Abstract
Background
Chlamydia trachomatis, an obligate intracellular pathogen, is a leading worldwide cause of ocular and urogenital diseases. Advances have been made in our understanding of the nine-member polymorphic membrane protein (Pmp) gene (pmp) family of C. trachomatis. However, there is only limited information on their biologic role, especially for biological variants (biovar) and clinical strains.
Methodology/Principal Findings
We evaluated expression for pmps throughout development for reference strains E/Bour and L2/434, representing different biovars, and for clinical E and L2 strains. Immunoreactivity of patient sera to recombinant (r)Pmps was also determined. All pmps were expressed at two hours. pmpA had the lowest expression but was up-regulated at 12 h for all strains, indicating involvement in reticulate body development. For pmpD, expression peaked at 36 h. Additionally, 57.7% of sera from infected and 0% from uninfected adolescents were reactive to rPmpD (p = 0.001), suggesting a role in immunogenicity. pmpF had the highest expression levels for all clinical strains and L2/434 with differential expression of the pmpFE operon for the same strains. Sera were nonreactive to rPmpF despite immunoreactivity to rMOMP and rPmpD, suggesting that PmpF is not associated with humoral immune responses. pmpFE sequences for clinical strains were identical to those of the respective reference strains. We identified the putative pmpFE promoter, which was, surprisingly, 100% conserved for all strains. Analyses of ribosomal binding sites, RNase E, and hairpin structures suggested complex regulatory mechanism(s) for this >6 Kb operon.
Conclusions/Significance
The dissimilar expression of the same pmp for different C. trachomatis strains may explain different strain-specific needs and phenotypic distinctions. This is further supported by the differential immunoreactivity to rPmpD and rPmpF of sera from patients infected with different strains. Furthermore, clinical E strains did not correlate with the E reference strain at the gene expression level, reinforcing the need for expansive studies of clinical strains.
Introduction
Chlamydia trachomatis is an obligate intracellular pathogen that is responsible for significant worldwide morbidity associated with ocular and sexually transmitted diseases (STD). The developmental cycle of the organism is biphasic beginning with the adhesion of the elementary body (EB), an infectious and metabolically inert form, to the host cell. After endocytosis, the EB differentiates ∼2 to 12 h post infection (p.i.) into a larger, non-infectious and metabolically active reticulate body (RB), which initiates intracellular replication by binary fission within a vacuole called an inclusion body. At ∼30 to 36 h p.i., RBs reorganize into new EBs, which are released by host cell lysis or exocytosis at 48 to 72 h p.i. that initiates another infectious cycle [1], [2].
The disease spectrum of C. trachomatis ranges from conjunctivitis and ocular trachoma to tubal factor infertility, ectopic pregnancy and infant pneumonitis [3], [4]. C. trachomatis serological variants (serovars) are grouped within two human biological variants (biovars) according to characteristics of disease presentation: the trachoma biovar, including serovars A to C and Ba, which cause conjunctivitis and trachoma, and serovars D to K and Ba, Da, Ia and Ja, which cause conjunctivitis, urogenital infections and infant pneumonitis, and the lymphogranuloma venereum (LGV) biovar (serovars L1 to L3 and L2a). The latter serovars are more invasive, causing genital ulceration, lymphadenitis and proctitis [3], [5]. However, serotyping of the major outer membrane protein (MOMP), and phylogenetic reconstructions of this protein and the corresponding gene (ompA) [6], [7] do not group serovars by trachoma, non-invasive urogenital or invasive LGV disease groups.
The molecular mechanisms behind these biological differences among serovars (or strains) are not well understood. Genome sequences of reference strains D/UW-3 [8] and A/Har-13 [9], as well as ongoing C. trachomatis genomic sequencing are providing information on specific genes and proteins that may explain tissue tropism and virulence differences for the three disease groups. C. trachomatis contains a partial tryptophan operon (trpRBA) where urogenital strains, but not trachoma strains, can synthesize tryptophan from mucosal substrates [10]. The toxin gene possesses an intact N-terminal region that encodes an active enzymatic domain for the urogenital strains but not for trachoma or LGV strains [11].
Research on the nine member polymorphic membrane protein (Pmp) gene (pmp) family has revealed phylogenetic reconstructions where six pmps (pmpB, pmpC, pmpF, pmpG, pmpH and pmpI) form clades that correspond to the three disease groups [12]–[14]. At the gene expression level, previous RT-PCR analyses of reference strains D/UW-3 and L2/434 [15], and microarray analysis of D/UW-3 [16] found that all nine pmps were transcribed starting at 8 h p.i. Yet, based on real-time quantitative (k)RT-PCR, we found expression as early as 2 h p.i. for pmpC for reference strains Ba/Apache-2, G/UW-57 and L2/434, and a differential expression profile with earlier up-regulation of pmpC for L2/434 [17]. Also, another study based on kRT-PCR, Kiselev et al. [18] detected pmpD expression as early as 2h p.i. for L2/434. Proteomics analyses have also shown that all Pmps of L2/434 are detected as outer membrane constituents [19]–[22]. There is also evidence that some Pmps are antigenic for human sera [15], [23]. We observed a heterogeneous immunoreactivity to recombinant (r)PmpC using sera from patients infected with different C. trachomatis strains, suggesting a role for PmpC in antigenic variation [17]. More recently, Pmps have been considered autotransporters based on bioinformatics analyses [24], [25]. Wehrl et al. [26] has experimentally demonstrated the autotransporter model for the C. pneumoniae ortholog of C. trachomatis PmpD, Pmp21. Further, using immunofluorescence microscopy, Western blotting and penicillin treatment, the results of Kiselev et al. [18] for L2/434 PmpD are in general agreement with the autotransporter model for this protein. PmpD has also been shown to be a species-common neutralizing antigen [27], while PmpF has been implicated as a potential target of the host immune response as it contains several predicted major histocompatibility (MHC) epitopes within the N-terminal domain [9].
Despite the potential importance of Pmps in chlamydial biology, there is a lack of expression data for the pmp genes as well as an insufficient understanding of the host immune response to their proteins. Here, we profile the expression of all pmps throughout development for reference strains E/Bour and L2/434, representing the two C. trachomatis biovars. We chose E/Bour because it is the most prevalent strain worldwide, although the mechanisms of its ecological success are not yet understood. L2/434 was selected as it has been widely studied with a plethora of biological information for comparative analyses. The biological uniqueness of these two strains in vivo is reflected in their differential tissue tropism, virulence and disease presentation. In light of our recent findings that reference strains do not represent the same genetic composition of clinical strains that are circulating among human populations today [28], we also compared the nine pmp expression levels for four C. trachomatis clinical strains, representing ompA genotypes of E and L2. Further, we examined the immunoreactivity of sera from adolescents with and without C. trachomatis urogenital infections against rPmps to further define their potential importance in human disease.
Materials and Methods
C. trachomatis cell culture of reference strains and clinical strains
C. trachomatis reference strains E/Bour and L2/434, three clinical strains belonging to ompA genotype E (designated as E/537C-05, E/S-141 and E/CS-500-96) and one clinical strain belonging to ompA genotype L2 were evaluated in this study. E/537C-05 and E/S-141 were collected from patients with vaginal discharge, E/CS-500-96 from a patient with cervicitis, and L2 from a patient with proctitis. Each was propagated in HeLa 229 cell monolayers using standard techniques as previously described [4], [29]. EBs were harvested at 48–72 h p.i. and purified by discontinuous density centrifugation in Renografin [30].
Confluent HeLa cells were either mock-infected or infected with a multiplicity of infection of one for each reference strain or clinical strain in SPG prior to incubation with culture medium [4], [29]. Eight T25 flasks (one for each time point of 2, 6, 12, 18, 24, 36, and 48 h and mock-infected) per strain were inoculated and placed at 37°C in 5% CO2 [17]. Cultured cells were harvested at each time point, and total RNA was extracted as previously described [17].
Reverse Transcription and Quantitative Real-Time PCR (kPCR)
RNA was quantified by O.D. measured at A260. cDNA was generated from 500 ng of each RNA sample using TaqMan RT Reagents (Applied Biosystems, Foster City, CA) and random hexamers, and was quantified by O.D. measured at A260.
Quantitation of pmp expression was achieved using the ABI 7000 SDS (Applied Biosystems), SYBR Green chemistry, and the standard curve method for relative quantitation, using reagents and thermocycling as previously described [17]. 16SrRNA was used as the endogenous control since normalizing the data against 16SrRNA provides a control for the number of organisms (EBs and RBs) and, therefore, for the differential growth rate of each strain. ompA was included as a quality control for kRT-PCR results since it has been widely used for gene expression studies [16], [17].
Primers for each of the nine pmps (Table 1) were designed using Primer Express (Applied Biosystems). Primers for ompA, 16SrRNA, and pmpC were used as previously designed (Table 1) [17].
Table 1. Oligonucleotide primers used for kRT-PCR.
| Gene | Primers | Primer sequence (5′ to 3′) | Gene Location | Base pair size |
| pmpA | pmpA-3 a | TGCTAGGGAAGATGTTGCAAATAG | 1434–1457 | 51 |
| pmpA-4 a | TGAACGGGTTGGTTAAAAATCG | 1484–1463 | ||
| pmpB | pmpB-5 a | CGACTATCAGCAAAAACACTGCTAA | 2120–2144 | 102 |
| pmpB-6 a | TAGCGGAGTTCTCAGAGATATTCAGTT | 2221–2195 | ||
| pmpC | pmpC-11 a | TTAGTGCTCCCTACAGACTCATCAA | 4150–4174 | 56 |
| pmpC-12 a | CCCGTCAGTACTATTTTCTGAGCTT | 4205–4181 | ||
| pmpD | pmpD-3 a | GCGTGTCGCTCTGGAAAATAAT | 4455–4476 | 51 |
| pmpD-4 a | ACTGTGCTGAAGTAAGAACTCCATTC | 4505–4480 | ||
| pmpE | pmpE-1 a | CATATGCGCTCTTCCGGATAC | 2140–2160 | 51 |
| pmpE-2 a | GTGTGTCTGCCCTGCTATCATC | 2190–2169 | ||
| pmpF | pmpF-5 a | TCCTATGTTTGATCGCATTGCT | 2520–2541 | 69 |
| pmpF-6 a | CTCCGCATGTTATGTGTTCCA | 2588–2566 | ||
| pmpG | pmpG-1 a , b | TGGGTTTCTGGAGTTTCGAATT | 2221–2242 | 51 |
| pmpG-2 a , b | ACCTAAAGCATCGCGGTCAT | 2271–2252 | ||
| pmpG-3 a | TGTGGCCCTGTACAATTCTTAGG | 1165–1187 | 52 | |
| pmpG-4 a | AAATCGCTCCACCATCATTAGC | 1216–1195 | ||
| pmpH | pmpH-15 a | TGCATACGCAGTATTTTAATGACAAA | 2486–2511 | 61 |
| pmpH-16 a | TGCCAATGACATTTCGAATGAT | 2546–2525 | ||
| pmpI | pmpI-1 a | GGAGAAGTGTGCGCATCGAT | 2176–2195 | 51 |
| pmpI-2 a | GAACAGTCCGGAACCATTGG | 2226–2207 | ||
| ompA | OmpA-9 c | TGCCGCTTTGAGTTCTGCTT | 33–52 | 75 |
| OmpA-10 c | GTCGATCATAAGGCTTGGTTCAG | 108–86 | ||
| 16SrRNA | 16SRNA-9 d | GCGAAGGCGCTTTTCTAATTTAT | 734–756 | 76 |
| 16SRNA-10 d | CCAGGGTATCTAATCCTGTTTGCT | 809–786 |
Each plate contained two replicates of each sample cDNA, three different negative controls and standard curves for each gene as previously described (17). For all experiments, the amount of target and control gene was determined from the respective standard curve by conversion of the mean threshold cycle values. Normalization was obtained by dividing the quantity of the target gene by the quantity of the control gene. The specificity of the amplified products was verified by analysis of the dissociation curves generated by the ABI7000 software based on the specific melting temperature for each amplicon. The results were based on three independent experiments for reference strains E/Bour and L2/434, and for the four clinical strains.
Genetic analysis of the pmpFE operon for C. trachomatis reference and clinical strains
Based on the considerable expression disparities between pmpF and pmpE (which belong to the same operon) for reference strain L2/434 and mostly for the clinical strains (see results below), we sequenced the pmpFE operon as well as the upstream 164 base pair (bp) pmpG/pmpF intergenomic region (IGR) that likely contains the operon regulatory region. In the C. trachomatis chromosome, pmpF and pmpE are located on the minus strand; pmpF is located upstream of pmpE, with a 2 bp IGR . pmpE was sequenced for the six strains (Genbank Accession Numbers EF490370 for E/537C-05, EF490371 for E/CS-500-96, EF490372 for E/S-141, EF490373 for L2, EF490374 for E/Bour, and EF490375 for L2/434), while pmpG/pmpF IGR and pmpF were sequenced only for the clinical strains (Genbank Accession Numbers EF490366 for L2, EF490367 for E/CS-500-96, EF490368 for E/S-141, and EF490369 for E/537C-05), as the sequences for the reference strains were available from our previous study (GenBank Accession Number AY887650 for E/Bour and AY887660 for L2/434) [12]. The amplification and sequencing strategies were performed as previously described [12], except for the pmpG/pmpF IGR, where we used primer 5′-ACT CGG ATC TCC TAT AAC AG-3′ for sequencing.
Since the transcription process can be strongly affected by the structure and sequence variability of promoter regions [31]–[34], a putative promoter search for the pmpFE operon was performed using EditSeq software (DNASTAR, Madison, WI) for sequences described in the literature and also by using two promoter prediction programs: http://www.fruitfly.org/seq_tools/promoter.html and http://www.prodoric.de/vfp/vfp_promoter.php. Given the expression dissimilarities obtained in this study for pmpF and pmpE, we searched for putative Shine-Dalgarno ribosome binding sequences (RBS) [35] as well as previously described chlamydial RBS [36]–[39] within this operon, since ribosomes can either prolong or shorten the lifetime of mRNA in response to events that occur during translation or termination processes [40]. The existence of putative consensus cleavage sites for RNase E [41]–[43], the major endonuclease that generally initiates mRNA degradation in most bacteria [44], was also examined within the pmpFE operon. These two analyses were performed using EditSeq software (DNASTAR). Putative stem-loop structures were searched throughout the pmpFE operon using GeneQuest software (DNASTAR) and RNAstructure software version 4.4 (http://rna.urmc.rochester.edu/rnastructure.html) due to the regulatory or processing role of stem-loop structures in premature transcription termination as well as in mRNA degradation and maturation mechanisms [45]–[49], respectively.
Immunoreactivity of patient sera against Pmp fusion proteins
We generated fusion proteins for PmpD and PmpF because the latter displayed such high mRNA expression for L2/434 and the clinical strains, and the former was expressed late in development for all strains under study, being the last up-regulated protein for four of the six strains analyzed. Also, PmpD has been associated with neutralizing epitopes [27]. The rMOMP fusion protein was available from a previous study [50]. The PET30 expression system (EMD Biosciences, San Diego, CA) was used for cloning PCR products containing pmpD or pmpF generated from strain E/Bour genomic DNA as we have described [17]. The forward and reverse primers were 5′-GACGACGACAAGATGAGTTCCGAGAAAGATATA-3′ and 5′-AATGCTGGATTGCGATTGATCTTTTAACCGGGCTTCTCCTC-3′ for pmpD, respectively, and 5′-GACGACGACAAGATGATTAAAAGAACTTCTCTA-3′ and 5′-AATGCAGGAGGAGCTCTGGTCTTTTAACCGGGCTTCTCCTC-3′ for pmpF, respectively. Sequencing confirmed that the insert was in frame with the S-tag and His-tag as we have described previously for rPmpC [17]. The clones were transformed into E. coli BL21 and induced using 0.1µM IPTG during the exponential growth phase. Ni-agarose (Sigma, St. Louis, MO) was used for fusion protein purification according to the package insert. Recombinant proteins were determined to be the correct molecular weight (calculated at ∼160.6 kDa for rPmpD and ∼112.3 kDa for rPmpF) by immunoblot using AP-conjugated S-protein, which binds to the S-tag peptide with a distinct band at the correct molecular weight for each. Optimal protein concentrations were determined and standardized using BCA (Pierce, Rockford, Ill) before analyzing the clinical sera. The optimal protein concentration for rPmpD was 50 ng and 100 ng for rPmpF. Sera from 39 consented female adolescents 14 to 19 years of age attending clinics in Oakland, CA, were used at a 1:50 dilution for immunoblotting as described previously [17]. The Institutional Review Board of Children's Hospital Oakland approved the study, and all patients provided written consent for all clinical samples that were obtained and used in this study. The blots were blocked with Blotto prior to reacting with patient sera and alkaline phosphatase-conjugated anti-human IgG (R&D Systems, Minneapolis, MN). The chemifluorescent substrate ECF (Amersham, Piscataway, NJ) was used to visualize reactive bands. Twenty six (67%) of the 39 adolescents were infected with a single ompA genotype: 3 Ba, 3 D, 8 E, 5 F, 1 G, 1 Ia, 2 J, and 3 K.
There was no evidence for mixed infections. The original cervical samples were used for sequencing (see ref 17) to best determine the presence of a mixed infection, since propagation may result in one strain overgrowing another. On inspection of the electropherograms, none of the samples had ambiguous results. All nucleotides were represented by single, clear peaks with extremely low background and without evidence for double peaks in a nucleotide position where different ompA genotypes differ, which might suggest a mixed infection.
Results
Real-time quantitation for pmp expression
The results of specificity assays revealed no non-specific products, and indicated the presence of the expected amplicon for each gene. Standard curves for all 11 genes had slope values between -3.1 and -3.5, which represents efficiencies between 93 and 100%. There were only minor variations in the slope for each standard curve among independent experiments, indicating a highly reproducible kPCR as we have also shown in previous experiments [17]. We defined the gene expression profile as the qualitative gene expression pattern throughout development where quantitative values are not considered. For example, one expression profile would show increasing expression up to a peak with tapering down of the expression after the peak. An expression peak was defined as the time point of the highest relative mRNA value. All quantitative expression comparisons refer to differences between the expression peak of each gene, even those occurring at different time points p.i.
Expression profile of the nine pmp genes throughout development for E/Bour and L2/434
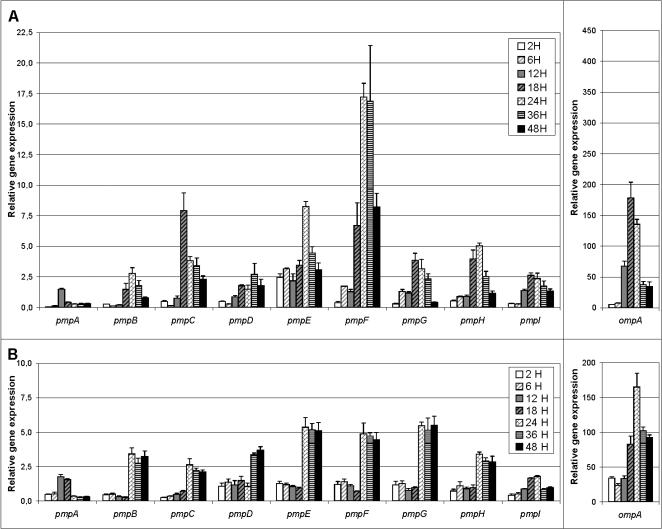
L2/434 had strikingly different mRNA levels among some pmps and also between different time points for the same pmp (Fig. 1A). pmpF had the highest relative mRNA expression, up to 11.5-fold higher than for pmpA, the least expressed gene. mRNA levels were detected at 2 h p.i. for all pmps, including pmpA where the scale limits visualization of the low mRNA expression, and peaked at different time points. For all pmps except pmpA, mRNA levels decreased consistently after the peak until 48 h.
Figure 1. Expression profile of the nine pmp genes and ompA throughout the development of C. trachomatis.
Reference strain L2/434 is represented in panel A and E/Bour in panel B. Values represent the mean±SEM based on three independent experiments for time points of 2, 6, 12, 18, 24, 36, and 48 h post infection. See methods for details.
The pmp expression profiles for E/Bour were more homogeneous than for L2/434, and, in some cases, mRNA levels were lower than for the corresponding L2/434 pmp (Fig. 1B). Similar to L2/434, mRNA levels were detected at 2 h p.i. for all pmp genes. pmpE, pmpF and pmpG showed the highest expression levels for strain E/Bour with up to 3.1-fold higher mRNA levels than for pmpA and pmpI, the least expressed genes. As for L2/434, pmpA and pmpD were the earliest and the latest up-regulated genes, respectively. In contrast to L2/434, all pmp genes except pmpA and pmpI had stable mRNA levels after the expression peak until 48 h. For this reason, the expression peak for E/Bour pmps was defined as the time point at which a noticeable expression increase occurred.
For both reference strains, ompA had remarkably higher mRNA values at all time points than for the pmps (Fig. 1). In contrast to most pmps, ompA revealed a similar gene expression profile for both reference strains.
Expression profile of the nine pmp genes throughout development for C. trachomatis clinical strains
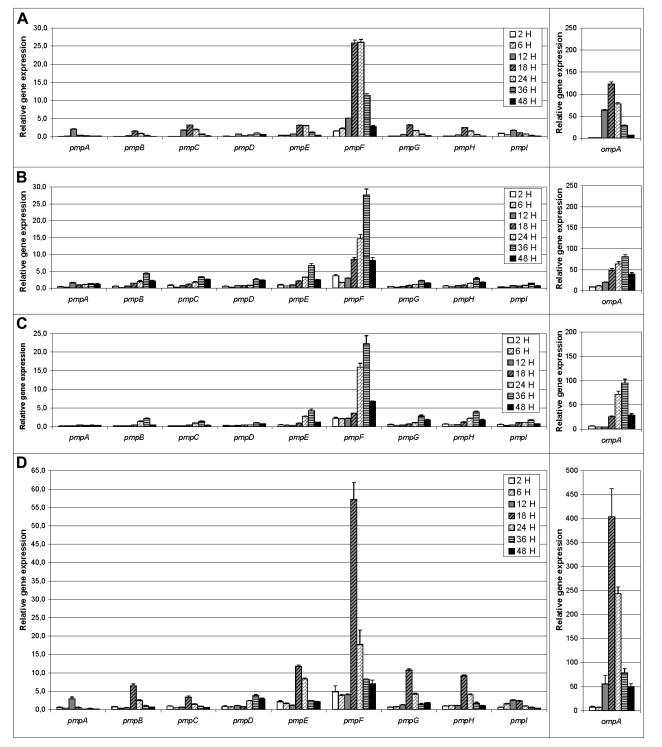
The four clinical strains had a similar pmp expression profile (Fig. 2), which showed decreasing mRNA levels after the expression peak to 48 h. mRNA levels were detected at 2 h p.i. for all pmps, although the scale limits visualization. Overall, pmps peaked at 18h for L2 (Fig. 2A) and E/CS-500-96 (Fig. 2D), and at 36h for clinical strains E/537C-05 (Fig. 2B) and E/S-141 (Fig. 2C). Similar to reference strains E and L2, pmpA was the first up-regulated gene for all clinical strains. In addition, pmpD was the last up-regulated gene for clinical strains L2 and E/CS-500-96 (Fig. 2A and 2D), and was also expressed late in development (together with other pmps) for the other two clinical E strains under study.
Figure 2. Expression profile of pmp and ompA genes throughout the development of C. trachomatis clinical strains.
(A) Strain L2 shares the same ompA genotype as L2/434; and strains E/537C-05 (B), E/S-141 (C) and E/CS-500-96 (D) share the same ompA genotype as E/Bour. Values represent the mean±SEM based on three independent experiments for time points of 2, 6, 12, 18, 24, 36, and 48 h post infection. See methods for details.
pmpF had the highest expression among all of the pmps for the clinical strains (Fig. 2). In fact, there was a 27-fold higher expression of pmpF compared with the least expressed gene (pmpD) for L2. For clinical E strains, there was a 19.2- and 22.6-fold higher expression of pmpF compared with the least expressed gene (pmpI) for E/537C-05 and E/CS-500-96 respectively, and a 54.2-fold higher expression than pmpA for E/S-141 Although no relevant dissimilarities were observed for pmpF between clinical L2 and L2/434, there were considerable expression differences among the clinical strains and E/Bour with up to 11.7-fold higher mRNA values for E/CS-500-96 than for E/Bour.
For ompA, mRNA levels peaked at 36 h for E/537C-05 and E/S-141, and at 18h for E/CS-500-96 and L2, declining thereafter (Fig. 2). The most striking example of differential mRNA levels between ompA and pmps occurred for E/S-141, where ompA had a 232.0-fold higher value compared with the least expressed gene, pmpA. However, all clinical strains except E/CS-500-96 had lower ompA expression levels for all time points compared with the corresponding reference strains.
Genetic analysis of pmpFE operon for C. trachomatis reference strains and clinical strains
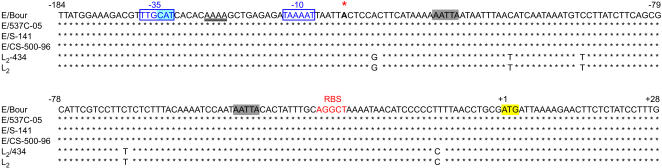
The pmpF, pmpE and pmpG/pmpF IGR sequences for the three clinical E strains were 100% similar to the corresponding E/Bour sequences, while L2 showed 4 nucleotide (nt) differences to L2/434 but only for pmpE. Compared to both L2 strains, the four E strains showed 317 (10.2%) nt and 106 (10.3%) amino acid (aa) differences for pmpF as well as 56 (1.9%) nt and 21 (20 to L2) (2.1%) aa differences for pmpE. For the pmpG/pmpF IGR, which comprises the ∼164 bp upstream region of pmpF, there were 5 nt differences between the L2 and the E strains, although none of them fell within the putative promoter region for the pmpFE operon (Fig. 3). The putative promoter is located within a 100% conserved stretch of the pmpG/pmpF IGR for both reference and all clinical strains (Fig. 3). The -10 promoter element (TAAAAT) identified in this study was identical to the one that was previously characterized for the L2/434 and D/UW-3 ltuB promoter, while the -35 region (TTGCAT) was 100% similar to the hctA promoter of the same chlamydial reference strains [32].
Figure 3. Predicted pmpF promoter sequence for reference and clinical strains.
Sequences are for reference strains E/Bour and L2/434, and clinical strains E/537C-05, E/S-141, E/CS-500-96, and L2. The predicted transcription promoter for pmpF is located within a 100% conserved region of the pmpG/pmpF IGR, where putative -10 and -35 elements are in blue characters and boxed. Potential A/T spacer region is underlined, and the predicted transcription start site is shown in a larger font below a red asterisk. The putative RBS for pmpF is in orange characters, and the putative RNase E cleavage sites are highlighted in grey. Numbers represent positions relative to the start codon of pmpF (highlighted in yellow). The start codon of pmpG is highlighted in blue.
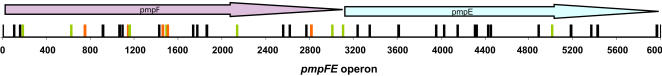
Analysis of the pmpFE operon sequence revealed several putative hairpin loop structures although the actual RNA folding in those regions functioning as a classic rho-independent type transcriptional terminator [49] cannot be assumed. At least 41 putative RNase E cleavage sites were identified throughout the pmpFE operon, 13 of which were not conserved between L2/434 (and L2) and the four E strains (Fig. 4). One of these non-conserved sites involved the pmpF/pmpE IGR, and is specific for the E strains. The search for an RBS revealed a perfect prokaryotic Shine-Dalgarno sequence (AGGAGG) located 17 nts upstream of the start codon of pmpE, which is approximately 3000 bp below the last bp in Figure 3 and, therefore, is not shown. This RBS is in close proximity to the above-described putative RNase cleavage site shared only by the four E strains. However, the best approach for a putative RBS sequence for pmpF has two mismatches when compared with the ones described in the literature, and is unusually distant from the start codon (Fig. 3). Two additional putative RNase E cleavage sites, one of which was in close proximity to this RBS, were identified within the pmpF regulatory region (Fig. 3).
Figure 4. Distribution/Location of the putative RNase E cleavage sites within the pmpFE operon coding sequence.
The sequence is for reference strains E/Bour and L2/434 and for clinical strains E/537C-05, E/S-141, E/CS-500-96 and L2. Black vertical lines represent all RNase E cleavage sites conserved among all strains under study; green vertical lines show the ones only conserved among the four “E” strains; orange vertical lines represent those specific solely for both L2 strains. Numbers represent nucleotide positions relative to the start codon of pmpF.
Immunoreactivity of patient sera with Pmp fusion proteins
Table 2 shows the clinical and microbiologic characteristics of the 39 adolescents enrolled in the study and the results of their serum immunoreactivity to rPmpD and rPmpF. All sera from patients infected with chlamydial clinical strains Ba, E, F and K (n = 15; 57.7%), but none with D, Ia, J or G (n = 11; 42.3%), were reactive to rPmpD while sera from uninfected patients were nonreactive with rPmpD (p = 0.001). Figure 5 shows the immunoblot results of representative sera from patients infected with Ba, D, E, F, G, Ia, J and K to rPmpD. Because pmpD is highly conserved among all reference strains [12], constructing rPmpD using the pmpD sequence of reference strain E/Bour should not have contributed to the observed differences in immunoreactivity. Further, cross-reactivity between strains was unlikely since the patients were infected with only a single strain, and sera that were reactive to rPmpD were not reactive to rPmpF. In our previous study, sera form the same individuals infected with clinical strains D, E and G reacted with rPmpC [17]. Surprisingly, none of the sera reacted to rPmpF (Figure 5), not even sera from the eight patients infected with strain E, although all sera from infected patients and one uninfected patient reacted with rMOMP as previously shown [17].
Table 2. Clinical and microbiologic characteristics of female adolescents from whom sera was used for determining the immunoreactivity against rPmpD and rPmpF.
| ompA genotypea (n) | Clinical diagnosisb (n) | Immunoreactivity of sera against recombinant fusion proteins | |
| rPmpD (%) | rPmpF (%) | ||
| Ba (3) | Cervicitis | 3/3 (100) | 0/3 (0) |
| D (3) | Cervicitis Dischargec (1/3) | 0/3 (0) | 0/3 (0) |
| E (8) | Cervicitis | 8/8 (100) | 0/8 (0) |
| F (5) | Cervicitis Dischargec (4/5) | 1/5 (20) | 0/5 (0) |
| G (1) | Cervicitis Dischargec (1/1) | 0/1 (0) | 0/1 (0) |
| Ia (1) | Cervicitis | 0/1 (0) | 0/1 (0) |
| J (2) | Cervicitis | 0/1 (0) | 0/1 (0) |
| K (3) | Cervicitis | 3/3 (100) | 0/3 (0) |
| Uninfected (13) | No clinical signs or symptoms | 0/13 (0) | 0/13 (0) |
Patients were adolescents 14–19 years of age who had a C. trachomatis infection with only one ompA genotype as described in methods or were uninfected;
The diagnosis of cervicitis was based on physical findings consistent with cervicitis as determined by the examining physician; all adolescents infected with C. trachomatis had cervicitis, and none of these patients complained of any symptoms;
A cervical discharge was noted by the examining physician; none of these patients had clinical signs or symptoms consistent with upper genital tract disease.
Figure 5. Dot-Blot of serum immunoreactivity against recombinant (r)PmpD and rPmpF.
Sera was obtained from adolescents singly infected and uninfected with a different C. trachomatis clinical strain as described previously [17] (see also methods). rPmpD and rPmpF concentrations were standardized for use on the blots. Immunoreactivity to each fusion protein for sera from patients infected with strain Ba (n = 3), D (n = 3), E (n = 8), F (n = 5), G (n = 1), Ia (n = 1), J (n = 2) or K (n = 3) are shown. Of note is that immunoreactivity was consistent for sera from patients infected with the same clinical strain except for strain F (Table 2); all eight patients infected with strain E were reactive to rPmpD.
Discussion
In this study, we determined the gene expression profile of the nine pmps throughout development for reference strains L2/434 and E/Bour, and four clinical strains belonging to ompA genotypes E and L2. The reference strains had significant gene expression differences where E/Bour had relatively lower mRNA levels and generally sustained expression from 24 to 48 h compared with L2/434 (Fig. 1). Surprisingly, in contrast to clinical L2, the three clinical E strains showed a dissimilar pmp expression profile to E/Bour (Fig. 2). These remarkable expression dissimilarities are generally supported by our recent comparative genomics findings where the laboratory adapted reference strains did not reflect the same genetic make-up of strains that are circulating among human populations today and currently exposed to immune selection [28].
It is well known that the developmental stages for reference E strains occur at later time points than for reference L2 strains [51] . This is supported by the ompA expression for E/Bour, which is shifted ∼6 hours later than for L2/434. However, the differential growth rate between these two reference strains does not explain the dissimilar pmp expression, as most pmps were up-regulated at the same time point for both (Fig. 1). For E/Bour, almost all of the pmps had increased expression during the second half of development with comparable mRNA levels at these stages, suggesting a similar involvement in RB division and RB to EB transformation. For L2/434, although most pmps showed a general up-regulation of transcription at the exponential growth phase of RB division when new membranes are being formed, pmpC, pmpE and pmpF appeared to play a more important role during this phase. Thus, the gene expression results of both E/Bour and L2/434 suggest their potential importance in membrane integrity. However, some Pmps may have more specific functions than others, depending on the chlamydial strain. In support of this, a proteomics study by Shaw et al. [21] detected five Pmps among reference strains A/HAR-13, D/UW-3, and L2/434, where PmpF was only detected for L2/434. In another proteomics study, only pmpE, pmpG, and pmpH were detected for L2/434 [22]. However, it is possible that these studies reflect a lack of sensitivity in detecting Pmps since a recent study was able to detect all Pmps for L2/434 [19].
Interestingly, pmpA had, in general, the lowest expression levels of all pmps at each time point except that it had one of the highest levels at 12 h p.i. (Fig. 1 and 2), suggesting a greater importance of PmpA during early stages of development. This is supported by shotgun proteomics where Skipp et al. [19] identified PmpA exclusively in RBs, whereas all other Pmps were detected in both RBs and EBs for L2/434. Additionally, for PmpD, the late up-regulation at 36 h corresponds to RB transformation into EBs, suggesting a role in EB outer membrane structure. In support of this, PmpD has a cysteine content considerably higher than any other Pmp [12]. There are 26 conserved cysteine residues in PmpD for all 19 C. trachomatis reference strains, while the mean for all other Pmps is only 13.9 [SE 2.3]. Cysteine residues are responsible for the highly disulfide cross-linked proteins of the outer membrane complex of EBs. Previous studies found that PmpD is surface located and cross-linked in the chlamydial outer membrane complex through disulfide bonds [52]. Furthermore, the N-terminal domain of C. pneumoniae Pmp21, the C. trachomatis PmpD ortholog, was shown to be non-covalently bound to other components of the EB surface [26]. Additionally, PmpD has shown species-specific neutralizing activity [27]. These collective data are supported by our findings that sera from C. trachomatis infected patients were reactive to rPmpD (Fig. 5). Our results were remarkably consistent for sera from patients infected with the same strain. For example, sera from all eight patients infected with strain E were reactive as were sera from three patients infected with strain Ba and three infected with strain K, although only one of the five patients with strain F were reactive; none of patients with strains D, Ia, J or G were reactive. Additional research is required to determine epitopes on PmpD that may correlate with the differential immune responses we observed.
Overall, considering both reference strains and clinical strains, pmpA and pmpI were the least expressed genes, while pmpF was the most highly expressed, although pmpE and pmpG also had similar expression levels for E/Bour. We previously found that PmpF is the most polymorphic protein among the C. trachomatis Pmps for both reference and clinical strains [28], [12]. Consistent with the observed protein diversity, phylogenetic analyses of PmpF grouped C. trachomatis strains by tissue tropism properties [12]. Further, comparative analyses of PmpF reveal distinct domains that may be associated with a specific disease group.
The outer membrane exposure of the N-terminus has been experimentally demonstrated for some C. pneumoniae Pmps [26], [53], suggesting that these proteins may be subjected to host immune pressure. The N-terminal half for C. trachomatis PmpF also contains numerous non-synonymous amino acid changes at locations of predicted MHC epitopes [9], indicating that it may be involved in eliciting a cellular immune response. Our findings that none of the sera from infected patients reacted with rPmpF suggest that this protein is not associated with the humeral immune response. Strain origin (E/Bour) of rPmpF did not seem to be an issue as sera from the eight patients infected with strain E were non-reactive. Furthermore, sensitivity was unlikely to be an issue given the immunoreactivity of the same sera with rPmpC and rMOMP, as we have previously described [17], and with rPmpD in this study. The occurrence of highly repeated GGAI motifs in the N-terminus suggests that Pmps may be associated with cell adhesion [54], which has been reported for Pmp21 of C. pneumoniae [26]. These cumulative findings suggest that Pmps are expressed with a differential immune response for patients infected with a specific strain. These findings and the remarkable pmpF expression dissimilarities among L2/434, E/Bour and the clinical strains suggest that there may be differential biological functions across strains and within the same strain for PmpF, either as a structural component to maintain membrane integrity, as part of a large pool of polymorphic antigens to elicit cellular immunity, or as an adhesin.
In our study, the pmpF sequences for the three clinical E strains were found to be 100% similar to the E/Bour sequence as was L2 to L2/434. Since it is highly unlikely that identical proteins have diverse functions, we hypothesized that there may be differential regulation at the promoter level or regulation involving variations in mRNA processing and/or degradation, which would yield distinct mRNA amounts according to strain-specific needs. It is well known that point mutations in regulatory regions, such as promoter regions and RBS, can affect transcription and translation levels. However, analysis of the putative promoter region and RBS for pmpF showed that they are 100% conserved for both reference and the clinical strains (Fig. 3), suggesting that the observed pmpF expression heterogeneity may result from variations in mRNA processing and/or degradation. In fact, regulatory systems of gene expression acting at both the transcriptional and translational levels are well represented in the chlamydial genome, including homologues of endoribonucleases E, III, G and P, exoribonucleases II and PNPase, and oligoribonuclease [8], [9]. These are known to control mRNA stability and processing as well as translational efficiency in other bacteria, such as Escherichia coli and Staphylococcus aureus [44], [48], [55]–[57]. The susceptibility of mRNA to ribonuclease attack may be influenced by events occurring not only at any stage during ribosome binding, but also during translation elongation or termination [40].
We identified two conserved putative RNase E cleavage sites in the pmpG/pmpF IGR, one of which is in close proximity to the putative RBS (Fig. 3). It is known that RBS sequence variability and sequestering by competitive regulatory proteins or conformational impediments can affect ribosome binding/loading and, thus, mRNA lifetime [40]. Considering this, a hypothetical initial cleavage by RNase E could reduce the affinity of the pmpF translation initiation region for ribosomes, thereby allowing subsequent mRNA degradation/processing by endo- and exonucleases, preferentially for E/Bour when compared to the other strains. A similar regulation has already been reported for sodB mRNA of E. coli at low iron concentrations [58]. However, this hypothetical mechanism, although possible, is speculative and lacks experimental evidence.
pmpF and pmpE belong to the same operon, yet had remarkably dissimilar mRNA levels for L2/434, and more so for all clinical strains with up to 8.4-fold higher expression for pmpF than for pmpE (Fig. 1A and 2). This did not occur for the pmpGH operon. We speculated that the expression heterogeneity within the pmpFE operon may be generated by premature termination of transcription, rapid mRNA processing, or mRNA degradation primarily of the downstream gene (pmpE) of this large operon transcript (>6 Kb). Similar regulatory mechanisms have already been suggested to explain the existence of multiple transcripts within other bacterial policistronic operons [55], such as those of Bacillus subtilis ara [59], Nitrosomonas europaea cbb [60], and Borrelia burgdorferi ospAB and bmpAB [61], [62].
Although we cannot assume that the putative stem-loop structures found within the pmpFE operon sequence may act as classic rho-independent type transcriptional terminators [49], the possibility of hairpin formation (a common phenomenon in mRNA, mainly on large transcripts) cannot be ignored nor can its hypothetical processing role in mRNA degradation and maturation be discounted. Furthermore, several putative RNase E cleavage sites were identified throughout the pmpFE operon (Fig. 4), which is expected for policistronic operons, although it is well known that RNase E cleaves mRNA only at a limited number of sites [55]. Interestingly, some of the RNase E sites were not conserved between L2 and E strains, suggesting that targeted mRNA degradation or rapid processing events may occur in this large transcript. Curiously, one of these non-conserved recognition sites involved solely the pmpF/pmpE IGR of the four E strains. Thus, if RNase E uses this cleavage site, subsequent degradation or processing events from this point would only occur for E strains and could hypothetically yield an mRNA decay of pmpE. Yet, as above, this mechanism is speculative and lacks experimental evidence. However, in a previous study, differential transcript quantities were reported for the MMSO genes of E. coli that contained a consensus RNase E cleavage site in the intergenic regions of the operon, suggesting complex mRNA processing [63].
Overall, the heterogeneous expression levels among pmps and among strains highlight the importance of this gene family in chlamydial biology. In particular, the unique expression disparity for the pmpFE operon with relatively high pmpF mRNA levels for 5 of the 6 strains under study, as well as the differential immunoreactivity of patient sera to rPmpD, suggest that some Pmps may explain phenotypic differences among strains for antigenicity, virulence and tissue tropism. Furthermore, our findings that clinical E strains do not correlate with reference strain E/Bour at the gene expression level are supported by our previously reported genomic data [28], reinforcing the need to examine clinical along with reference strains to advance our understanding of the role of pmps in chlamydial biology and disease pathogenesis.
Acknowledgments
We would like to express our gratitude to the clinicians, staff and patients (and their parents) of the teen clinics in Oakland for their willingness to participate in the study.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from Fundação Para a Ciência e Tecnologia and FEDER (POCTI/39822/MGI/2001) (to M.J. Borrego), and Public Health Service Grants from the National Institutes of Health, R01-AI39499 and R01-AI059647 (to D. Dean).
References
- 1.Moulder JW. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991;55:143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward ME. The chlamydial development cycle. In: Barron AL, editor. The Microbiology of Chlamydia. Boca Raton, , FL: CRC Press, Inc; 1988. pp. 71–97. [Google Scholar]
- 3.Dean D. Chlamydia trachomatis Sexually Transmitted Diseases. In: Connor DH, Schwartz DA, Chandler FW, editors. Pathology of Infectious Diseases. Stamford, , CT: Appleton and Lange Publishers; 1997. pp. 473–490. [Google Scholar]
- 4.Dean D, Powers VC. Persistent Chlamydia trachomatis infections resist apoptotic stimuli. Infect Immun. 2001;69:2442–2447. doi: 10.1128/IAI.69.4.2442-2447.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fields PI, Barnes RC. The Genus Chlamydia. In: Balows A, Truper HG, Dworkin M, Harder W, Schleifer KH, editors. The Prokaryotes. New York: Springer-Verlag; 1992. pp. 3691–3709. [Google Scholar]
- 6.Fitch WM, Peterson EM, de la Maza LM. Phylogenetic analysis of the outer-membrane-protein genes of Chlamydiae, and its implication for vaccine development. Mol Biol Evol. 1993;10:892–913. doi: 10.1093/oxfordjournals.molbev.a040048. [DOI] [PubMed] [Google Scholar]
- 7.Dean D, Millman K. Molecular and mutation trends analyses of omp1 alleles for serovar E of Chlamydia trachomatis. Implications for the immunopathogenesis of disease. J Clin Invest. 1997;99:475–483. doi: 10.1172/JCI119182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stephens RS, Kalman S, Lammel C, Fan J, Marathe R, et al. Genome sequence of an obligate intracellular pathogen of humans: Chlamydia trachomatis. Science. 1998;282:754–759. doi: 10.1126/science.282.5389.754. [DOI] [PubMed] [Google Scholar]
- 9.Carlson JH, Porcella SF, McClarty G, Caldwell HD. Comparative genomic analysis of Chlamydia trachomatis oculotropic and genitotropic strains. Infect Immun. 2005;73:6407–6418. doi: 10.1128/IAI.73.10.6407-6418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caldwell HD, Wood H, Crane D, Bailey R, Jones RB, et al. Polymorphisms in Chlamydia trachomatis tryptophan synthase genes differentiate between genital and ocular isolates. J Clin Invest. 2003;111:1757–1769. doi: 10.1172/JCI17993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson JH, Hughes S, Hogan D, Cieplak G, Sturdevant DE, et al. Polymorphisms in the Chlamydia trachomatis cytotoxin locus associated with ocular and genital isolates. Infect Immun. 2004;72:7063–7072. doi: 10.1128/IAI.72.12.7063-7072.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomes JP, Nunes A, Bruno WJ, Borrego MJ, Florindo C, et al. Polymorphisms in the Nine Polymorphic Membrane Proteins of Chlamydia trachomatis across All Serovars: Evidence for Serovar Da Recombination and Correlation with Tissue Tropism. J Bacteriol. 2006;188:275–286. doi: 10.1128/JB.188.1.275-286.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stothard DR, Toth GA, Batteiger BE. Polymorphic membrane protein H has evolved in parallel with the three disease-causing groups of Chlamydia trachomatis. Infect Immun. 2003;71:1200–1208. doi: 10.1128/IAI.71.3.1200-1208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomes JP, Bruno WJ, Borrego MJ, Dean D. Recombination in the genome of Chlamydia trachomatis involving the polymorphic membrane protein C gene relative to ompA and evidence for horizontal gene transfer. J Bacteriol. 2004;186:4295–4306. doi: 10.1128/JB.186.13.4295-4306.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindquist EA, Stephens RS. Transcriptional activity of a sequence variable protein family in Chlamydia trachomatis. In: Stephens RS, Byrne GI, Christiansen G, Clarke IN, Grayston JT, et al., editors. Chlamydial Infections Proceedings of the Ninth International Symposium on Human Chlamydial Infection. Napa, , California: International Chlamydia Symposium; 1998. pp. 259–262. [Google Scholar]
- 16.Belland RJ, Zhong G, Crane DD, Hogan D, Sturdevant D, et al. Genomic transcriptional profiling of the developmental cycle of Chlamydia trachomatis. Proc Natl Acad Sci U S A. 2003;100:8478–8483. doi: 10.1073/pnas.1331135100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomes JP, Hsia RC, Mead S, Borrego MJ, Dean D. Immunoreactivity and differential developmental expression of known and putative Chlamydia trachomatis membrane proteins for biologically variant serovars representing distinct disease groups. Microbes Infect. 2005;7:410–420. doi: 10.1016/j.micinf.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 18.Kiselev AO, Stamm WE, Yates JR, Lampe MF. Expression, Processing, and Localization of PmpD of Chlamydia trachomatis Serovar L2 during the Chlamydial Developmental Cycle. PLoS ONE. 2007;2:e568. doi: 10.1371/journal.pone.0000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skipp P, Robinson J, O'Connor CD, Clarke IN. Shotgun proteomic analysis of Chlamydia trachomatis. Proteomics. 2005;5:1558–1573. doi: 10.1002/pmic.200401044. [DOI] [PubMed] [Google Scholar]
- 20.Mygind PH, Christiansen G, Roepstorff P, Birkelund S. Membrane proteins PmpG and PmpH are major constituents of Chlamydia trachomatis L2 outer membrane complex. FEMS Microbiol Lett. 2000;186:163–169. doi: 10.1111/j.1574-6968.2000.tb09098.x. [DOI] [PubMed] [Google Scholar]
- 21.Shaw AC, Gevaert K, Demol H, Hoorelbeke B, Vandekerckhove J, et al. Comparative proteome analysis of Chlamydia trachomatis serovar A, D and L2. Proteomics. 2002;2:164–186. doi: 10.1002/1615-9861(200202)2:2<164::aid-prot164>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 22.Tanzer RJ, Hatch TP. Characterization of outer membrane proteins in Chlamydia trachomatis LGV serovar L2. J Bacteriol. 2001;183:2686–2690. doi: 10.1128/JB.183.8.2686-2690.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hsia Rc, Ahmed I, Batteiger B, Sekkides O, Ridgway G, et al. Differential immune response to polymorphic membrane proteins in STD patients. In: Saikku P, editor. Helsinki, Finland: Societa Editrice Esculapio; 2000. Aug 20–23, 2000, p. 219. [Google Scholar]
- 24.Henderson IR, Lam AC. Polymorphic proteins of Chlamydia spp.–autotransporters beyond the Proteobacteria. Trends Microbiol. 2001;9:573–578. doi: 10.1016/s0966-842x(01)02234-x. [DOI] [PubMed] [Google Scholar]
- 25.Henderson IR, Navarro-Garcia F, Nataro JP. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 1998;6:370–378. doi: 10.1016/s0966-842x(98)01318-3. [DOI] [PubMed] [Google Scholar]
- 26.Wehrl W, Brinkmann V, Jungblut PR, Meyer TF, Szczepek AJ. From the inside out–processing of the Chlamydial autotransporter PmpD and its role in bacterial adhesion and activation of human host cells. Mol Microbiol. 2004;51:319–334. doi: 10.1046/j.1365-2958.2003.03838.x. [DOI] [PubMed] [Google Scholar]
- 27.Crane DD, Carlson JH, Fischer ER, Bavoil P, Hsia RC, et al. Chlamydia trachomatis polymorphic membrane protein D is a species-common pan-neutralizing antigen. Proc Natl Acad Sci U S A. 2006;103:1894–1899. doi: 10.1073/pnas.0508983103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomes JP, Bruno WJ, Nunes A, Santos N, Florindo C, et al. Evolution of Chlamydia trachomatis diversity occurs by widespread interstrain recombination involving hotspots. Genome Res. 2007;17:50–60. doi: 10.1101/gr.5674706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dean D, Suchland R, Stamm W. Evidence for long-term cervical persistence of Chlamydia trachomatis by omp1 genotyping. J Infect Dis. 2000;182:909–916. doi: 10.1086/315778. [DOI] [PubMed] [Google Scholar]
- 30.Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaumburg CS, Tan M. Mutational analysis of the Chlamydia trachomatis dnaK promoter defines the optimal -35 promoter element. Nucleic Acids Res. 2003;31:551–555. doi: 10.1093/nar/gkg150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaumburg CS, Tan M. A positive cis-acting DNA element is required for high-level transcription in Chlamydia. J Bacteriol. 2000;182:5167–5171. doi: 10.1128/jb.182.18.5167-5171.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Douglas AL, Hatch TP. Mutagenesis of the P2 promoter of the major outer membrane protein gene of Chlamydia trachomatis. J Bacteriol. 1996;178:5573–5578. doi: 10.1128/jb.178.19.5573-5578.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan M, Gaal T, Gourse RL, Engel JN. Mutational analysis of the Chlamydia trachomatis rRNA P1 promoter defines four regions important for transcription in vitro. J Bacteriol. 1998;180:2359–2366. doi: 10.1128/jb.180.9.2359-2366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shine J, Dalgarno L. The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974;71:1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephens RS, Wagar EA, Edman U. Developmental regulation of tandem promoters for the major outer membrane protein gene of Chlamydia trachomatis. J Bacteriol. 1988;170:744–750. doi: 10.1128/jb.170.2.744-750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de la Maza LM, Fielder TJ, Carlson EJ, Markoff BA, Peterson EM. Sequence diversity of the 60-kilodalton protein and of a putative 15-kilodalton protein between the trachoma and lymphogranuloma venereum biovars of Chlamydia trachomatis. Infect Immun. 1991;59:1196–1201. doi: 10.1128/iai.59.3.1196-1201.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allen JE, Stephens RS. Identification by sequence analysis of two-site posttranslational processing of the cysteine-rich outer membrane protein 2 of Chlamydia trachomatis serovar L2. J Bacteriol. 1989;171:285–291. doi: 10.1128/jb.171.1.285-291.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Danilition SL, Maclean IW, Peeling R, Winston S, Brunham RC. The 75-kilodalton protein of Chlamydia trachomatis: a member of the heat shock protein 70 family? Infect Immun. 1990;58:189–196. doi: 10.1128/iai.58.1.189-196.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deana A, Belasco JG. Lost in translation: the influence of ribosomes on bacterial mRNA decay. Genes Dev. 2005;19:2526–2533. doi: 10.1101/gad.1348805. [DOI] [PubMed] [Google Scholar]
- 41.Ehretsmann CP, Carpousis AJ, Krisch HM. Specificity of Escherichia coli endoribonuclease RNase E: in vivo and in vitro analysis of mutants in a bacteriophage T4 mRNA processing site. Genes Dev. 1992;6:149–159. doi: 10.1101/gad.6.1.149. [DOI] [PubMed] [Google Scholar]
- 42.Robertson HD, Dickson E, Dunn JJ. A nucleotide sequence from a ribonuclease III processing site in bacteriophage T7 RNA. Proc Natl Acad Sci U S A. 1977;74:822–826. doi: 10.1073/pnas.74.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chelladurai BS, Li H, Nicholson AW. A conserved sequence element in ribonuclease III processing signals is not required for accurate in vitro enzymatic cleavage. Nucleic Acids Res. 1991;19:1759–1766. doi: 10.1093/nar/19.8.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deutscher MP. Degradation of RNA in bacteria: comparison of mRNA and stable RNA. Nucleic Acids Res. 2006;34:659–666. doi: 10.1093/nar/gkj472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meinken C, Blencke HM, Ludwig H, Stulke J. Expression of the glycolytic gapA operon in Bacillus subtilis: differential syntheses of proteins encoded by the operon. Microbiology. 2003;149:751–761. doi: 10.1099/mic.0.26078-0. [DOI] [PubMed] [Google Scholar]
- 46.Mader U, Hennig S, Hecker M, Homuth G. Transcriptional organization and posttranscriptional regulation of the Bacillus subtilis branched-chain amino acid biosynthesis genes. J Bacteriol. 2004;186:2240–2252. doi: 10.1128/JB.186.8.2240-2252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Hoon MJ, Makita Y, Nakai K, Miyano S. Prediction of transcriptional terminators in Bacillus subtilis and related species. PLoS Comput Biol. 2005;1:e25. doi: 10.1371/journal.pcbi.0010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kushner SR. mRNA decay in prokaryotes and eukaryotes: different approaches to a similar problem. IUBMB Life. 2004;56:585–594. doi: 10.1080/15216540400022441. [DOI] [PubMed] [Google Scholar]
- 49.Washio T, Sasayama J, Tomita M. Analysis of complete genomes suggests that many prokaryotes do not rely on hairpin formation in transcription termination. Nucleic Acids Res. 1998;26:5456–5463. doi: 10.1093/nar/26.23.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hessel T, Dhital SP, Plank R, Dean D. Immune response to chlamydial 60-kilodalton heat shock protein in tears from Nepali trachoma patients. Infect Immun. 2001;69:4996–5000. doi: 10.1128/IAI.69.8.4996-5000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyairi I, Mahdi OS, Ouellette SP, Belland RJ, Byrne GI. Different Growth Rates of Chlamydia trachomatis Biovars Reflect Pathotype. J Infect Dis. 2006;194:350–357. doi: 10.1086/505432. [DOI] [PubMed] [Google Scholar]
- 52.Kiselev AO, Johnson ML, Ballweber LB, Stamm WE, Lampe MF. Surface location of PmpD, a polymorphic membrane protein of Chlamydia trachomatis serovar L2. In: Schachter J, Christiansen G, Clarke IN, Hammerschlag MR, Kaltenboeck B, et al., editors. San Francisco: International Chlamydia Symposium; 2002. pp. 567–570. [Google Scholar]
- 53.Vandahl BB, Pedersen AS, Gevaert K, Holm A, Vandekerckhove J, et al. The expression, processing and localization of polymorphic membrane proteins in Chlamydia pneumoniae strain CWL029. BMC Microbiol. 2002;2:36. doi: 10.1186/1471-2180-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grimwood J, Stephens RS. Computational analysis of the polymorphic membrane protein superfamily of Chlamydia trachomatis and Chlamydia pneumoniae. Microb Comp Genomics. 1999;4:187–201. doi: 10.1089/omi.1.1999.4.187. [DOI] [PubMed] [Google Scholar]
- 55.Kennell D. Processing endoribonucleases and mRNA degradation in bacteria. J Bacteriol. 2002;184:4645–4657; discussion 4665. doi: 10.1128/JB.184.17.4645-4657.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mian IS. Comparative sequence analysis of ribonucleases HII, III, II PH and D. Nucleic Acids Res. 1997;25:3187–3195. doi: 10.1093/nar/25.16.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huntzinger E, Boisset S, Saveanu C, Benito Y, Geissmann T, et al. Staphylococcus aureus RNAIII and the endoribonuclease III coordinately regulate spa gene expression. Embo J. 2005;24:824–835. doi: 10.1038/sj.emboj.7600572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Afonyushkin T, Vecerek B, Moll I, Blasi U, Kaberdin VR. Both RNase E and RNase III control the stability of sodB mRNA upon translational inhibition by the small regulatory RNA RyhB. Nucleic Acids Res. 2005;33:1678–1689. doi: 10.1093/nar/gki313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sa-Nogueira I, Nogueira TV, Soares S, de Lencastre H. The Bacillus subtilis L-arabinose (ara) operon: nucleotide sequence, genetic organization and expression. Microbiology. 1997;143 ( Pt 3):957–969. doi: 10.1099/00221287-143-3-957. [DOI] [PubMed] [Google Scholar]
- 60.Wei X, Sayavedra-Soto LA, Arp DJ. The transcription of the cbb operon in Nitrosomonas europaea. Microbiology. 2004;150:1869–1879. doi: 10.1099/mic.0.26785-0. [DOI] [PubMed] [Google Scholar]
- 61.Liang FT, Caimano MJ, Radolf JD, Fikrig E. Borrelia burgdorferi outer surface protein (osp) B expression independent of ospA. Microb Pathog. 2004;37:35–40. doi: 10.1016/j.micpath.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 62.Ramamoorthy R, McClain NA, Gautam A, Scholl-Meeker D. Expression of the bmpB gene of Borrelia burgdorferi is modulated by two distinct transcription termination events. J Bacteriol. 2005;187:2592–2600. doi: 10.1128/JB.187.8.2592-2600.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yajnik V, Godson GN. Selective decay of Escherichia coli dnaG messenger RNA is initiated by RNase E. J Biol Chem. 1993;268:13253–13260. [PubMed] [Google Scholar]