sAPP have been reported in a diversity of cell types (16–18). BK channel activity may also be stimulated via tyrosine phosphorylation by prolactin-activated JAK2 tyrosine kinase (19).
The activation of BK channels by neurotrophins resulting in an increased outward K+ current may lead to membrane hyperpolarization and decreased neuronal excitability in specific neurons. Our functional study indicating that neurotrophins can modulate neuronal activity should be seen on the background of reports showing that NT-3 and NGF are expressed in the fetal and adult brain (1), that these neurotrophins are released in response to activity (4–6), and that TrkA and TrkC receptors are found in various regions of the brain (3). Recently it was demonstrated that another regulatory polypeptide, sAPP can suppress action potentials and hyperpolarize neurons by activating high-conductance, charybdotoxin-sensitive K+ channels (18). sAPP activated both Kv and BK channels via signaling pathways that may include cGMP, nitric oxide, Ca2+, and protein dephosphorylation. Others have shown that neurotrophins may enhance spontaneous and excitatory synaptic transmission in neuronal cultures and slices. NT-3 and BDNF increased the neuronal firing rate and the amplitude of excitatory postsynaptic currents in cortical and hippocampal neurons within 2–3 min after the application of neurotrophins (9, 11). In the adult hippocampus, NT-3 and NGF induced a sustained enhancement of synaptic strength for 2–3 hr (10). In addition, BDNF and NT-3 rapidly potentiated the frequency of miniature synaptic events at developing neuromuscular junctions in amphibia (7, 8). Taken together, our data and previous studies suggest that neurotrophins are capable of altering neuronal excitability and synaptic strength acutely preceding the long-term actions on neuronal survival and differentiation.
Acknowledgments
We thank Dr. J. Hounsgaard for advice and discussions. We are grateful to Dr. Steen Dissing for helping out with the Ca2+ imaging study. The study was supported by the Danish Health Research Council, the Danish Cancer Society, the Lundbeck Foundation, and Copenhagen County.
Footnotes
Abbreviations: NGF, nerve growth factor; NT-3, neurotrophin-3; BDNF, brain-derived neurotrophic factor; CNTF, ciliary neurotrophic factor; IGF-I, insulin-like growth factor 1; BK channel, calcium-dependent potassium channel; PLC, phospholipase C; TEA, tetraethylammonium; sAPP, secreted Alzheimer β-amyloid precursor protein.
References
- 1.Korsching J. J Neurosci. 1993;13:2739–2748. doi: 10.1523/JNEUROSCI.13-07-02739.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Snider W D. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 3.Barbacid M. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- 4.Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. EMBO J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu B, Yokoyama M, Dreyfus C F, Black I B. Proc Natl Acad Sci USA. 1991;88:6289–6292. doi: 10.1073/pnas.88.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patterson S L, Grover L M, Schwartzkroin P A, Bothwell M. Neuron. 1992;9:1081–1088. doi: 10.1016/0896-6273(92)90067-n. [DOI] [PubMed] [Google Scholar]
- 7.Lohof A M, Ip N Y, Poo M M. Nature (London) 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 8.Stoop R, Poo M-M. J Neurosci. 1996;16:3256–3264. doi: 10.1523/JNEUROSCI.16-10-03256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine E S, Dreyfus C F, Black I B, Plummer M R. Proc Natl Acad Sci USA. 1995;92:8074–8077. doi: 10.1073/pnas.92.17.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang H, Schuman E M. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 11.Kim H G, Wang T, Olafsson P, Lu B. Proc Natl Acad Sci USA. 1994;91:12341–12345. doi: 10.1073/pnas.91.25.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams P R, Constanti A, Brown D A, Clark R B. Nature (London) 1982;296:746–749. doi: 10.1038/296746a0. [DOI] [PubMed] [Google Scholar]
- 13.Storm J F. J Physiol (London) 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robitaille R, Charlton M P. J Neurosci. 1992;12:297–305. doi: 10.1523/JNEUROSCI.12-01-00297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robitaille R, Garcia M L, Kaczorowski G L, Charlton M P. Neuron. 1993;11:645–655. doi: 10.1016/0896-6273(93)90076-4. [DOI] [PubMed] [Google Scholar]
- 16.White R E, Schonbrunn A, Armstrong D L. Nature (London) 1991;351:570–573. doi: 10.1038/351570a0. [DOI] [PubMed] [Google Scholar]
- 17.White R E, Lee A B, Shcherbatko A D, Lincoln T M, Schonbrunn A, Armstrong D L. Nature (London) 1993;361:263–266. doi: 10.1038/361263a0. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa K, Barger S W, Blalock E M, Mattson M M. Nature (London) 1996;379:74–78. doi: 10.1038/379074a0. [DOI] [PubMed] [Google Scholar]
- 19.Prevarskaya N B, Skryma R N, Vacher P, Daniel N, Djiane J, Dufy B. J Biol Chem. 1995;270:24292–24299. doi: 10.1074/jbc.270.41.24292. [DOI] [PubMed] [Google Scholar]
- 20.Reinhart P H, Chung S, Martin B L, Brautigan D L, Levitan I B. J Neurosci. 1991;11:1627–1635. doi: 10.1523/JNEUROSCI.11-06-01627.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esguerra M, Wang J, Foster C D, Adelman J P, North R A, Levitan I B. Nature (London) 1994;369:563–565. doi: 10.1038/369563a0. [DOI] [PubMed] [Google Scholar]
- 22.Drejer J, Honoré T, Schousboe A. J Neurosci. 1987;7:2910–2916. doi: 10.1523/JNEUROSCI.07-09-02910.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knaus H G, McManus O B, Lee S H, Schmalhofer W A, Garcia-Calvo M, Helms L M H, Sanchez M, Giangiacomo K M, Reuben J P, Smith A B, III, Kaczorowski G J, Garcia M L. Biochemistry. 1994;33:5819–5828. doi: 10.1021/bi00185a021. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh A, Carnahan J, Greenberg M E. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen F C, Wang E-M, Gammeltoft S. J Neurochem. 1991;56:12–21. doi: 10.1111/j.1471-4159.1991.tb02556.x. [DOI] [PubMed] [Google Scholar]
- 26.Ip N Y, McClain J, Barrezueta N X, Aldrich T H, Pan L, Li Y, Wiegand S J, Friedman B, Davis S, Yancopoolous G D. Neuron. 1993;10:89–102. doi: 10.1016/0896-6273(93)90245-m. [DOI] [PubMed] [Google Scholar]
- 27.Greene L A, Kaplan D R. Neurobiology. 1995;5:579–587. doi: 10.1016/0959-4388(95)80062-x. [DOI] [PubMed] [Google Scholar]
- 28.Knüsel B, Hefti F. J Neurochem. 1992;59:1987–1996. doi: 10.1111/j.1471-4159.1992.tb10085.x. [DOI] [PubMed] [Google Scholar]
- 29.Smallridge R C, Kiang J G, Gist I D, Fein H G, Galloway R J. Endocrinology. 1992;131:1883–1888. doi: 10.1210/endo.131.4.1396332. [DOI] [PubMed] [Google Scholar]
- 30.Bialojan C, Takai A. Biochem J. 1988;256:283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Widmer H R, Kaplan D R, Rabin S J, Beck K D, Hefti F, Knüsel B. J Neurochem. 1993;60:2111–2123. doi: 10.1111/j.1471-4159.1993.tb03496.x. [DOI] [PubMed] [Google Scholar]