Abstract
The recent flood of research concerning pollutants in personal environmental and biological samples—blood, urine, breastmilk, household dust and air, umbilical cord blood, and other media—raises questions about whether and how to report results to individual study participants.
Clinical medicine provides an expert-driven framework, whereas community-based participatory research emphasizes participants’ right to know and the potential to inform action even when health effects are uncertain. Activist efforts offer other models.
We consider ethical issues involved in the decision to report individual results in exposure studies and what information should be included. Our discussion is informed by our experience with 120 women in a study of 89 pollutants in homes and by interviews with other researchers and institutional review board staff.
ON JANUARY 29, 2003, readers opened The New York Times to a full-page advertisement that featured a photograph of Andrea Martin, a 56-year-old mother and the founder of the Breast Cancer Fund, with a headline boxed like a cigarette label across her chest: “Warning: Andrea Martin Contains 59 Cancer-Causing Industrial Chemicals.”1 The ad reported on a study by Environmental Working Group (EWG) and Mt Sinai Medical School that reported finding an average of 90 pollutants in blood samples from 9 volunteers who were tested for 200 environmental chemicals. Details on the EWG Web site put a human face on “the pollution in people” by revealing each volunteer’s test results.2 A month later, the US Centers for Disease Control and Prevention (CDC) published its Second National Report on Human Exposure to Environmental Chemicals, an extensive assessment of personal exposure statistics for a representative sample of the US population, that included measurement of 116 pollutants in participants’ blood and urine.3
These reports marked the beginning of a flood of personal exposure information. Scientific journals, activist Web sites, and the news media were soon reporting on contaminants in personal environmental and biological samples—for example, flame retardants in breastmilk,4 pesticides in umbilicalcord blood,5,6 endocrine-disrupting compounds in homes,7 phthalates in cars,8 and chemicals in a family tested by the Oakland Tribune.9 The Third National Report on Human Exposure to Environmental Chemicals in 2005 reported on 148 chemicals in more than 5000 people.10 National screening will expand to 473 chemicals in 2009, and biomonitoring programs are beginning in several states.
These efforts rest on new chemical analytic methods that enable the detection of ever-lower concentrations of an increasing number of chemicals for which animal and cell studies show troubling biological effects. However, human exposure concentrations, chemical sources, health effects, and exposure-reduction strategies are not yet understood. The new methods and data advance environmental epidemiology and environmental health policy, and they are powerful communication and mobilization tools. However, the methods and data raise ethical and technical issues about how to interpret and report results to study participants and their communities when the health implications of exposures are uncertain. The National Academy of Sciences’ (NAS’s) report, Human Biomonitoring for Environmental Chemicals, notes that chemical testing technologies have advanced faster than ethical guidelines and methods for interpreting and communicating results, and it recommends sharing information about multiple approaches in order to develop best practices.11
These issues are of particular importance to our study team because of the household exposure study of endocrine-disrupting compounds we are conducting. As part of the Cape Cod (Massachusetts) Breast Cancer and Environment Study,12–14 we tested for 89 endocrine-disrupting compounds in household air and dust from 120 homes and tested a urine sample from the woman in each home who participated in the breast cancer study. The endocrine-disrupting compounds tested for included phthalates, alkylphenols, parabens, polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), pesticides, and other phenolic endocrine-disrupting compounds.7 We are continuing the study in Cape Cod and have expanded the study in Northern California.
Our approach in the household exposure study is to draw on a community-based participatory research framework15 and “right-to-know” ethic; we report aggregated results in scientific journals, at public meetings, and in the news media and offered participants the opportunity to receive their individual results. However, we have found few models for reporting personal exposures to study participants; indeed, some researchers and institutional review boards have argued against reporting individual results when the clinical implications are unclear.
Because of the dearth of models and the questions about the ethics of reporting, we examined ethical frameworks and interviewed other researchers, institutional review board members, and our study participants about their perspectives. Here, we examine several ethical perspectives on whether to report individual-level exposure results and then consider how to report these results in a community-based participatory research context. We draw qualitatively on all our interviews with researchers, institutional review board staff, and our study participants to date; we plan to report on participant interviews again when they are complete. Our goal here is to stimulate dialogue about individual report-back issues (reporting an individual’s own results to her or him), which are pressing, given the expansion of individual-level exposure measures of emerging pollutants.
DECIDING WHETHER TO REPORT INDIVIDUAL RESULTS
The guidelines for protecting human participants in research rest on 4 principles. Autonomy includes the right to know as a basis for self-determination in acting on research results. Beneficence and nonmalfeasance encompass the researcher’s responsibility to maximize good and minimize harm. Justice refers to the distribution of benefits and harms to different groups.16 The principles of autonomy and justice favor reporting individual results to study participants. Beneficence guides researchers to consider benefits, such as empowering individuals and communities to take actions to reduce exposures, protect their health, and participate more fully in public health research and policy, all of which are expressions of the values of democracy. The concept of nonmalfeasance directs researchers to consider the potential that report-back may result in experiences such as fear, worry, or stigma; legal and economic complications, such as effects on health insurance or property values from knowing about a suspect chemical; and the possible unintended promotion of unnecessary or counterproductive interventions. Because responsible report-back is expensive (financial costs include creating a customized report for each individual and, often, a face-to-face meeting), unintended harm may be created from the use of resources that would otherwise be spent on health or services.
Clinical medicine, community-based participatory research, and activist campaigns offer alternative perspectives for analyzing potential benefits and harms.
CLINICAL MEDICINE
Reporting blood and urine concentrations of chemicals, including pollutants such as lead, is commonplace in medical practice, so researchers understandably turn to clinical medicine as a model for reporting individual results. In the clinical medicine model, individual test results are reported to the participant by a medical practitioner when the results are considered clinically relevant because expert judgment has determined that the results are associated with an adverse health outcome or because the results trigger intervention on the basis of medical guidelines or legal mandate.
Deck and Kosatsky17 applied this standard in a study of sport fishing in which they measured environmental pollutants in blood, hair, and urine: “In clinical practice, full disclosure does not require that all results be communicated but rather those findings which, in the clinician’s judgment, raise the possibility of the need for some action to be taken.”17(p227) In this model, action is predicated on knowing the relationship between the bio-marker and health outcome: if the exposure–health effect relationship is not known, “it does not seem prudent to communicate such information [individual exposure], since doing so does not enhance autonomy and has potential for causing more harm than good.”17(p227)
The clinical medicine model gives more importance to the expert-researcher’s role in avoiding possible harm from reporting uncertain information and gives less importance to the study participants’ ability to process complex and uncertain scientific information and respond autonomously. One particular concern in the context of recent toxicology is that the clinical approach does not enable precautionary action by participants, because they would not learn their individual results even if the evidence suggested that there are health effects below the action level, as is the case for lead, for example.18,19 Additionally, clinical medicine offers a narrow view of the potential for beneficial action. Clinical medicine is usually focused on medical intervention, although it extends to exposure reduction for lead and mercury, contaminants for which an adverse effect level in humans has been established.
Changes in medical practice over the past few decades suggest that this constrained standard of report-back oversimplifies evolving doctor–patient relationships because patients are taking a more active role in directing their care.20,21 For example, patients today often track their own medical results, such as blood pressure and cholesterol, even when they are below a clinical action criterion. In a dramatic change, medical practice has moved away from hiding cancer diagnosis and prognosis to protect patients from the perceived harm of bad news. The present standard of candid discussion is based on observations that patients can benefit from open communication with care-givers and others and dying patients can take actions—such as preparing a will, planning their memorial, making decisions about their care, and saying good-bye to family and friends.22
Individual report-back as done in the CDC National Exposure Report is a step toward more open communication within a clinical medical model about chemical exposure. Along with numerous medical results, such as blood pressure, hemoglobin counts, and bone density, participants received results for a small number of environmental chemicals, including arsenic, cadmium, lead, and mercury. Results were flagged as “high” if they exceeded a health-based criterion.23,24 Participants with concentrations above a health guideline received a letter telling them their results may have health implications and instructing them to consult a doctor. Participants may also have received information about an environmental measurement, for example if their drinking water exceeded the US Environmental Protection Agency (EPA) tri-halomethane standard. However, no report-back was done for the vast majority of environmental chemicals tested (G. M. McQuillan, written communication, September 14, 2006).
COMMUNITY-BASED PARTICIPATORY RESEARCH
Community-based participatory research conceptualizes research as a joint effort of researchers, community members, and study participants with shared decisionmaking and ownership of information.25,26 The spirit of this relationship is expressed in a study of farm-worker pesticide exposures by Quandt et al., in which the authors argue that individuals have a right to know their results, because “It is ethical to return information to the ‘owner’ of that information.”27(p642)
A collaborative study design and interpretation process is used in the community-based participatory research model to make decisions that take into account what community partners and study participants want. Extending this model to report-back, alternatives for reporting results would first be discussed by community representatives and researchers within the community-based participatory research team, then in a broader circle, for example with additional community leaders and in public meetings. In this context, research partners have a responsibility to facilitate informed participation by clarifying what information the proposed study methods can and cannot provide. In an exposure study for emerging pollutants, this means communicating that results are likely to show detectable concentrations of environmental chemicals, that the presence of pollutants does not necessarily result in harm, and that the study is not designed to find relationships with health effects. Researchers should communicate strengths or uncertainties in scientific knowledge about the relationship of exposure to health risk and articulate that exposure assessment is an important preliminary step toward revealing health effects in the future, while in the meantime, offering help to identify emissions sources and exposure-reduction strategies.
In addition, research partners must consider in advance how report-back may affect the confidentiality of the individual’s results. If individuals learn their own results, might they be obligated to disclose them to others—e.g., if results point to a source that is regulated, such as a work-place, or reveal a possible hazard that may affect others?
The researcher–community consultation is then reflected in the recruiting scripts and informed consent. In designing these protocols, community-based participatory research begins from a presumption that study participants should be empowered to make their own decisions about receiving individual results.28 However, this does not mean a hands-off ethic for researchers with respect to the principles of beneficence and nonmalfeasance. Rather, the informed consent confers an opportunity and a responsibility for researchers and community partners to jointly articulate the potential community and individual benefits and harms considered by the study team as possible sequelae of reporting results.
In our Household Exposure Study, community leaders, including the Massachusetts Breast Cancer Coalition, strongly advocated for report-back, even though health effects and even typical exposure levels are unknown for many of the contaminants we measured. Given the opportunity to learn their individual results, 116 of 120 participants (97%) requested them. Our interviews indicate that their experiences with receiving their results included curiosity about what we would find, motivation to adopt exposure-reduction strategies, reflections on the disease experience of a family member, and deeper analysis of “toxic trespass.”
OTHER APPROACHES TO REPORT-BACK
Not all personal exposure report-back falls into the clinical medicine and community-based participatory research categories. Activist exposure studies, such as the EWG Body Burden project,2 represent another approach, which is focused on population-level benefits of personal exposure monitoring. Seeking to educate the public and influence policy, EWG and other groups have conducted highly visible studies, often with volunteers appearing publicly along with their results. Activist studies have also used unidentified samples or commingled samples from multiple individuals.29 As in the national and state biomonitoring programs, the focus is on revealing exposure trends in populations.
Looking across government programs, activist campaigns, and community-based participatory research studies like ours, a critical mass of personal exposure-monitoring reports could significantly transform environmental health research, regulatory policy, and public health approaches to disease prevention by both humanizing and quantifying “invisible” issues of contamination. The process and content of community and individual report-back are important dimensions in planning exposure studies, and these dimensions also influence the impact of the studies.
HOW AND WHAT TO REPORT
Ethical considerations extend beyond the decision about whether to report individual results to the inidividual to how and what to report. A first responsibility is to report in ways that are understandable.30,31 Methods for communicating risk—including strategies for building trust, respecting cultural context, and considering cognitive processes (i.e., how people process information)—are the subject of a large body of literature that informs effective reporting. The NAS biomonitoring report includes a recent overview.11 Because NAS and others have extensively addressed these aspects of risk communication elsewhere, we focus here on the content of what is reported back.
From the community-based participatory research perspective, the question of how to report individual results begins with an understanding of what people want and need to know to guide action. We have investigated what study participants want to know by fielding numerous questions from women in our study, community leaders, and the news media, and through discussion with public advisory committees. Access to this input is possible because of a 10-year history of building relationships and trust in the community. Based on our work to date, we developed a set of questions that captured participants’ inquiries and assessed what research data help answer these questions (Table 1 ▶).
1.
Answers to Typical Participant Questions About Their Personal Exposure Results
| Question | Information |
| Description | |
| What did you find? | List of detected chemicals |
| How much? | Concentration shown in a table or graph |
| Analysis/Comparison | |
| Is that high? | Study participant’s result shown in relation to the distribution of others in the study or a reference group such as the CDC Exposure Report |
| Is it safe? | Study participant’s result shown in relation to a health- based regulatory guideline and concentrations associated with health effects in epidemiological Studies |
| What should I focus on? | Results for multiple chemicals shown in relationship to each other |
| Where did the chemical come from? | List of types of products or processes that commonly contain or emit detected chemicals, such as combustion and auto exhaust |
| Recommendation | |
| What can/should I do? | Individual and community exposure-reduction strategies, precautionary strategies, research needs |
Note. CDC = Centers for Disease Control and Prevention.
At the most basic level, participants want to know what target chemicals we found in their homes, and a list of detected chemicals provides the answer. We can also answer the question, “How much?” by reporting the numerical concentration in a table or bar graph. To make this information meaningful for action, we must go beyond description to more difficult analytic and comparative questions.
Analysis and Comparisons in Reporting
Questions about “meaning” are difficult to answer for emerging pollutants for which health effects may not have been investigated and are not known. For 30 of 89 chemicals in our study, no measurements from indoor environments had previously been reported. For 39 chemicals, we found an existing federal health-based guideline; however, many were outdated, and none were based on potential hormonal effects, which was the mechanism of interest and the reason for including the chemicals in our study. For example, the current EPA guideline for dibutyl phthalate is based on a 1953 study in which the end point was mortality32; this standard is inadequate given the wealth of new information on its hormonally mediated developmental toxicity.
In the absence of a health guideline that directly answers the question, “Is it safe,” the CDC National Report on Human Exposure is a valuable resource that allows individuals to compare their results to a representative sample of the US population. For pollutants not included in national studies, the study population itself can be used as a comparison for individual results.
However, comparisons to national or other study groups could lead both researchers and participants to “normalize” (interpret a situation as normal or routine) problematic contaminant levels and even construe them as safe. Interviews with participants in our Household Exposure Study show that they do evaluate their results against others in the study. These observations lead us to consider research designs that include the assessment of exposure in the absence of a source, because this method would provide a better comparison to background levels. For example, for chemicals with mostly indoor sources, outdoor concentrations in air or soil provide a useful comparison.
We are currently experimenting with information-rich visual report-back accompanied by pared-down verbal summaries. The visual report communicates “what,” “how much,” and comparisons to the full distribution of other individuals in the study and a federal health guideline, if one exists (Figure 1 ▶). The verbal summaries distill key messages from the 139 results displayed in the graphs.33 We found that summaries take substantial time for senior scientists to produce and require extensive toxicological and epidemiological knowledge and integrative thinking to evaluate what is most important. We took this as an indication that helping participants interpret their results is a responsibility of the research team. At the same time, participants in our study autonomously processed and interpreted the extensive, complex, and less “filtered” graphs.33
FIGURE 1—
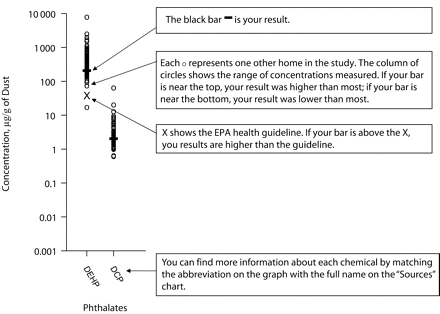
Sample instruction page illustrating the graphic reporting of individual results in the Cape Cod Household Exposure Study.
Recommendations to Study Participants
In our experience, participants want the reporting of results to include the researchers’ recommendations for action, which, like the verbal summaries, integrate study data with broader scientific and practical knowledge. We believe that addressing this need is an ethical responsibility but is a significant scientific, ethical, and communications challenge.
In some studies, involving health care providers in the tailoring of the report-back of the results may be valuable, although we expect that this will require substantial training and briefing for clinicians, particularly in research on emerging pollutants. Often, community-based participatory research teams include members of the local medical community, who can help make the connections between environmental health and individual clinical medicine.
In our study of indoor chemical concentrations, the primary avenue for individual action is to identify the products or activities that are sources of exposure and remove or reduce them. We included a table of chemical sources in our report-back packet so participants could identify the household products or practices that produce the chemicals we detected,34 and our verbal summaries highlighted strategies that are thought to reduce exposures. Recommendations for community-level public health action are also desirable. We informed study participants about how a particular chemical is regulated in the United States and Europe and provided information about policy actions by our community partner Massachusetts Breast Cancer Coalition. We also regularly cosponsor community forums in which participants can interact with advocacy organizations.
Identifying sources is difficult for many of our target compounds, including phthalates, parabens, and phenols, for which the dominant sources and the formulations of specific products are unknown. In addition, we encountered a number of puzzles that have become the subject of follow-up research. For example, we found very high concentrations of PCBs in some homes with no evidence of electrical equipment that might be a source. In addition, we unexpectedly detected the potent carcinogen 2,3 dibromopropanol in 10% of homes. This compound is a breakdown product of the flame retardant tris (2,3-dibromo-propyl) phosphate, which was banned from children’s sleepwear in 1977 but still used in other textiles, so we tested reserved dust samples for the parent compound to confirm it was the source. Additional testing narrowed the source to certain rooms, and we are considering “biopsies” of specific textiles so source objects could be removed. This follow-up resulted in an extended, interactive report-back.
The questions that arose from our research illustrate that just as the health effects of emerging pollutants are uncertain, the efficacy of exposure-reduction strategies may also be unknown. We caution researchers to acknowledge this uncertainty and not allow the wish to “fix” things and reduce worry to lead to unsubstantiated reassurance or recommendations. Figure 2 ▶ shows conceptually how increasing certainty about health effects and exposure reduction (i.e., high knowledge of exposure-effect relationships combined with high knowledge of exposure reduction methods) leads to clear recommendations both for public health and for individual action, whereas decreasing certainty in these dimensions (i.e., low knowledge of exposure-effect relationships combined with low knowledge of exposure reduction methods) leads to recommendations for further research and pre-cautionary exposure reduction. Placing target compounds on the graph clarifies responsible communications. For example, it makes researchers more conscious of the impulse to deemphasize health risks in situations in which exposure-reduction strategies are unknown, or to overstate the efficacy of exposure reduction when health risks are clear. It may also motivate researchers to articulate what is not known and to work to fill the knowledge gaps.
FIGURE 2—
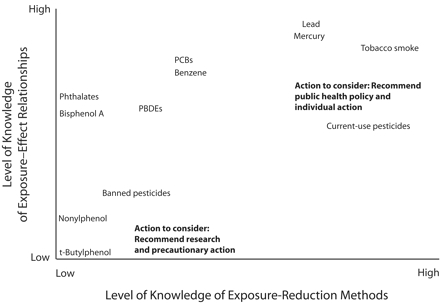
Conceptual graph of the types of actions to recommend in situations with high versus low knowledge about exposure–health effect relationships and exposure-reduction methods.
Note. PBDEs = polybrominated diphenyl ethers, PCBs = polychlorinated biphenyls. High certainty (i.e., high knowledge of exposure-effect relationships combined with high knowledge of exposure reduction methods) supports recommendations for public health policies and individual behavior change. Low certainty (i.e., low knowledge of exposure-effect relationships combined with low knowledge of exposure reduction methods) supports recommendations for further research and consideration of precautionary action.
CONCLUSION
Personal exposure assessment has developed into a key tool for science, risk assessment, and public health policy, especially as the environmental health sciences investigate low-dose chemical effects and the presence of emerging contaminants from consumer products, ambient air, and drinking water. Creative research to assess personal exposures has advanced significantly, but the evolution of study methods has outpaced the development of ethical standards for reporting results to communities and individual study participants. A majority of studies do not report individual exposure results and rely on outdated standards based in clinical medicine. Newer models experiment with reporting results as part of community-researcher partnerships that emphasize participants’ right to know and right to act, and in some cases, highly visible results reporting is central to activist “data judo”35 strategies to stimulate policy change.
In the long run, balancing benefits and harms from individual report-back of personal environmental and biological monitoring should be informed by empirical research on whether such reporting supports empowerment or causes unconstructive fear. Recent studies raise specific questions: Does breastmilk monitoring affect breastfeeding practices among study participants? Do people who choose to receive their results later regret their decision? Does report-back, in fact, help people reduce exposures?
By seeking input from researchers, community partners, study participants, policymakers, and health care providers, and by testing report-back strategies, we can develop guidelines for future practice. In shaping these guidelines, personal exposures are best understood in a public health ethical context that recognizes both individuals and communities as stakeholders and values public input, empowerment, and support for action.36 Researchers have both a responsibility to anticipate and minimize harm and to maximize benefit. Report-back has the potential to generate experiential learning about environmental pollutants, generating broad benefits in improved public understanding, individual risk reduction, influence on corporate practices, and increased participation in environmental public health policy.
Acknowledgments
Preparation of this article was supported by the National Institute of Environmental Health Sciences (grant 1 R25 ES013258-01) and the National Science Foundation (grant SES-0450837).
The article was developed through a community-based participatory research collaboration of researchers and community partners at Silent Spring Institute, Brown University, and Communities for a Better Environment.
Human Participant Protection This article refers to research protocols and interviews conducted by the authors as part of research approved by the Brown University institutional review board.
Peer Reviewed
Contributors J. G. Brody, R. Morello-Frosch, P. Brown, and R. A. Rudel originated the study. J. G. Brody led the writing with substantial input from the other authors. R. G. Altman and M. Frye conducted interviews with study participants and other researchers. C. A. Osimo and C. Perez were the primary liaisons to community leaders and study participants. L. M. Seryak reviewed informed consent practices.
References
- 1.Environmental Working Group. WARNING: Andrea Martin contains 59 cancer-causing industrial chemicals. Available at: http://www.ewg.org/reports/bodyburden1/pdf/NYT_final.pdf. Accessed April 11, 2006.
- 2.Environmental Working Group. Body burden: the pollution in people. Available at: http://www.ewg.org/reports/bodyburden/index.php. Accessed April 11, 2006.
- 3.Centers for Disease Control and Prevention. Second National Report on Human Exposure to Environmental Chemicals. Atlanta, Ga: Division of Laboratory Science, National Center for Environmental Health; 2003.
- 4.Hooper K, She J. Lessons from the Polybrominated Diphenyl Ethers (PBDEs): precautionary principle, primary prevention, and the value of community-based body-burden monitoring using breast milk. Environ Health Perspect. 2003;111:109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whyatt RM, Barr DB, Camann DE, et al. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect. 2003;111: 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whyatt RM, Rauh V, Barr DB, et al. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ Health Perspect. 2004;112:1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudel RA, Camann DE, Spengler JD, Korn LR, Brody JG. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine disrupting compounds in indoor air and dust. Environ Sci Technol. 2003;37:4543–4553. [DOI] [PubMed] [Google Scholar]
- 8.Gearhart J, Posselt H Toxic at any speed: Chemicals in cars and the need for safe alternatives. Ann Arbor, Mich: The Ecology Center; 2006. Available at: http://www.ecocenter.org/dust/ToxicAtAnySpeed.pdf. Accessed June 15, 2007.
- 9.Fischer D. A body’s burden: our chemical legacy. Available at: http://www.insidebayarea.com/bodyburden. Accessed April 11, 2006.
- 10.Centers for Disease Control and Prevention. Third National Report on Human Exposure to Environmental Chemicals. Atlanta, Ga: Centers for Disease Control and Prevention; 2006. Available at: http://www.cdc.gov/exposurereport/3rd/default.htm. Accessed May 22, 2006.
- 11.National Research Council. Human Biomonitoring for Environmental Chemicals. Washington, DC: The National Academies Press; 2006.
- 12.Brody JG, Tickner J, Rudel RA. Community-initiated breast cancer and environment studies and the precautionary principle. Environ Health Perspect. 2005;113:920–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown P, McCormick S, Mayer B, et al. ‘A lab of our own’: environmental causation of breast cancer and challenges to the dominant epidemiological paradigm. Sci Technol Human Values. 2006;31:499–536. [Google Scholar]
- 14.McCormick S, Brody J, Brown P, Polk R. Public involvement in breast cancer research: an analysis and model for future research. Int J Health Serv. 2004;34:625–646. [DOI] [PubMed] [Google Scholar]
- 15.Israel BA, Eng E, Schulz AJ, Parker EA, eds. Methods in Community-Based Participatory Research for Health. San Francisco; Calif: Jossey-Bass; 2005.
- 16.The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. Available at: http://ohsr.od.nih.gov/guidelines/belmont.html. Accessed May 19, 2006. [PubMed]
- 17.Deck W, Kosatsky T. Communicating their individual results to participants in an environmental exposure study: insights from clinical ethics. Environ Res. 1999;80:S223–S229. [DOI] [PubMed] [Google Scholar]
- 18.Canfield R, Henderson C, Cory-Slechta D, Cox C, Jusko T, Lanphear B. Intellectual impairment in children with blood lead concentrations below 10 μg per deciliter. N Engl J Med. 2003; 348:1517–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellinger D. Lead. Pediatrics. 2004; 113(suppl 4):1016–1022. [PubMed] [Google Scholar]
- 20.Bury M. Researching patient-professional interactions. J Health Serv Res Policy. 2004;9(suppl 1):48–54. [DOI] [PubMed] [Google Scholar]
- 21.Brown P, Zavestoski S, eds. Social Movements in Health. Malden, Mass: Blackwell Publishing; 2005.
- 22.Kasper A, Ferguson S, eds. Breast Cancer: Society Shapes an Epidemic. New York, NY: St Martin’s Press; 2000.
- 23.National Center for Health Statistics. National Health and Nutrition Examination Survey: Final Report of Findings. Available at: http://www.cdc.gov/nchs/data/nhanes/report_findings_july_05.pdf. Accessed September 22, 2006.
- 24.National Center for Health Statistics. National Health and Nutrition Examination Survey: Health Professionals. Available at: http://www.cdc.gov/nchs/about/major/nhanes/hlthprofess.htm. Accessed September 22, 2006.
- 25.Minkler M, Wallerstein N, eds. Community-based Participatory Research for Health. San Francisco, Calif: Jossey-Bass; 2003.
- 26.Israel BA, Parker EA, Rowe Z, et al. Community-based participatory research: lessons learned from the Centers for Children’s Environmental Health and Disease Prevention Research. Environ Health Perspect. 2005; 113:1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quandt SA, Doran AM, Rao P, Hoppin JA, Snively BM, Arcury TA. Reporting pesticide assessment results to farmworker families: development, implementation, and evaluation of a risk communication strategy. Environ Health Perspect. 2004;112:636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morello-Frosch R, Brody J, Frye M, et al. The right to know, the right to act, and the right not-to-know: ethical and scientific dilemmas of reporting data in biomonitoring and environmental exposure studies. Presented at: the American Sociological Association Annual Meeting; August 11, 2006; Montreal, Quebec.
- 29.Costner P, Thorpe B, McPherson A. Sick of dust: chemicals in common products–a needless health risk in our homes. Available at: http://www.safer-products.org/downloads/Dust%20Report.pdf. Accessed May 19, 2006.
- 30.Needham LL, Sexton K. Assessing children’s exposure to hazardous environmental chemicals: an overview of selected research challenges and complexities. J Expo Anal Environ Epidemiol. 2000;10:611–629. [DOI] [PubMed] [Google Scholar]
- 31.Sandman PM. Emerging communication responsibilities of epidemiologists. J Clin Epidemiol. 1991;44(supp 1): 41S–50S. [DOI] [PubMed] [Google Scholar]
- 32.US Environmental Protection Agency, Integrated Risk Information System (IRIS). Dibutyl phthalate (CASRN 84–74–2). Available at: http://www.epa.gov/iris/subst/0038.htm. Accessed May 19, 2006.
- 33.Silent Spring Institute. Sample of Household Exposure Study report-back materials. Available at: http://www.silentspring.org/newweb/research/report-back_sample.pdf. Accessed May 19, 2006.
- 34.Silent Spring Institute. Sources of chemicals in the Silent Spring Institute Household Exposure Study. Available at: http://www.silentspring.org/newweb/research/Source%20Table_sept05.pdf. Accessed May 19, 2006.
- 35.Morello-Frosch R, Pastor M Jr, Sadd JL, Porras C, Prichard M. Citizens, science, and data judo: leveraging secondary data analysis to build a community–academic collaborative for environmental justice in Southern California. In: Israel BA, Eng E, Schulz AJ, Parker EA, eds. Methods in Community-Based Participatory Research for Health. San Francisco: Jossey-Bass; 2005;371–392.
- 36.American Public Health Association. APHA Public Health Code of Ethics. Available at: http://www.apha.org/codeofethics/ethics.htm. Accessed April 11, 2006.


