Abstract
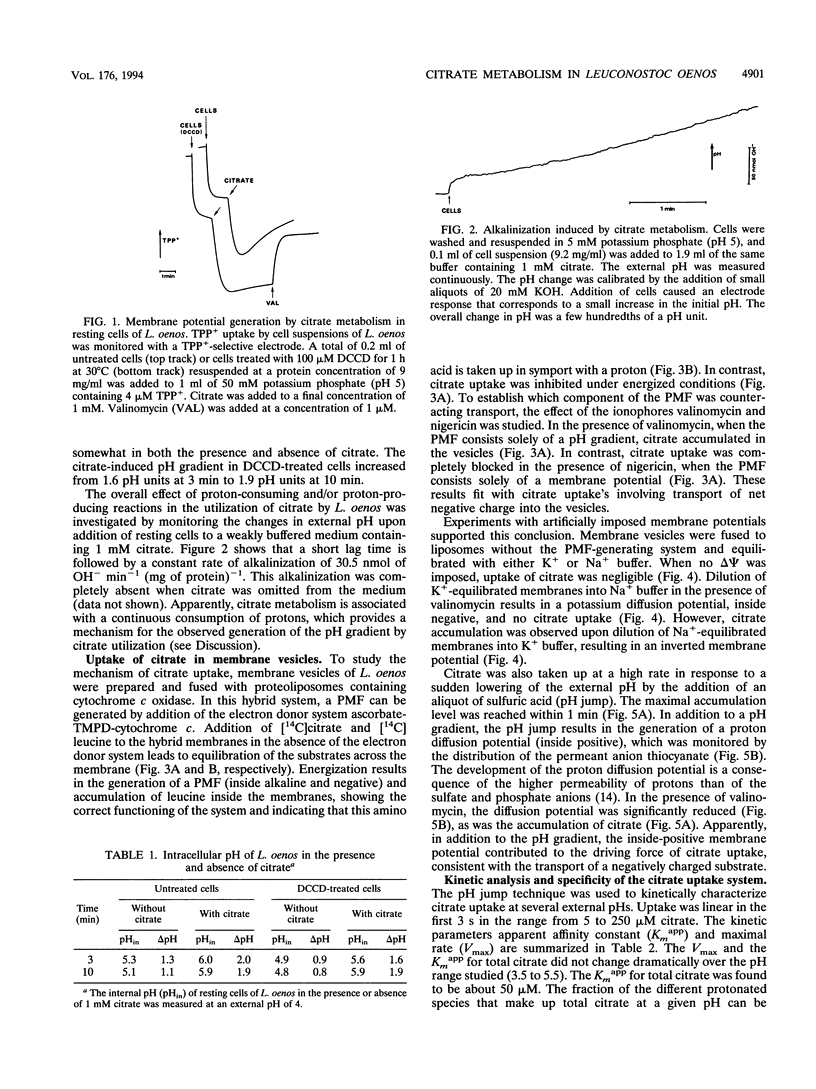
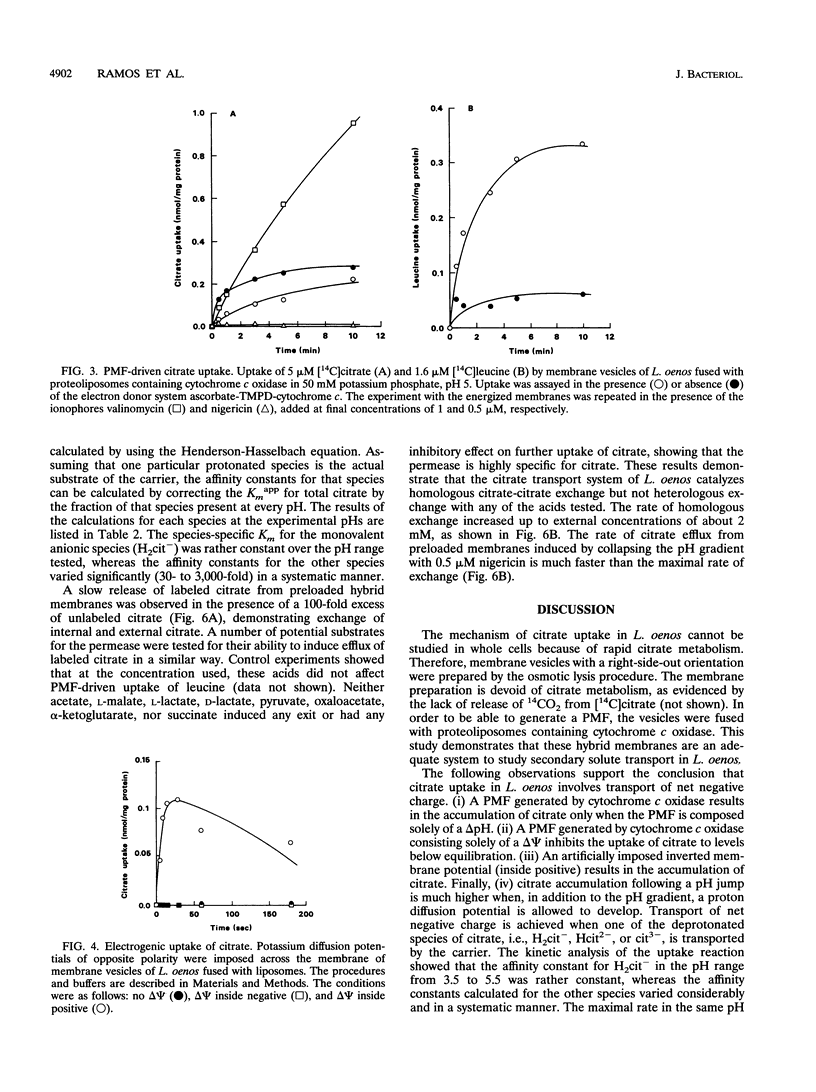
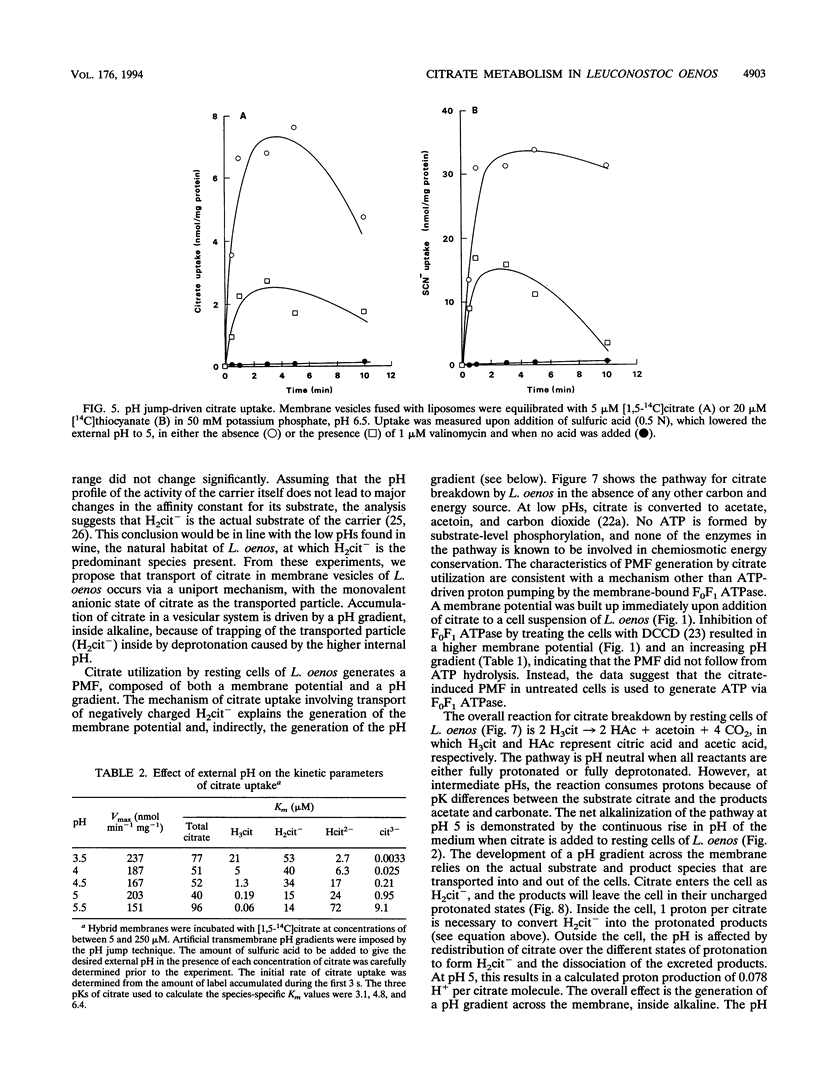
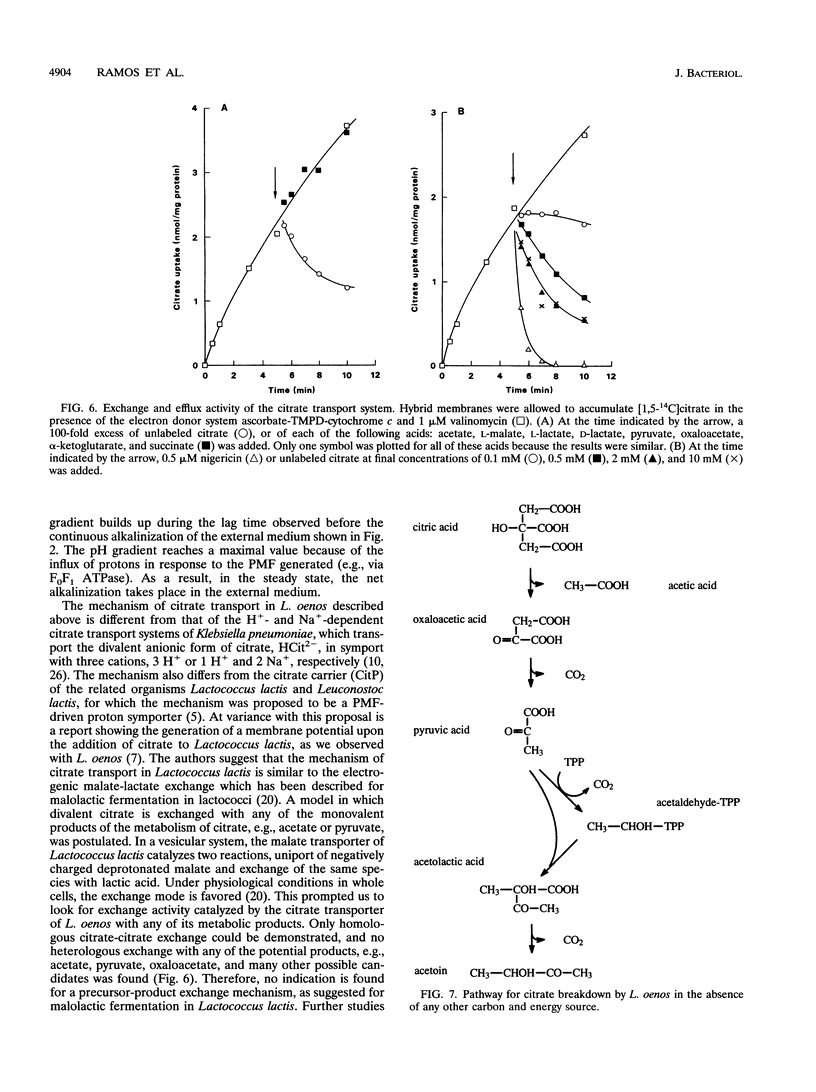
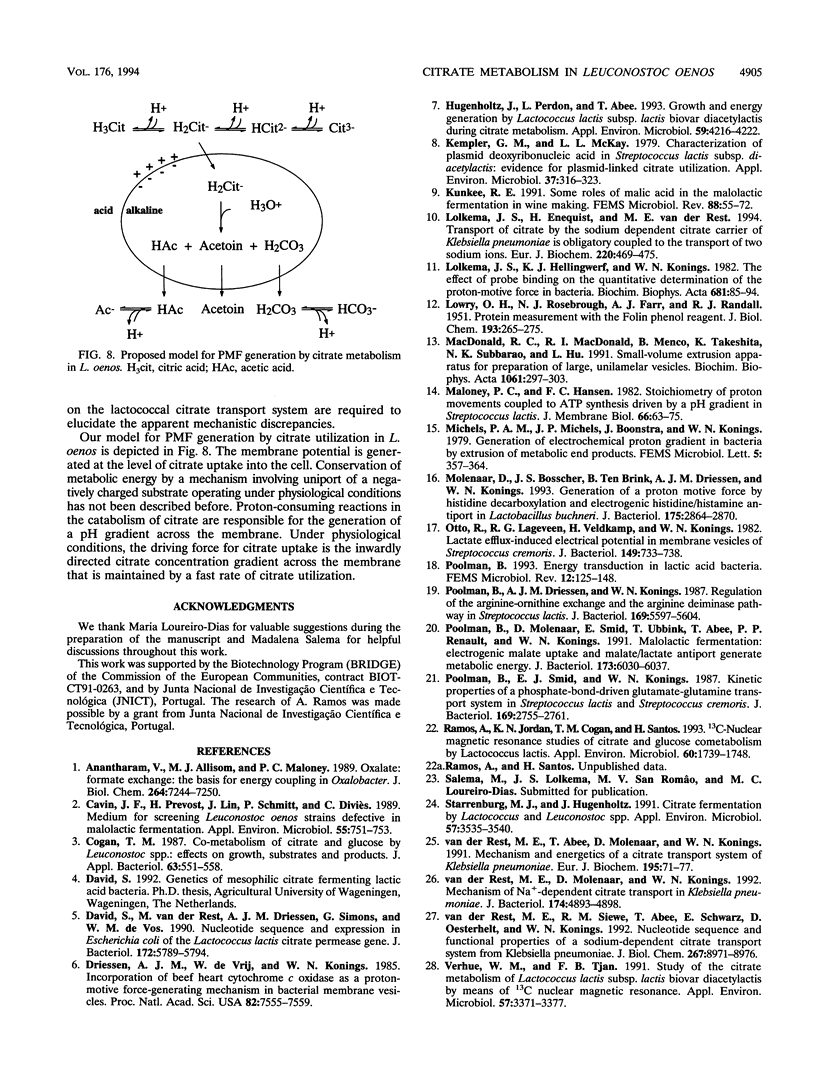
The mechanism and energetics of citrate transport in Leuconostoc oenos were investigated. Resting cells of L. oenos generate both a membrane potential (delta psi) and a pH gradient (delta pH) upon addition of citrate. After a lag time, the internal alkalinization is followed by a continuous alkalinization of the external medium, demonstrating the involvement of proton-consuming reactions in the metabolic breakdown of citrate. Membrane vesicles of L. oenos were prepared and fused to liposomes containing cytochrome c oxidase to study the mechanism of citrate transport. Citrate uptake in the hybrid membranes is inhibited by a membrane potential of physiological polarity, inside negative, and driven by an inverted membrane potential, inside positive. A pH gradient, inside alkaline, leads to the accumulation of citrate inside the membrane vesicles. Kinetic analysis of delta pH-driven citrate uptake over a range of external pHs suggests that the monovalent anionic species (H2cit-) is the transported particle. Together, the data show that the transport of citrate is an electrogenic process in which H2cit- is translocated across the membrane via a uniport mechanism. Homologous exchange (citrate/citrate) was observed, but no evidence for a heterologous antiport mechanism involving products of citrate metabolism (e.g., acetate and pyruvate) was found. It is concluded that the generation of metabolic energy by citrate utilization in L. oenos is a direct consequence of the uptake of the negatively charged citrate anion, yielding a membrane potential, and from H(+)-consuming reactions involved in subsequent citrate metabolism, yielding a pH gradient. The uptake of citrate is driven by its own concentration gradient, which is maintained by efficient metabolic breakdown (metabolic pull).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anantharam V., Allison M. J., Maloney P. C. Oxalate:formate exchange. The basis for energy coupling in Oxalobacter. J Biol Chem. 1989 May 5;264(13):7244–7250. [PubMed] [Google Scholar]
- Cavin J. F., Prevost H., Lin J., Schmitt P., Divies C. Medium for Screening Leuconostoc oenos Strains Defective in Malolactic Fermentation. Appl Environ Microbiol. 1989 Mar;55(3):751–753. doi: 10.1128/aem.55.3.751-753.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S., van der Rest M. E., Driessen A. J., Simons G., de Vos W. M. Nucleotide sequence and expression in Escherichia coli of the Lactococcus lactis citrate permease gene. J Bacteriol. 1990 Oct;172(10):5789–5794. doi: 10.1128/jb.172.10.5789-5794.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driessen A. J., de Vrij W., Konings W. N. Incorporation of beef heart cytochrome c oxidase as a proton-motive force-generating mechanism in bacterial membrane vesicles. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7555–7559. doi: 10.1073/pnas.82.22.7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugenholtz J., Perdon L., Abee T. Growth and Energy Generation by Lactococcus lactis subsp. lactis biovar diacetylactis during Citrate Metabolism. Appl Environ Microbiol. 1993 Dec;59(12):4216–4222. doi: 10.1128/aem.59.12.4216-4222.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempler G. M., McKay L. L. Characterization of Plasmid Deoxyribonucleic Acid in Streptococcus lactis subsp. diacetylactis: Evidence for Plasmid-Linked Citrate Utilization. Appl Environ Microbiol. 1979 Feb;37(2):316–323. doi: 10.1128/aem.37.2.316-323.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lolkema J. S., Enequist H., van der Rest M. E. Transport of citrate catalyzed by the sodium-dependent citrate carrier of Klebsiella pneumoniae is obligatorily coupled to the transport of two sodium ions. Eur J Biochem. 1994 Mar 1;220(2):469–475. doi: 10.1111/j.1432-1033.1994.tb18645.x. [DOI] [PubMed] [Google Scholar]
- MacDonald R. C., MacDonald R. I., Menco B. P., Takeshita K., Subbarao N. K., Hu L. R. Small-volume extrusion apparatus for preparation of large, unilamellar vesicles. Biochim Biophys Acta. 1991 Jan 30;1061(2):297–303. doi: 10.1016/0005-2736(91)90295-j. [DOI] [PubMed] [Google Scholar]
- Maloney P. C., Hansen F. C., 3rd Stoichiometry of proton movements coupled to ATP synthesis driven by a pH gradient in Streptococcus lactis. J Membr Biol. 1982;66(1):63–75. doi: 10.1007/BF01868482. [DOI] [PubMed] [Google Scholar]
- Molenaar D., Bosscher J. S., ten Brink B., Driessen A. J., Konings W. N. Generation of a proton motive force by histidine decarboxylation and electrogenic histidine/histamine antiport in Lactobacillus buchneri. J Bacteriol. 1993 May;175(10):2864–2870. doi: 10.1128/jb.175.10.2864-2870.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto R., Lageveen R. G., Veldkamp H., Konings W. N. Lactate efflux-induced electrical potential in membrane vesicles of Streptococcus cremoris. J Bacteriol. 1982 Feb;149(2):733–738. doi: 10.1128/jb.149.2.733-738.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Driessen A. J., Konings W. N. Regulation of arginine-ornithine exchange and the arginine deiminase pathway in Streptococcus lactis. J Bacteriol. 1987 Dec;169(12):5597–5604. doi: 10.1128/jb.169.12.5597-5604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B. Energy transduction in lactic acid bacteria. FEMS Microbiol Rev. 1993 Sep;12(1-3):125–147. doi: 10.1111/j.1574-6976.1993.tb00015.x. [DOI] [PubMed] [Google Scholar]
- Poolman B., Molenaar D., Smid E. J., Ubbink T., Abee T., Renault P. P., Konings W. N. Malolactic fermentation: electrogenic malate uptake and malate/lactate antiport generate metabolic energy. J Bacteriol. 1991 Oct;173(19):6030–6037. doi: 10.1128/jb.173.19.6030-6037.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Smid E. J., Konings W. N. Kinetic properties of a phosphate-bond-driven glutamate-glutamine transport system in Streptococcus lactis and Streptococcus cremoris. J Bacteriol. 1987 Jun;169(6):2755–2761. doi: 10.1128/jb.169.6.2755-2761.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos A., Jordan K. N., Cogan T. M., Santos H. C Nuclear Magnetic Resonance Studies of Citrate and Glucose Cometabolism by Lactococcus lactis. Appl Environ Microbiol. 1994 Jun;60(6):1739–1748. doi: 10.1128/aem.60.6.1739-1748.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starrenburg M. J., Hugenholtz J. Citrate Fermentation by Lactococcus and Leuconostoc spp. Appl Environ Microbiol. 1991 Dec;57(12):3535–3540. doi: 10.1128/aem.57.12.3535-3540.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Rest M. E., Abee T., Molenaar D., Konings W. N. Mechanism and energetics of a citrate-transport system of Klebsiella pneumoniae. Eur J Biochem. 1991 Jan 1;195(1):71–77. doi: 10.1111/j.1432-1033.1991.tb15677.x. [DOI] [PubMed] [Google Scholar]
- Verhue W. M., Tjan F. S. Study of the Citrate Metabolism of Lactococcus lactis subsp. lactis Biovar Diacetylactis by Means of C Nuclear Magnetic Resonance. Appl Environ Microbiol. 1991 Nov;57(11):3371–3377. doi: 10.1128/aem.57.11.3371-3377.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Rest M. E., Molenaar D., Konings W. N. Mechanism of Na(+)-dependent citrate transport in Klebsiella pneumoniae. J Bacteriol. 1992 Aug;174(15):4893–4898. doi: 10.1128/jb.174.15.4893-4898.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Rest M. E., Siewe R. M., Abee T., Schwarz E., Oesterhelt D., Konings W. N. Nucleotide sequence and functional properties of a sodium-dependent citrate transport system from Klebsiella pneumoniae. J Biol Chem. 1992 May 5;267(13):8971–8976. [PubMed] [Google Scholar]



