Abstract
A defect in the structure of the obese gene is responsible for development of obesity in the ob/ob mouse. The product of expression of the gene is the protein hormone leptin. Leptin causes weight loss in ob/ob and normal mice, it is secreted by adipocytes, and it is an important controller of the size of fat stores by inhibiting appetite. The ob/ob mouse is infertile and has a pattern of gonadotropin secretion similar to that of prepubertal animals. Consequently, we hypothesized that leptin might play a role in the control of gonadotropin secretion and initiated studies on its possible acute effects on hypothalamic–pituitary function. After a preincubation period, hemi-anterior pituitaries of adult male rats were incubated with leptin for 3 hr. Leptin produced a dose-related increase in follicle-stimulating hormone (FSH) and luteinizing hormone (LH) release, which reached peaks with 10−9 and 10−11 M leptin, respectively. Gonadotropin release decreased at higher concentrations of leptin to values indistinguishable from that of control pituitaries. On the other hand, prolactin secretion was greatly increased in a dose-related manner but only with leptin concentrations (10−7–10−5 M). Incubation with leptin of median eminence–arcuate nuclear explants from the same animals produced significant increases in LH-releasing hormone (LHRH) release only at the lowest concentrations tested (10−12–10−10 M). As the leptin concentration was increased, LHRH release decreased and was significantly less than control release at the highest concentration tested (10−6 M). To determine if leptin can also release gonadotropins in vivo, ovariectomized females bearing implanted third ventricle cannulae were injected with 10 μg of estradiol benzoate s.c., followed 72 hr later by microinjection into the third ventricle of leptin (0.6 nmol in 5 μl) or an equal volume of diluent. There was a highly significant increase in plasma LH, which peaked 10–50 min after injection of leptin. Leptin had no effect on plasma FSH concentrations, and the diluent had no effect on either plasma FSH or LH. Thus, leptin at very low concentrations stimulated LHRH release from hypothalamic explants and FSH and LH release from anterior pituitaries of adult male rats in vitro and released LH, but not FSH, in vivo. The results indicate that leptin plays an important role in controlling gonadotropin secretion by stimulatory hypothalamic and pituitary actions.
Keywords: obese mouse, follicle-stimulating hormone, luteinizing hormone, luteinizing hormone-releasing hormone
In late 1994, Zhang et al. (1) published an extremely important paper in which the structure of the mouse obese (ob) gene and its human homologue by positional cloning was described. The protein named leptin is the product of the ob gene. The obesity in the ob/ob mouse is due to a mutation in the gene, resulting in failure of secretion of leptin from adipocytes. Leptin causes weight loss in mice by reducing food intake and increasing energy expenditure (2–5). Furthermore, ob gene expression is increased in human obesity (6–9) and in various animal models of obesity (10). The accumulated evidence suggests that leptin is important in controlling body weight.
Not only is the ob/ob mouse infertile, but it has atrophic reproductive organs (11). Gonadotropin secretion is impaired and very sensitive to the negative feedback of gonadal steroids, much as in prepubertal animals (12). It has recently been shown that chronic treatment with leptin can induce recovery in the reproductive system in the ob/ob mouse by promoting growth and function of the reproductive organs and fertility (13, 14) by increasing secretion of gonadotropins (15).
The critical weight hypothesis of the development of puberty states that when body weight reaches a certain level, puberty occurs (16). This hypothesis in its original form does not hold since, if rats are underfed, puberty is delayed, but with access to food, rapid weight gain leads to onset of puberty at weights well below the critical weight under normal nutritional conditions (17). Therefore, puberty is probably induced when fat stores reach a certain level (16, 17). We hypothesized that when fat stores reach the critical point, there is increased release of leptin from adipocytes into the bloodstream. Leptin then acts on hypothalamic cells to stimulate release of luteinizing hormone (LH)-releasing hormone (LHRH), thereby triggering gonadotropin release. The subsequent release of follicle-stimulating hormone (FSH) and LH stimulates gonadal steroid secretion, leading to development of the reproductive tract and induction of puberty.
Therefore, we initiated studies of the possible effects of leptin on hypothalamic–pituitary function. We hypothesized that leptin would also play a role in control of gonadotropin secretion in adult animals, and we therefore studied its effects on the release of FSH and LH from hemi-anterior pituitaries (APs) and also its possible action in releasing LHRH from medial basal hypothalamic explants in vitro. To determine if leptin is active in vivo, we used a model which we have often employed to evaluate stimulatory effects of peptides on LH release, namely the ovariectomized, estrogen-primed rat (18). We microinjected the protein into the third ventricle (3V) of conscious animals bearing implanted 3V cannulae and also catheters in the external jugular vein extending to the right atrium, so that we could remove blood samples before and after the injection of leptin and measure its effect on the concentrations of plasma FSH and LH.
MATERIALS AND METHODS
Adult male and female rats of the Sprague–Dawley strain (Holtzmann, Madison, WI; 200–250 g) were housed two per cage under controlled conditions of temperature (23–25°C) and lighting (on from 0500 to 1700 hr). The animals had free access to a pellet diet and tap water.
In Vitro Studies.
After acclimatization for 5 or more days in the vivarium, male rats were killed by decapitation.
Incubation of APs.
After removal of the posterior lobe, the anterior pituitary was bisected longitudinally, and each AP was incubated in a tube containing 0.5 ml of Krebs–Ringer bicarbonate (1 mg/ml ascorbic acid; pH 7.4) buffer (KRB) in an atmosphere of 95% O2/5% CO2 in a Dubnoff shaker (50 cycles per min) for a period of 60 min. Following this preincubation, APs were incubated for 3 hr in fresh KRB buffer alone or KRB containing graded concentrations of leptin [recombinant human leptin, a gift to David York (Pennington Biomedical Research Center) from CIBA Pharmaceutical]. The medium was then aspirated and stored frozen at −20°C until measurements of FSH, LH, and prolactin were made by radioimmunoassay (RIA) using kits supplied by the National Institute of Digestive Diabetes and Kidney Disease (NIDDK).
Incubation of Median Eminence (ME)–Arcuate Nuclear (Arc) Explants.
The explants were dissected by a single cut so as to give an explant containing the ME, pituitary stalk, overlying arcuate nucleus (AN), and adjacent tissue. The cut extended from just caudal to the optic chiasm back to just caudal to the point of separation of the pituitary stalk. This produced a piece of tissue ≈2 mm rostrocaudally, 0.25 mM deep, and extending 0.25 mm bilaterally from the midline. Each ME–Arc explant was preincubated in a tube containing 0.5 ml of KRB for 30 min before replacement with fresh medium or medium containing leptin or diluent (KRB). After incubation for 30 min, the medium was aspirated, saved, and replaced with an equal volume of fresh medium containing the same concentration of leptin. The incubation was continued for an additional 30 min. At that time, the medium was aspirated, boiled for 10 min in a water bath, and stored frozen, before determination of LHRH by RIA using a highly specific antibody to LHRH kindly donated by A. Barnea (University of Texas Southwestern Medical Center, Dallas). The minimal detectable LHRH was 0.2 pg per tube, and the curve was linear to 100 pg. All in vitro experiments were repeated at least three times. The results presented are from representative experiments.
In Vivo Studies.
Adult female rats from Holtzmann (200–250 g) were ovariectomized under halothane anesthesia 5 or more days after receipt and were used 3–4 weeks later.
An indwelling cannula was implanted in the 3V 6–8 days before experiments, using the technique of Antunes-Rodrigues and McCann (19) and tribromoethanol anesthesia. Three days before the experiment, the rats were injected s.c. with 10 μg of estradiol benzoate in oil (0.1 ml). One day before the experiment, while the rat was anesthetized with halothane, an indwelling catheter was introduced into the right external jugular and advanced to the right atrium for collection of blood samples, according to the technique of Harms and Ojeda (20). Immediately after collection of the first blood sample (0.3 ml), 10 μg of leptin dissolved in 5 μl of KRB or KRB alone was microinjected into the 3V for 60 sec. Additional (0.3 ml) blood samples were collected every 10 min for 120 min. An equal volume of saline was injected immediately after removal of each blood sample to restore blood volume.
After centrifugation, the plasma was stored at −20°C before RIA for FSH, LH, and prolactin, and expressed in terms of the NIDDK RP-3, RP-2, and RP-3 reference preparations, respectively.
Statistics.
Results were analyzed by one-way analysis for variance with repeated measures and significance of differences between means was determined with the Student–Newman–Keuls test. The differences between the means of two groups were calculated by Student’s t test.
RESULTS
Effect of Leptin on LH Release.
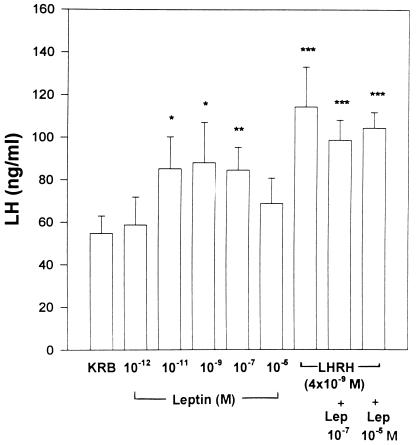
Leptin induced a bell-shaped dose–response curve of LH release from incubated APs (Fig. 1). The minimal effective dose was 10−11 M, and release remained on a plateau as the concentration of leptin was increased to 10−7 M. Release declined with the highest concentration tested (10−5 M) and was no longer significantly increased. The release of LH by leptin was not significantly less than that caused by LHRH of a similar concentration (4 × 10−9 M). Under these conditions, there was no additional release of LH when leptin (10−7 or 10−5M) was incubated together with LHRH (4 × 10−9 M). In certain other experiments, there was an additive effect when leptin was incubated with LHRH; however, this effect was not uniformly seen. The results indicate that leptin was only slightly less effective in releasing LH than LHRH itself.
Figure 1.
The effect of leptin on LH release from APs incubated in vitro. There were eight individual APs incubated in each group. In this and subsequent figures, the height of each column represents the mean response with each treatment. The vertical line above the column = 1 SEM. Asterisks indicate significance versus KRB control: ∗, P < 0.05; ∗∗, P < 0.01; and ∗∗∗, P < 0.001.
The Effect of Leptin on FSH Release.
FSH release from these same glands showed a similar pattern as that of LH, except that the sensitivity in terms of FSH release was much less than that for LH (Fig. 2). The minimal effective dose for FSH release was 10−9 M, whereas it was 10−11 M for LH. The responses were roughly of the same magnitude at the effective concentration as obtained with LH, and the response was equivalent to that observed with 4 × 10−9 M LHRH. Combination of LHRH with an ineffective concentration of leptin, which released FSH at a level just below significance, gave a clear additive effect on FSH release (Fig. 2).
Figure 2.
The effect of leptin on FSH release from APs incubated in vitro.
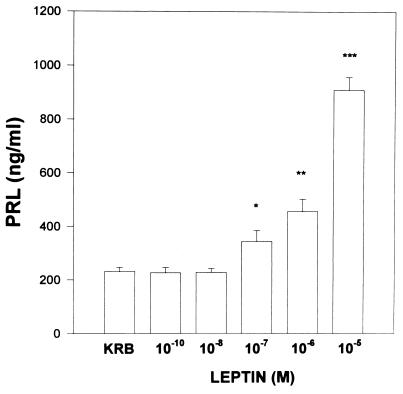
Effect of Leptin on Prolactin Release.
The results with prolactin were in contrast to those with FSH and LH in that the maximal response (a 4.5-fold increase) was seen with the highest concentration tested (10−5 M) and prolactin release increased in a dose-related manner with concentrations >10−7 M leptin (Fig. 3).
Figure 3.
The effect of leptin on prolactin release from APs incubated in vitro.
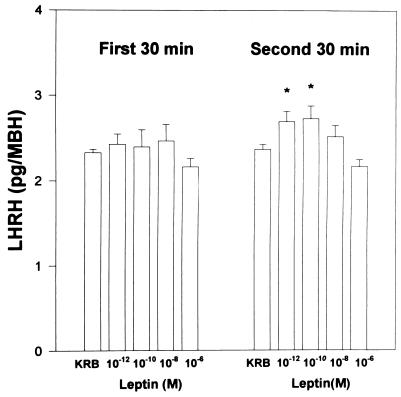
Effect of Leptin on LHRH Release from ME–Arc Explants.
There was no significant effect of leptin (10−12–106 M) on LHRH release from ME–Arc explants during the first 30 min of incubation; however, during the second 30 min, the lowest concentrations (10−12 and 10−10M) produced a plateaued significant increase in LHRH release. The overall significance, combining the results with the two stimulatory concentrations, was P < 0.01 versus LHRH release from explants incubated with buffer alone. The release of LHRH decreased at higher concentrations and was no longer significantly different from that of control explants incubated in KRB. There was a significant decrease in LHRH release with the highest concentration tested (10−6 M; Fig. 4).
Figure 4.
The effect of leptin on the release of LHRH from ME–Arc explants. ∗, P < 0.05 (versus KRB control). Since the results were similar for both 10−12 and 10−10 M leptin, the values were pooled and were highly significantly different from the LHRH release in the KRB-incubated control explants (P < 0.01).
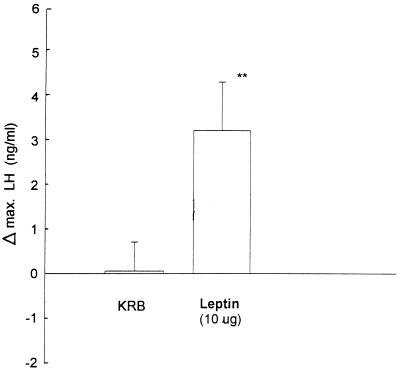
The Effect of Intraventricularly Injected Leptin on Plasma Gonadotropin Concentrations in Ovariectomized, Estrogen-Primed Rats.
Injection into the 3V of the diluent for leptin (KRB, 5 μl) had no effect on pulsatile LH release, but the injection of leptin (10 μg, 0.6 nmol) uniformly increased plasma LH concentrations with a variable time lag ranging from 10 to 50 min, so that the maximal increase in LH from the starting value was highly significant (P < 0.01) and constituted a mean increase of 60% (Fig. 5). In contrast, neither the diluent nor leptin significantly altered plasma FSH concentrations. Therefore, in contrast to the leptin-induced release in LH release, FSH release was not altered. During the second hour of sampling, there were no significant changes in plasma gonadotropin concentrations between the diluent and leptin-injected animals.
Figure 5.
The maximum increase in plasma LH during the first hour after injection of leptin [10 μg (0.6 nmol) in 5 μl of KRB] or an equal volume of the diluent (KRB) into the 3V of ovariectomized rats injected with 10 μg of estradiol benzoate s.c. 72 hr before the experiment.
DISCUSSION
Our results show that leptin has a very potent effect on the anterior pituitary in vitro. It stimulates FSH and LH release within 3 hr of incubation. The effect was sometimes additive with LHRH, and there was no significant difference between the FSH and LH release from equivalent concentrations of LHRH and leptin. On the other hand, there was a much more pronounced stimulation of prolactin release at high concentrations, exceeding all induced increases we have observed previously. This was even more remarkable, considering that prolactin release is already augmented from anterior pituitaries in vitro because of removal of inhibitory hypothalamic control (21).
We were surprised to see such remarkable increases in gonadotropin release from the incubated pituitaries, which could be of physiologic significance. Leptin receptors in the anterior pituitary have not been reported; however, on the basis of our results, they must be present on secretory cells of the gland, like the gonadotropes.
Presumably, when leptin is secreted by the adipocytes, it circulates to the hypothalamus and diffuses from the hypophyseal portal capillaries into the ME, a circumventricular organ, lacking the blood–brain barrier. The remaining leptin in the portal capillaries is transported by the long portal veins to the anterior pituitary. The arterial blood supply to the neural lobe delivers leptin to this lobe of the pituitary directly. From there, it is transported to the anterior lobe by the short portal vessels, which account for the remaining 30% of the blood flow to the anterior lobe. In the anterior lobe, leptin combines with ob/obb receptors (22) to augment both FSH and LH release and may also augment the response to LHRH.
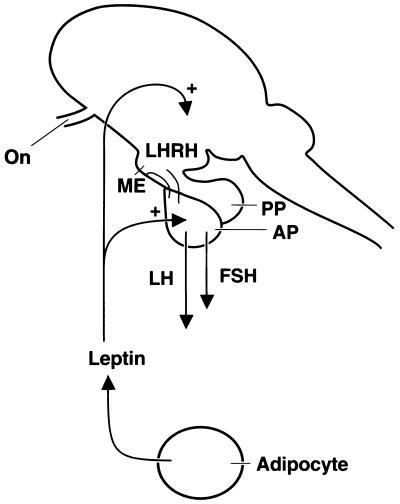
There was a significant release of LHRH from ME–Arc explants at the lowest concentrations of leptin (10−12 and 10−10 M) tested that occurred after a delay of 30–60 min. Other peptides or cytokines that we have evaluated have become active within 30 min (23). The reason for the delay with leptin is not apparent. Therefore, leptin stimulates gonadotropin release by both hypothalamic and pituitary actions (Fig. 6).
Figure 6.
Diagram of the probable action of leptin to stimulate LHRH and gonadotropin release. On, optic nerve; PP, posterior pituitary.
Leptin released LH, but not FSH, with a variable time delay (10–50 min), after 3V injection in ovariectomized, estrogen-primed rats. Presumably this effect was caused by LHRH release; however, a direct effect on the gonadotropes to release LH is also likely, in view of its potency to release LH from pituitaries incubated in vitro. The difference in time delay for the release of LH in individual rats may be caused by differences in the distance from the cannula tip in the 3V to the AN, the presumed site of action of leptin.
Leptin appears to reach the brain via a transport mechanism (24) mediated by the ob/oba receptors (22) in the choroid plexus (22). These receptors have an extensive extracellular domain, but a greatly truncated intracellular domain (22), and mediate transport of the hormone by a saturable mechanism (24). Following uptake into the cerebrospinal fluid through the choroid plexus, leptin is carried by the flow of cerebrospinal fluid to the 3V, where it either diffuses into the hypothalamus through the ependymal layer lining the ventricle and combines with ob/obb receptors (25) on paraventricular nuclei (PVN) and AN neurons or on receptors on terminals of the responsive of neurons that extend to the ventricular wall.
The ob/obb receptor has a large intracellular domain that presumably mediates the action of the protein (25). These receptors are widespread throughout the brain (25, 26), but particularly localized in the region of the PVN and AN. Leptin activates Stat3 within 30 min after intraventricular injection (25). Stat3 is a protein important in conveying information to the nucleus to initiate DNA-directed mRNA synthesis. Following injection of bacterial lipopolysaccharide, Stat3 is also activated, but in this case, the time delay is 90 min, presumably because lipopolysaccharide induces interleukin 1β mRNA in the same areas, namely the PVN and AN (27). Interleukin 1β mRNA would then cause production of interleukin 1β, which would activate Stat3. On entrance into the nucleus, Stat3 would activate or inhibit DNA-directed mRNA synthesis. In the case of leptin, it induces corticotropin-releasing hormone mRNA in the PVN, whereas in the AN, it decreases neuropeptide Y (NPY) mRNA, resulting in increased corticotropin-releasing hormone synthesis, and presumably release in the PVN, and decreased NPY synthesis and release in the AN, respectively (26). Indeed, leptin receptors have been localized over NPY neurons in the AN (28). Presumably, combination of leptin with these transducing receptors also either increases or decreases the firing rate of that particular neuron, leading to increased or decreased release, respectively, of its neuropeptide. In the case of the AN–ME area, leptin may also enter the ME by diffusion between the tanycytes to reach its receptors on the AN (24), or, alternatively, it may combine with its receptors on terminals of neurons projecting to the tanycytes. Activation or inhibition of these neurons would induce LHRH release.
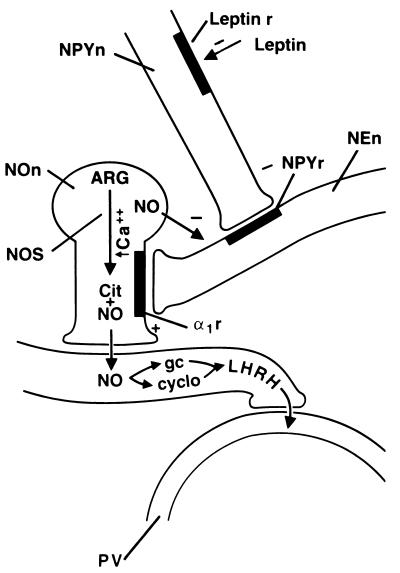
The complete pathway of leptin action in the MBH to stimulate LHRH release is not yet elucidated, but a hypothetical pathway is as follows (Figs. 6 and 7). AN neurons bearing ob/obb receptors may project from the ME to the tanycyte–portal capillary junction. Leptin would either combine with its receptors on the terminals that transmit information to the cell bodies in the AN or diffuse to the AN to combine with its receptors on the perikarya of AN neurons. Since leptin decreases NPY mRNA and presumably NPY biosynthesis in NPY neurons in the AN, we postulate that leptin causes a decrease in NPY release. Since, NPY inhibited LH release in intact and castrated male and ovariectomized female rats (29, 30), we hypothesize that NPY decreases the release of LHRH by inhibiting the noradrenergic neurons that have been shown to mediate pulsatile release of LHRH (31, 32). Therefore, when the release of NPY is inhibited by leptin, noradrenergic impulses are generated that act on α1 receptors on the NO-ergic neurons, causing the release of NO, which diffuses to the LHRH terminals and activates LHRH release by activating guanylate cyclase and cyclooxygenase1, as shown in our prior experiments (31, 32). The LHRH enters the portal vessels and is carried to the anterior pituitary gland, where it acts to stimulate FSH and particularly LH release by combining with its receptors on the gonadotropes. The release of LH and to a lesser extent FSH is further increased by the direct action of leptin on the pituitary gland.
Figure 7.
Hypothetical mechanism of action of leptin to cause stimulation of LHRH release. For explanation, please see text. NPYn, NPY neuron; Leptin r, leptin receptor; NPYr, NPY receptor; NEn, noradrenergic neuron terminal; NOn, nitricoxidergic neuron; ARG, arginine; NOS, NO synthase; Cit, citrulline; α1r, α1 adrenergic receptor; gc, guanylate cyclase; cyclo, cyclooxygenase; PV, portal vessel; +, stimulation; −, inhibition.
We hypothesize that leptin may be a critical factor in induction of puberty as the animal nears the so-called critical weight. Either metabolic signals reaching the adipocytes, or signals related to their content of fat cause the release of leptin, which increases LHRH and gonadotropin release, thereby initiating puberty and finally ovulation and onset of estrous cycles. In the male, the system would work similarly; however, there is no preovulatory LH surge brought about by the positive feedback of estradiol. Sensitivity to leptin is undoubtedly under gonadal steroid control (A.W., W.H.U., and S.M.M., unpublished work).
During fasting, the leptin signal is removed and LH pulsatility and reproductive function decline quite rapidly. Indeed, it has just been reported that fasting in mice causes cessation of estrous cycles, which can be restored by refeeding or administration of leptin for 2 days, which is accompanied by an increase in plasma LH (33). In women with anorexia nervosa, we hypothesize that decreased food intake leads to decreased leptin secretion, which results in reduced gonadotropin release, causing a reversion to the prepubertal state. The prepubertal state can be reversed by increased caloric intake and resultant leptin secretion from the adipocytes.
The consequences to gonadotropin secretion of overproduction of leptin, as has already been demonstrated in human obesity (6–9), are not clear. There are often reproductive abnormalities in this circumstance and whether they are due to excess leptin production or other factors remains to be determined. It is noteworthy that the highest concentration of leptin tested in vitro decreased, instead of increasing, LHRH. Thus, leptin may have a powerful influence on reproduction throughout the lifespan of the individual by actions on the hypothalamus and pituitary.
Acknowledgments
We thank Judy Scott and Jason Holland for their excellent secretarial assistance. This work was supported by National Institutes of Health Grants DK43900 and MH51853.
Footnotes
Abbreviations: LH, luteinizing hormone; LHRH, LH-releasing hormone; FSH, follicle-stimulating hormone; 3V, third ventricle; AP, hemi-anterior pituitary; KRB, Krebs–Ringer bicarbonate buffer; ME, median eminence; Arc, arcuate nuclear; AN, arcuate nucleus; PVN, paraventricular nuclei; NPY, neuropeptide Y.
References
- 1.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 2.Pelleymounter M A, Cullen M J, Baker M B, Hecht R, Winters D, Boone T, Collins F. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 3.Halaas J L, Kajiwala K S, Maffei M, Cohen S L, Chait B T, Rabinowitz D, Lallone R L, Burley S K, Friedman J M. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 4.Campfield L A, Smith F J, Guisez Y, Devos R, Burn P. Science. 1995;269:546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 5.Stephens T W, Basinski M, Bristow P K, Bue-Valleskey J M, Burgett S G, et al. Nature (London) 1995;377:530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- 6.Considine R V, Considine E L, Williams C J, Nyce M R, Magosin S A, Bauer T L, Rosato E L, Caro J F. J Clin Invest. 1995;95:2986–2988. doi: 10.1172/JCI118007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mazsuzaki H, Ogawa Y, Isse N, Satoh N, Okazaki T, et al. Diabetes. 1995;44:855–858. doi: 10.2337/diab.44.7.855. [DOI] [PubMed] [Google Scholar]
- 8.Lonngist F, Arner P, Nordfors L, Schalling M. Nat Med. 1995;1:950–953. doi: 10.1038/nm0995-950. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton B S, Paglia D, Kwan A Y M, Deitel M. Nat Med. 1995;1:953–956. doi: 10.1038/nm0995-953. [DOI] [PubMed] [Google Scholar]
- 10.Murakami T, Shima K. Biochem Biophys Res Commun. 1995;209:944–952. doi: 10.1006/bbrc.1995.1589. [DOI] [PubMed] [Google Scholar]
- 11.Swerdloff R, Batt R, Bray G. Endocrinology. 1976;98:1359–1364. doi: 10.1210/endo-98-6-1359. [DOI] [PubMed] [Google Scholar]
- 12.Swerdloff R, Peterson M, Vera A, Batt R, Heber D, Bray G. Endocrinology. 1978;103:542–547. doi: 10.1210/endo-103-2-542. [DOI] [PubMed] [Google Scholar]
- 13.Chehad F F, Lim M E, Ronghua L. Nat Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 14.Cioffi J, Shafer A, Zupancic T, Smith-Gbur J, Mikhail A, Platika D, Snodgrass H. Nat Med. 1996;2:585–588. doi: 10.1038/nm0596-585. [DOI] [PubMed] [Google Scholar]
- 15.Barash I A, Cheung C C, Weigle D S, Ren H, Kabigting E B, Kuijper J L, Clifton D K, Steiner R A. Endocrinology. 1996;137:3144–3147. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- 16.Frisch R E, McArthur Science. 1974;185:949–951. doi: 10.1126/science.185.4155.949. [DOI] [PubMed] [Google Scholar]
- 17.Ronnekleiv O K, Ojeda S R, McCann S M. Biol Reprod. 1978;19:414–424. doi: 10.1095/biolreprod19.2.414. [DOI] [PubMed] [Google Scholar]
- 18.Mani S K, Allen J M C, Rettori V, O’Malley B W, Clark J H, McCann S M. Proc Natl Acad Sci USA. 1994;91:6468–6472. doi: 10.1073/pnas.91.14.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antunes-Rodrigues J, McCann S M. Proc Soc Exp Biol Med. 1970;133:1464–1470. doi: 10.3181/00379727-133-34713. [DOI] [PubMed] [Google Scholar]
- 20.Harms P C, Ojeda S R. J Appl Physiol. 1974;36:391–392. doi: 10.1152/jappl.1974.36.3.391. [DOI] [PubMed] [Google Scholar]
- 21.Duvilanski B H, Zambruno C, Seilicovich A, Pisera D, Lasaga M, Diaz M C W, Belova N, Rettori V, McCann S M. Proc Natl Acad Sci USA. 1995;92:170–174. doi: 10.1073/pnas.92.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cioffi J, Shafer A, Zupancic T, Smith-Gbur J, Mikhail A, Platika D, Snodgrass H. Nat Med. 1996;2:585–588. doi: 10.1038/nm0596-585. [DOI] [PubMed] [Google Scholar]
- 23.Karanth S, Lyson K, McCann S M. Proc Natl Acad Sci USA. 1993;90:3383–3387. doi: 10.1073/pnas.90.8.3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banks W A, Kastin A J, Huang W, Jaspan J B, Maness L M. Peptides. 1996;17:305–311. doi: 10.1016/0196-9781(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz M W, Seeley R J, Campfield L A, Burn P, Baskin D G. J Clin Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaisse C, Halaas J L, Horvath C M, Darnell J E, Jr, Stoffel M, Friedman J M. Nat Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 27.Wong M-L, Bongiorno P B, Rettori V, McCann S M, Licinio J. Proc Natl Acad Sci USA. 1997;94:227–232. doi: 10.1073/pnas.94.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mercer J G, Hoggard N, Williams L M, Lawrence C B, Hannah L T, Morgan P J, Trayhunt P. J Neuroendocrinol. 1996;8:733–735. doi: 10.1046/j.1365-2826.1996.05161.x. [DOI] [PubMed] [Google Scholar]
- 29.Reznikov A G, McCann S M. Neuroendocrinology. 1993;57:1148–1154. doi: 10.1159/000126481. [DOI] [PubMed] [Google Scholar]
- 30.McDonald J, Lumpkin M D, Samson W K, McCann S M. Proc Natl Acad Sci USA. 1985;82:561–564. doi: 10.1073/pnas.82.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rettori V, Belova N, Dees W L, Nyberg C L, Gimeno M, McCann S M. Proc Natl Acad Sci USA. 1993;90:10130–10134. doi: 10.1073/pnas.90.21.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Canteros G, Rettori V, Franchi A, Genaro A, Cebral E, Saletti A, Gimeno M, McCann S M. Proc Natl Acad Sci USA. 1995;92:3416–3420. doi: 10.1073/pnas.92.8.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahima R S, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier J S. Nature (London) 1996;328:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]