Abstract
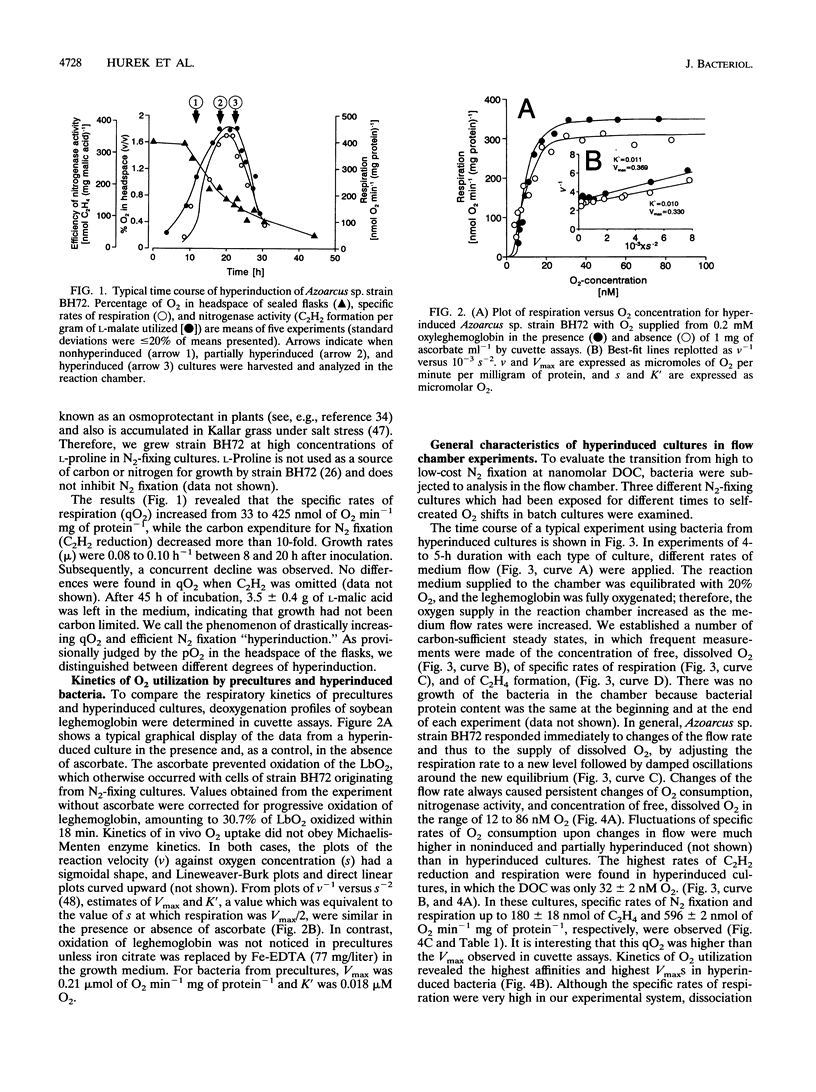
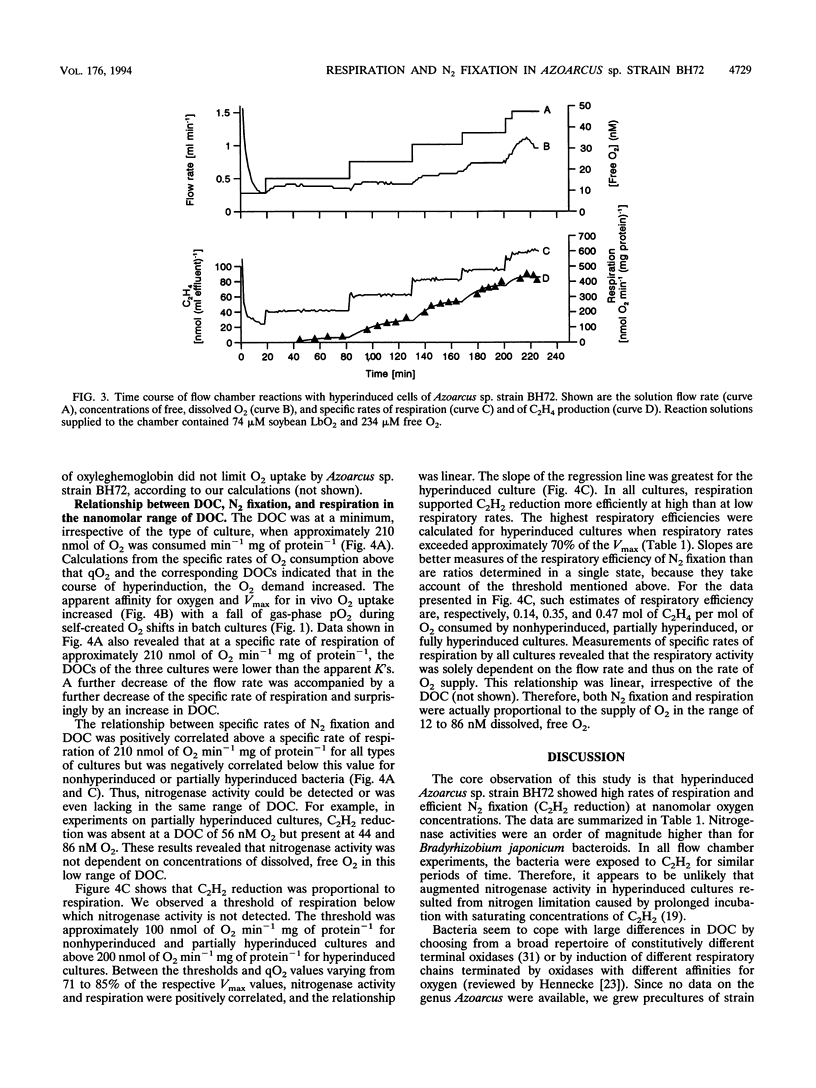
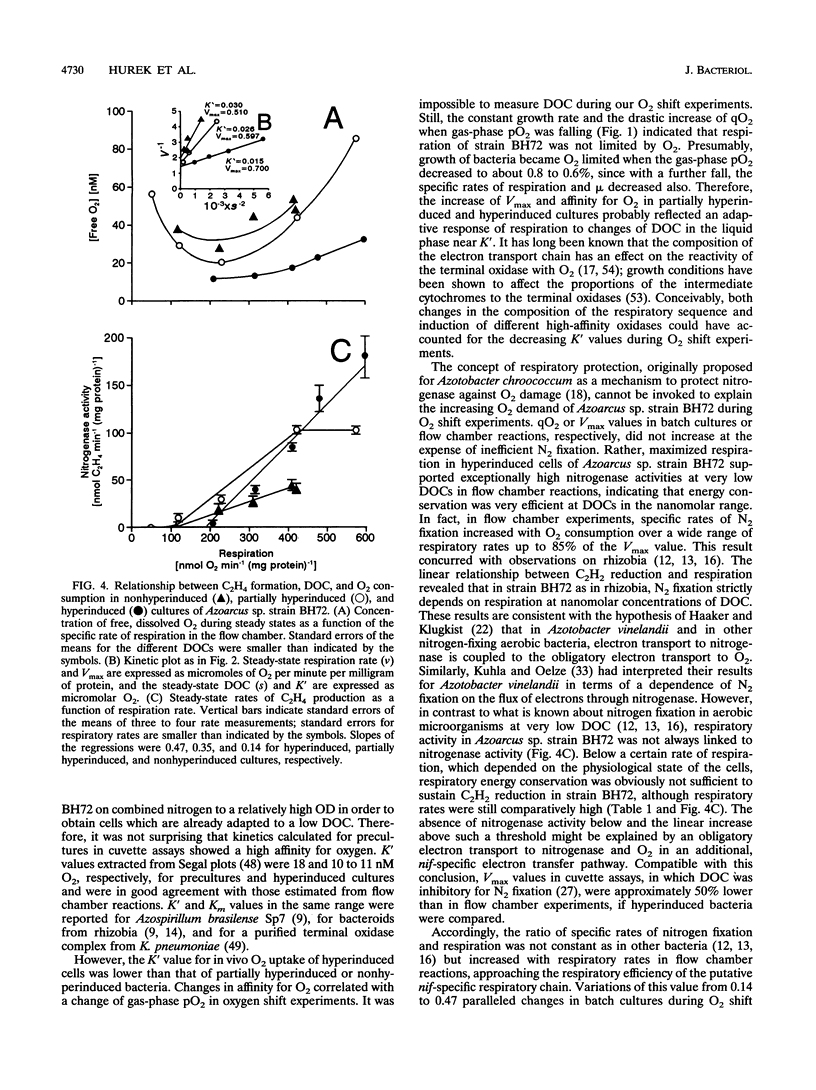
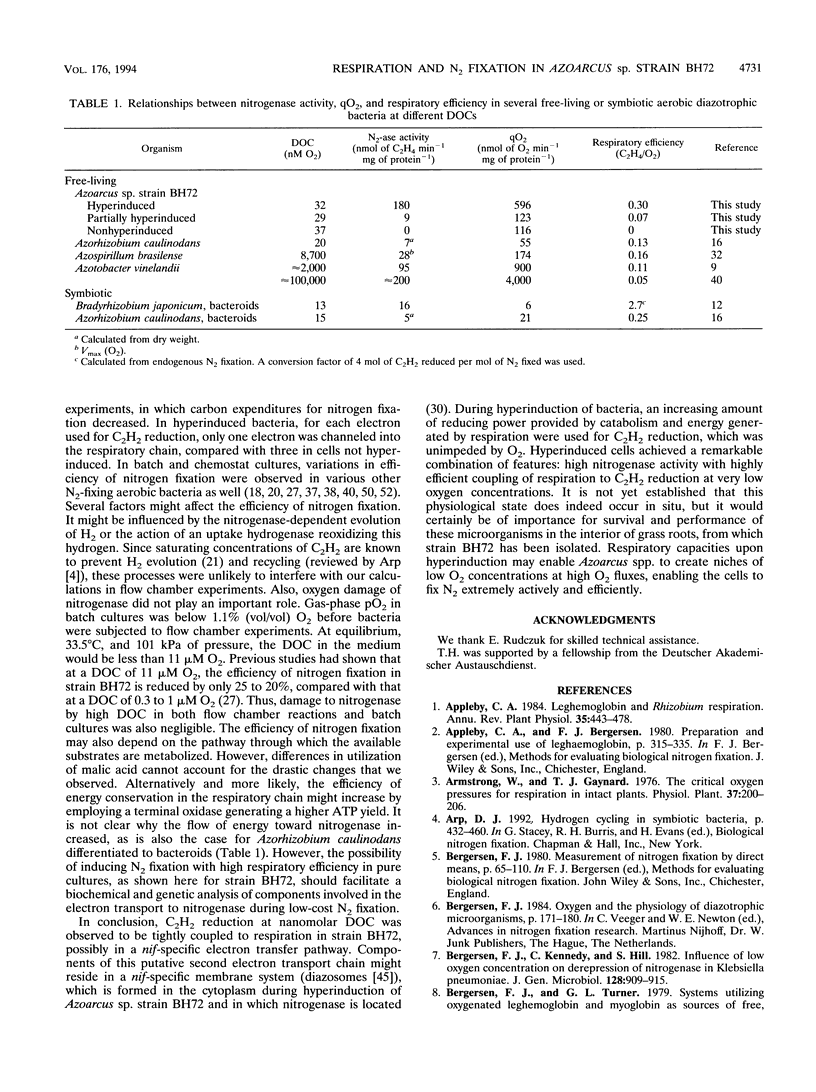
Azoarcus sp. strain BH72 is an aerobic diazotrophic bacterium that was originally found as an endophyte in Kallar grass. Anticipating that these bacteria are exposed to dissolved O2 concentrations (DOCs) in the nanomolar range during their life cycle, we studied the impact of increasing O2 deprivation on N2 fixation and respiration. Bacteria were grown in batch cultures, where they shifted into conditions of low pO2 upon depletion of O2 by respiration. During incubation, specific rates of respiration (qO2) and efficiencies of carbon source utilization for N2 reduction increased greatly, while the growth rate did not change significantly, a phenomenon that we called "hyperinduction." To evaluate this transition from high- to low-cost N2 fixation in terms of respiratory kinetics and nitrogenase activities at nanomolar DOC, bacteria which had shifted to different gas-phase pO2s in batch cultures were subjected to assays using leghemoglobin as the O2 carrier. As O2 deprivation in batch cultures proceeded, respiratory Km (O2) decreased and Vmax increased. Nitrogenase activity at nanomolar DOC increased to a specific rate of 180 nmol of C2H4 min-1 mg of protein-1 at 32 nM O2. Nitrogenase activity was proportional to respiration but not to DOC in the range of 12 to 86 nM O2. Respiration supported N2 fixation more efficiently at high than at low respiratory rates, the respiratory efficiency increasing from 0.14 to 0.47 mol of C2H4 mol of O2 consumed-1. We conclude that (i) during hyperinduction, strain BH72 used an increasing amount of energy generated by respiration for N2 fixation, and (ii) these bacteria have a high respiratory capacity, enabling them to develop ecological niches at very low pO2, in which they may respire actively and fix nitrogen efficiently at comparatively high rates.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergersen F. J., Kennedy C., Hill S. Influence of low oxygen concentration on derepression of nitrogenase in Klebsiella pneumoniae. J Gen Microbiol. 1982 May;128(5):909–915. doi: 10.1099/00221287-128-5-909. [DOI] [PubMed] [Google Scholar]
- Bergersen F. J., Turner G. L. Supply of O2 regulates O2 demand during utilization of reserves of poly-beta-hydroxybutyrate in N2-fixing soybean bacteroids. Proc Biol Sci. 1992 Aug 22;249(1325):143–148. doi: 10.1098/rspb.1992.0096. [DOI] [PubMed] [Google Scholar]
- David K. A., Fay P. Effects of long-term treatment with acetylene on nitrogen-fixing microorganisms. Appl Environ Microbiol. 1977 Dec;34(6):640–646. doi: 10.1128/aem.34.6.640-646.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S., Turner G. L., Bergersen F. J. Synthesis and activity of nitrogenase in Klebsiella pneumoniae exposed to low concentrations of oxygen. J Gen Microbiol. 1984 May;130(5):1061–1067. doi: 10.1099/00221287-130-5-1061. [DOI] [PubMed] [Google Scholar]
- Hurek T., Reinhold-Hurek B., Van Montagu M., Kellenberger E. Root colonization and systemic spreading of Azoarcus sp. strain BH72 in grasses. J Bacteriol. 1994 Apr;176(7):1913–1923. doi: 10.1128/jb.176.7.1913-1923.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurek T., Reinhold B., Fendrik I., Niemann E. G. Root-Zone-Specific Oxygen Tolerance of Azospirillum spp. and Diazotrophic Rods Closely Associated with Kallar Grass. Appl Environ Microbiol. 1987 Jan;53(1):163–169. doi: 10.1128/aem.53.1.163-169.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitts C. L., Ludwig R. A. Azorhizobium caulinodans respires with at least four terminal oxidases. J Bacteriol. 1994 Feb;176(3):886–895. doi: 10.1128/jb.176.3.886-895.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhla J., Oelze J. Dependence of nitrogenase switch-off upon oxygen stress on the nitrogenase activity in Azotobacter vinelandii. J Bacteriol. 1988 Nov;170(11):5325–5329. doi: 10.1128/jb.170.11.5325-5329.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Lloyd D., James K., Williams J., Williams N. A membrane-covered photobacterium probe for oxygen measurements in the nanomolar range. Anal Biochem. 1981 Sep 1;116(1):17–21. doi: 10.1016/0003-2697(81)90315-8. [DOI] [PubMed] [Google Scholar]
- Nelson L. M., Knowles R. Effect of oxygen and nitrate on nitrogen fixation and denitrification by Azospirillum brasilense grown in continuous culture. Can J Microbiol. 1978 Nov;24(11):1395–1403. doi: 10.1139/m78-223. [DOI] [PubMed] [Google Scholar]
- Okon Y., Houchins J. P., Albrecht S. L., Burris R. H. Growth of Spirillum lipoferum at constant partial pressures of oxygen, and the properties of its nitrogenase in cell-free extracts. J Gen Microbiol. 1977 Jan;98(1):87–93. doi: 10.1099/00221287-98-1-87. [DOI] [PubMed] [Google Scholar]
- Post E., Kleiner D., Oelze J. Whole cell respiration and nitrogenase activities in Azotobacter vinelandii growing in oxygen controlled continuous culture. Arch Microbiol. 1983 Jan;134(1):68–72. doi: 10.1007/BF00429410. [DOI] [PubMed] [Google Scholar]
- Reinhold B., Hurek T., Fendrik I. Cross-reaction of predominant nitrogen-fixing bacteria with enveloped, round bodies in the root interior of kallar grass. Appl Environ Microbiol. 1987 Apr;53(4):889–891. doi: 10.1128/aem.53.4.889-891.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold B., Hurek T., Fendrik I. Strain-specific chemotaxis of Azospirillum spp. J Bacteriol. 1985 Apr;162(1):190–195. doi: 10.1128/jb.162.1.190-195.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhold B., Hurek T., Niemann E. G., Fendrik I. Close association of azospirillum and diazotrophic rods with different root zones of kallar grass. Appl Environ Microbiol. 1986 Sep;52(3):520–526. doi: 10.1128/aem.52.3.520-526.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Hill S., Anthony C. The purification, characterization and role of the d-type cytochrome oxidase of Klebsiella pneumoniae during nitrogen fixation. J Gen Microbiol. 1990 Jan;136(1):171–180. doi: 10.1099/00221287-136-1-171. [DOI] [PubMed] [Google Scholar]
- WHITE D. C. Cytochrome and catalase patterns during growth of Haemophilus parainfluenzae. J Bacteriol. 1962 Apr;83:851–859. doi: 10.1128/jb.83.4.851-859.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE D. C. FACTORS AFFECTING THE AFFINITY FOR OXYGEN OF CYTOCHROME OXIDASES IN HEMOPHILUS PARAINFLUENZAE. J Biol Chem. 1963 Nov;238:3757–3761. [PubMed] [Google Scholar]


