Abstract
Background
Low-function alleles of the serotonin transporter promoter polymorphism (5HTTLPR) have been linked to various psychopathological entities, especially in individuals exposed to prior stressors. In women with bulimic syndromes, we explored associations with personality pathology of 5HTTLPR and prior sexual or physical maltreatment.
Methods
Ninety-two women with bulimic syndromes were genotyped for 5HTTLPR short (S) and long (LG and LA) alleles and were then assessed for eating symptoms, dimensional personality disturbances, history of sexual or physical abuse and borderline personality disorder (BPD).
Results
With a classification based on a biallelic model of 5HTTLPR (i.e., presence or absence of at least 1 S-allele copy), multiple regression analyses indicated significant proportions of variance in stimulus seeking and insecure attachment to be explained by abuse × genotype interaction effects, with greater psychopathology always occurring in S-allele carriers who had been abused. Likewise, a logistic regression analysis linked BPD to significant main effects of genotype and abuse. Analyses that aggregated carriers according to a triallelic model of 5HTTLPR (i.e., presence or absence of at least 1 copy of a presumably low-function S or LG allele) produced similar patterns but no statistically significant effects.
Conclusions
Traits such as sensation seeking and insecure attachment are, on average, elevated in 5HTTLPR S-allele carriers with bulimic syndromes who report prior physical or sexual maltreatment. These results add to the literature associating pronounced psychopathological manifestations, with conjoint effects of stress and the 5HTTLPR polymorphism.
Medical subject headings: bulimia nervosa, genotypes, serotonin
Abstract
Contexte
On a établi un lien entre des allèles à faible fonction du polymorphisme du promoteur du transporteur de la sérotonine (5HTTLPR) et diverses entités psychopathologiques, en particulier chez des sujets exposés à des facteurs de stress antérieurs. Chez les femmes qui présentent des syndromes boulimiques, nous avons étudié des liens entre la pathologie du 5HTTLPR liée à la personnalité et des sévices sexuels ou physiques antérieurs.
Méthodes
Chez 92 femmes qui présentaient des syndromes boulimiques, on a déterminé le génotype pour les allèles courts (S) et longs (LG et LA) du 5HTTLPR et on a évalué ensuite leurs symptômes alimentaires, les troubles de la personnalité dimensionnels, les antécédents de violence sexuelle ou physique et le trouble de la personnalité limite (TPL).
Résultats
Avec une classification fondée sur un modèle biallélique du 5HTTLPR (c.-à-d. présence ou absence d'au moins une copie de l'allèle S), des analyses de régression multiple ont indiqué des pourcentages significatifs de variance au niveau de la recherche des stimulus et de l'attachement insécurisé qu'on explique par les effets de l'interaction entre la violence selon le génotype, les porteurs de l'allèle S victimes auparavant de violence présentant toujours une plus grande psychopathologie. De même, une analyse de régression logistique a établi un lien entre le TPL et les effets principaux importants du génotype et de la violence. Des analyses basées sur une agrégation des porteurs en fonction des modèles trialléliques du 5HTTLPR (c.-à-d. présence ou absence d'au moins une copie d'un allèle S ou LG présumément à faible fonction) a produit des tendances semblables, mais aucun effet statistiquement significatif.
Conclusions
Des caractéristiques comme la recherche de sensations et l'attachement insécurisé sont en moyenne élevées chez les sujets porteurs de l'allèle S du 5HTTLPR qui présentent des syndromes boulimiques et déclarent avoir déjà subi des sévices physiques ou sexuels. Ces résultats s'ajoutent aux publications qui établissent un lien entre des manifestations pathologiques prononcées et les effets conjugués du stress et du polymorphisme du 5HTTLPR.
Introduction
Syndromes in the spectrum of bulimia nervosa (BN) coaggregate with various psychopathological traits, including impulsivity, novelty seeking, harm avoidance, compulsivity and perfectionism.1,2 Bulimic individuals also show heterogeneous trait profiles, with studies documenting subgroups conforming to such descriptors as “dysregulated (impulsive),” “overregulated (compulsive)” and “psychologically intact.”3,4 Variants characterized by “dysregulation” (i.e., showing pronounced impulsivity, novelty seeking and affective instability) have, however, been thought to implicate stronger “doses” of certain constitutional and developmental susceptibilities, including deficits in central serotonin (5-hydroxytryptamine [5-HT]) functioning5,6 and heightened exposure to childhood abuse.3,6 We examined the relevance to “dysregulation” in women with bulimic syndromes of a polymorphism believed to influence 5-HT transporter activity and past sexual or physical abuse.
Serotonin function in bulimic syndromes
The 5-HT system regulates mood, social behaviour, impulsivity and eating behaviour,5,6 creating an obvious rationale for the idea that this system acts in binge-eating syndromes. Consistent with this idea, studies of people who regularly binge (with or without purging) document disorder-relevant alterations in 5-HT metabolism, receptor sensitivity and transporter activity. Studies using single photon emission computer tomography have shown reduced central 5-HT transporter availability in women with BN7 and binge eating disorder,8 whereas studies using platelet measures have suggested altered peripheral 5-HT reuptake in actively bulimic people, in formerly bulimic people9 and even in the unaffected relatives of bulimic people.10 All of the preceding suggest that reduced 5-HT transporter kinetics may contribute (although perhaps not specifically or universally) to risk of bulimic behaviour. Consistent with this notion, 1 study associates the short (S) allele of the 5-HT transporter promoter region polymorphism (5HTTLPR) (presumably linked to relatively low 5-HT reuptake activity) with BN.11 Other studies do not, however, replicate such effects.12,13
One basis for inconsistent association of 5HTTLPR with bulimic phenotypes may be that this polymorphism corresponds to behavioural phenotypes that are systematically, but not universally, associated with bulimic syndromes (i.e., that only indirectly heighten susceptibility to bulimic eating). Consistent with this notion, bulimic patients who carry the S allele of 5HTTLPR have been reported to display such bulimia-linked personality traits as affective instability, impulsivity, insecure attachment14 or harm avoidance.13 Low-activity alleles of polymorphisms reported to affect 5-HT transporter activity (including the 5HTTLPR S allele, the 5HTTLPR S-10 haplotype or the 10-repeat allele in intron 2 variable number tandem repeat (VNTR) have, in other contexts, been associated with impulsivity,15,16 novelty seeking,17 affective instability,18 suicidality19 and borderline personality disorder (BPD).20
Sexual and physical abuse
Data show that about one-third of bulimic adults report unwanted sexual experiences during childhood and adolescence, and 50% or more report childhood physical abuse,21,22 suggesting an additional association between bulimic disturbances and history of maltreatment. Although such data point to an important convergence, when compared with rates in women showing other forms of maladjustment, childhood abuse is not uniquely elevated in samples of women with bulimia.21,22 Further, childhood abuse is observed to be a stronger correlate of affective instability, impulsivity or self-injuriousness in bulimic patients than it is of the severity of these patients' bingeing or purging.23,24 An implication may be that maltreatment is an extenuating factor, but not a specific causal agent, associated with specific psychopathological manifestations seen in some individuals with bulimic syndromes.
Putative gene (5HTTLPR)–environment interactions
Raising the additional notion that psychopathological potentials associated with 5HTTLPR may be modulated by environmental stressors, several studies report that depressive symptoms are increased in 5HTTLPR S-allele carriers who report a history of adverse or stressful life events.25–27 One study specifically reports elevated depression in adult carriers of the S allele who had been abused in childhood.27 Inspired by such findings, we explored the hypothesis that pathological personality trait expressions in women with bulimia would be influenced by 5HTTLPR variations and prior experiences of sexual or physical maltreatment. Given the findings associating childhood abuse in BN with increased personality pathology and self-injuriousness23,24 and linking 5-HT transporter hypoactivity with greater recklessness, borderline features and self-mutilation,6,16 we anticipated strongest gene–environment effects on personality characteristics compatible with the “dysregulated–impulsive” or “dramatic– erratic” spectrum.
5HTTLPR variations
The 5HTTLPR polymorphism has traditionally been conceptualized as being biallelic, with long (L) and S allele variants thought to correspond to relatively high or low production of the 5-HT transporter protein.15,18 However, some recent data suggest the existence of a low-frequency L-allele variant, LG, (with an adenine to guanine in its sequence) whose functioning may be comparable to that of the S allele.28,29 In other words, 5HTTLPR may be triallelic, with S and LG alleles representing “low-function” variants and an LA allele conferring expectedly higher function. Because the functional significance of the allelic variations described yet needs to be ascertained, we tested models that were consistent with biallelic and triallelic formulations.
Methods
Participants
This study was approved by the Douglas Institute Research Ethics Board, and all study participants gave informed consent. We recruited women with bulimia spectrum disorders through a specialized eating disorders (ED) program, using the following criteria: body mass index (BMI) of 17.5 to 30 and meeting criteria for a bulimia spectrum ED in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV)30 but not binge eating disorder or anorexia nervosa and not pregnant. After exclusions, we completed assays in 92 women with bulimic syndromes, 70 (76.1%) of whom met DSM-IV criteria for BN-purging subtype, 4 (4.3%) for BN-nonpurging subtype and 18 (19.6%) for a bulimia spectrum ED not otherwise specified (because they binged or purged at less than the requisite twice weekly). Minimum binge frequency was 1 episode per month over the past 3 months; mean binge episodes and binge days per month in our sample (25.37, standard deviation [SD] 20.85 d/mo] and 15.36, SD 7.86, respectively) were substantially higher. Thus, we felt the sample to be typical of women with bulimia seeking treatment and note the finding that threshold and subthreshold bulimic variants are equivalent on many clinical dimensions.31 Subjects' mean age was 25.24, SD 6.37, years and mean BMI was 22.38, SD 2.65, kg/m2, respectively. Limiting recruitment to unmedicated patients was impractical (and undesirable on grounds of representativeness), so we included 24 women (26.1% of the sample) who were taking a psychoactive medication when tested. Statistical procedures were applied to control for confounding effects of medications upon symptom presentation (see Results). Given the sample size, we controlled only for the effects owing to presence or absence of adjunctive medication and not for effects of individual medication types or families. An earlier report14 described findings in 48 (52.2%) of the 92 cases described in the present sample, but it did not report on developmental experiences (i.e., childhood abuse) or gene–environment interactions.
Measures
ED diagnoses and symptoms were assessed with the widely used eating disorders examination (EDE)32 and eating attitudes test (EAT-26).33 We also computed subjects' BMI (kg/m2). To achieve a comprehensive, dimensional assessment of personality pathology, while avoiding risks of overparameterization, we selected specific subscales from the Dimensional Assessment of Personality Pathology-Basic Questionnaire (DAPP-BQ)34 to broadly sample overregulated and dysregulated spectrum personality traits that are frequently ascribed to bulimic syndromes.1,2 The resulting battery measured compulsivity (i.e., orderliness and conscientiousness), restricted expression (i.e., low self-disclosure or restricted affect), anxiousness (trait anxiety), stimulus seeking (i.e., novelty seeking or quest for excitement), affective instability (i.e., mood lability, overreactivity) and insecure attachment (i.e., reactivity to separations, abandonment fears or intense needs for closeness). To complement our assessment, we added the Barrat Impulsivity Scale (BIS, version 11)35 and the Centre for Epidemiological Studies-Depression (CES-D) scale.36
Borderline personality disorder (BPD) was assessed in 90 of the 92 participants with the Structured Clinical Interview for DSM-IV Axis-II Disorders,37 excluding the criterion referring to overeating. Audits on interrater reliability for a BPD or non-BPD distinction, conducted in sets of 12, 14 and 33 interviews selected from 3 recent study periods in our laboratory, produced kappas (and percent agreements) of 0.80 (91.7%), 0.81 (92.86%) and 0.77 (90.91%). Because interviews for other axis II diagnoses were not exhaustively completed on all participants, this report treats BPD diagnoses alone.
Childhood abuse was assessed with the Childhood Trauma Interview (CTI),38 a roughly 30-minute structured interview on experiences of abuse before age 18 years. Interrater reliability for indices reflecting the nature, severity, frequency and duration of trauma were very good. CTI indices also showed solid convergence with other measures of abuse. Construct validity is supported by logical associations with syndromes having theoretical links to trauma exposure.38 We used CTI severity indices to isolate experiences of unambiguous physical or sexual maltreatment occurring at or before age 18 years (in conformity with the standard CTI protocol); however, 65% of instances reported occurred before age 13 years. Sexual abuse was defined as “sexualized experiences involving repeated sexual contacts occurring at least 3 times within 1 year, or more extreme experiences (e.g., oral sex or penetration) happening at least once.” We defined physical abuse as “experiences of blatant hitting, occurring at least 3 times within 1 year, or at least 1 instance of extreme physical abuse, implicating such acts as indiscriminate hitting with an object.”
Genotyping
DNA samples, obtained from whole blood, were amplified by polymerase chain reaction (PCR) in a total volume of 20 μL, which contained 100 ng of genomic DNA, 200 μmol of dNTPs, 10 pmol each of the forward and reverse primer, 1 unit of Taq DNA polymerase (Qiagen, Alameda, Calif.), 1 × PCR buffer and 1 × Q solution (Qiagen). The forward primer (5′-ATG CCA GCA CCT AAC CCC TAA TGT-3′) and reverse primer (5′-GG ACC GCA AGG TGG GCG GGA-3′) were used to amplify a region encompassing 5HTTLPR; L and S alleles were then resolved on a 2% agarose gel. The PCR protocol involved preheating the samples at 94°C for 5 minutes, followed by 35 cycles of denaturation at 94°C (30 s), annealing at 64°C (30 s), and extension at 72°C (45 s), as well as a final hold of 5 minutes at 72°C. The LG and LA alleles were subsequently studied by enzymatic digestion of 7 μL of the above-mentioned PCR product, using 5 units of Moraxella spI and incubating at 37°C for a minimum of 3 hours. The LG and LA alleles were resolved on a 2% agarose gel.
Data analyses
Preliminary analyses (not reported here) revealed no systematic differences on clinical variables between homozygotes or heterozygotes for low-function alleles. Given this, and previous evidence for the dominance of low-function alleles,15,29 all subsequent analyses applied dichotomous classifications for the genotype variable, according to both biallelic (S/S or S/L v. L/L) and triallelic (S/S, S/LG, S/LA, LG/LA or LG/LG v. LA/LA) conceptualizations. To explore effects of genotype and abuse (abuse: present or absent) on dimensional symptom variables while accounting for possible effects of psychoactive medications (medicated or unmedicated), we applied hierarchical multiple regressions on each variable of interest. Each regression successively tested the genotype main effect (step 1), the abuse main effect (step 2) and the genotype × abuse interaction (step 3) and then controlled for the medication main effect (step 4). For categorical BPD diagnoses, we used logistic regression analysis to conduct parallel tests for main and interaction effects of genotype and abuse factors and the main medication effect.
Results
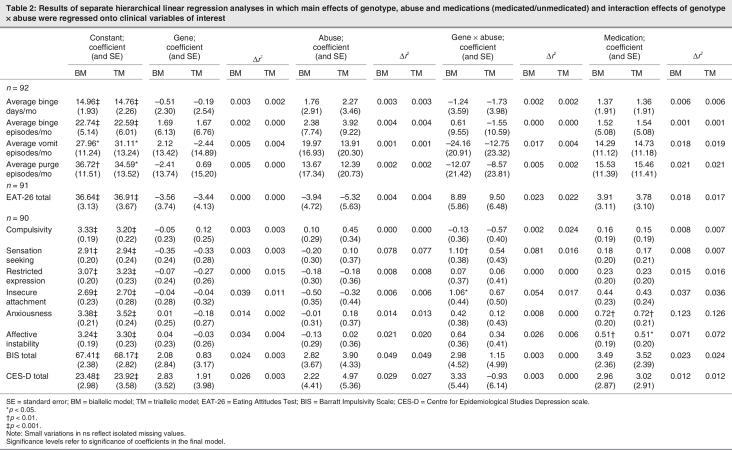
Treating 5HTTLPR in a conventional, biallelic fashion, frequencies of S/S, S/L and the L/L genotypes, respectively, occurring in 18 (19.6%), 43 (46.7%) and 31 (33.7%) of our participants conformed with the Hardy–Weinberg equilibrium (X21 = 0.20, not significant). With a triallelic model, we observed S/S, S/LG, S/LA, LG/LA, LG/LG and LA/LA genotypes, respectively, to occur in 18 (19.6%), 10 (10.9%), 33 (35.9%), 8 (8.7%), 1 (1.1%) and 22 (23.9%) of our participants. Frequencies of groups who were carriers of 2, 1 or no low-function (i.e., S or LG) alleles also conformed with Hardy–Weinberg equilibrium (X21 = 0.99, not significant). When crossed with the abuse factor, the biallelic model led us to form the following groups: not abused, no S allele (n = 18); not abused, S allele (n = 37); abused, no S allele (n = 13); and abused, S allele (n = 24). The triallelic model led to the following groups: not abused, no S or LG allele (n = 13); not abused, S or LG allele (n = 42); abused, no S or LG allele (n = 9); and abused, S or LG allele (n = 28). To illustrate the results, Table 1 shows (for groups formed with each classification) mean and SD, age and BMI and mean scores on dimensional measures of eating symptoms (monthly binge, vomit and purge frequencies; EAT-26) and psychopathology (stimulus seeking, affective instability, insecure attachment, compulsivity, restricted expression, anxiousness, impulsivity, depression). The purge variable combined monthly episodes of vomiting or abuse of laxatives or diuretics. In the case of binge, vomit and purge variables, we limited isolated outliers to the sample mean plus 2 SDs. One-way analyses of variance showed no group differences in age or BMI.
Table 1
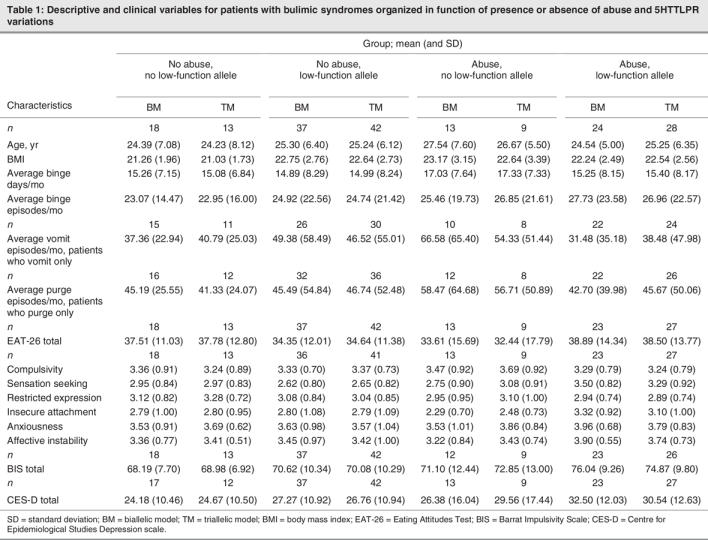
Effects on clinical variables were tested with multiple regression procedures (described above). Table 2 summarizes the final model obtained with each regression analysis. (Significant results obtained at successive steps are reported in the text). On eating symptoms, no main or interaction effects of gene, abuse or medication variables were indicated. To ensure that observed deviations from normality did not influence results for binge, vomit and purge frequencies, we conducted a second tier of analyses (not shown in Table 2) in which scores were log-transformed. Results for gene, abuse, gene–abuse interaction and medication effects remained nonsignificant.
Table 2
On psychopathological indices, there were various significant results. Using a biallelic (S v. no S) classification, significant abuse and (or) gene × abuse effects were obtained on stimulus seeking and insecure attachment (Table 2). In the case of stimulus seeking, introducing abuse (at step 2) yielded a significant 7.8% increment in variance accounted for (Fchange [1,87] = 7.41, p < 0.01) and resulted in a significant regression equation at that step (F2,87 = 3.83, p < 0.03). Adding the genotype–abuse interaction at the subsequent step explained an additional 8.1% of variance (Fchange [1,86] = 8.29, p < 0.01), and increased the significance of the overall regression equation at that step (F3,86 = 5.53, p < 0.01). The variance in stimulus seeking explained by gene, abuse and gene × abuse effects totaled 16.2%. Likewise, for insecure attachment, introduction of the gene × abuse effect (at step 3) added a significant, incremental 5.4% to variance previously explained (Fchange [1,86] = 5.20, p < 0.03) and yielded a significant regression equation at that step (F3,86 = 3.19, p < 0.03). Total variance in insecure attachment explained by gene, abuse and gene × abuse effects was 9.9%. In both cases, results indicated pathological elevations in S-allele carriers who had been abused (see Table 1). On impulsivity, we obtained no genotype or genotype × abuse effects. However, addition of abuse at step 2 explained a significant incremental 4.9% of variance (Fchange [1,87] = 4.60, p < 0.04) and yielded a significant regression equation at that step (F2,87 = 3.45, p < 0.04). The analyses described confirmed independence of effects from those attributable to psychoactive medications. Not surprisingly, significant medication effects showed that patients who were more symptomatic of anxiousness or affective instability were more likely to be medicated (see Table 2). Although results pointed in the same direction (see Table 1), no significant gene, abuse or gene × abuse effects were obtained in parallel analyses based on a triallelic gene model (see Table 2). Significant incremental abuse effects were again detected on stimulus seeking and impulsivity at step 2, respectively, accounting for 7.7% and 4.9% increments in the variance explained (Fchange [1,87] = 7.28, p < 0.01 and Fchange [1,87] = 4.45, p < 0.04, respectively). Medication effects showed that patients who were more symptomatic of anxiousness or affective instability were more often medicated.
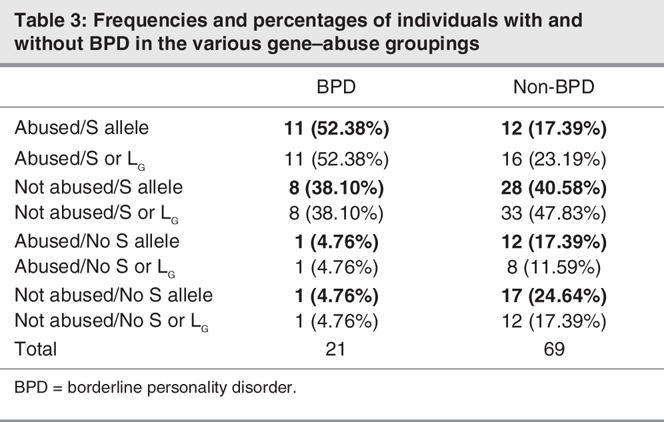
Of the 90 participants who completed BPD interviews, 21 (23.3%) met BPD criteria. Table 3 shows numbers (and proportions) of cases with and without BPD who fell into various genotype × abuse groupings. A first logistic regression analysis, testing for main and interaction effects of gene (biallelic) and abuse factors and the main medication effect on the BPD classification, detected no significant genotype × abuse interaction. Consequently, a final analysis tested for main effects alone. Results showed the S allele to be a significant predictor of BPD (odds ratio [OR] 7.79, 95% confidence interval [CI] 1.60–37.91; p < 0.02). Of 21 individuals with BPD, 19 (90.5%) were S-allele carriers, compared with 40 (58.0%) of the individuals without BPD. The abuse effect was also statistically significant (OR 3.27, 95% CI 1.09–9.81; p < 0.04). Of 21 individuals with BPD, 12 (57.1%) had been abused, compared with 24 (34.8%) without BPD. The risk of membership in the BPD category was thus associated with independent effects of the genetic (S allele) susceptibility and prior maltreatment. Any apparent tendencies toward increased likelihood of medication in BPD patients did not reach statistical significance (OR 2.37, 95% CI 0.75–7.50; p = 0.14). (Wide CIs associated with all effects noted are likely to be attributable to 2 cells containing only 1 participant). Results based on a triallelic model (shown in bold font) showed combined S and LG alleles to predict BPD at a trend level (OR 4.11, 95% CI 0.84–20.06; p < 0.09), while the abuse effect was again statistically significant (OR 2.92, 95% CI 1.02–8.37; p < 0.05). Tendencies toward increased medication in BPD patients did not reach statistical significance (OR 2.32, 95% CI 0.76–7.06; p = 0.14).
Table 3
Discussion
We tested the prediction that conjoint effects of variations in the 5HTTLPR polymorphism and history of sexual or physical maltreatment would influence the likelihood of “dramatic–erratic” phenotypes in women with bulimic syndromes. This prediction was inspired partly by findings in populations with and without bulimia that associate the 5HTTLPR S allele and a positive abuse history with increased behavioural instability or personality pathology14–19,23 and partly by a literature suggesting that prior abuse mitigates psychopathological potentials associated with 5HTTLPR.25,27 In line with predictions, we detected significant genotype–abuse interaction effects on measures of stimulus seeking and insecure attachment: both suggest that women with bulimia who were carriers of the 5HTTLPR S allele when they reported past sexual or physical maltreatment showed greater propensities toward psychopathological expressions. Likewise, although we detected no interaction effects, we observed apparently additive main effects of genotype and abuse that had value in accounting for BPD diagnoses. In other words, S-allele carriers who were previously abused tended to show elevated personality pathology.
Given a relatively small sample size, caution is required surrounding the interpretation of results of multiple comparisons. At the same time, to the extent that variables on which significant effects are obtained (i.e., stimulus seeking, insecure attachment, BPD) are all associated with the same underlying clinical constructs — namely, dramatic–erratic or borderline disturbances — observed effects tend to cross-validate one another. Further, because the simple correlation between the stimulus-seeking and insecure attachment scales is quite modest (r = 0.25) in our data, findings cannot be due solely to collinearities among measures. Considering all of these factors, we are encouraged to interpret our results as indicating that, in bulimic syndromes, S-allele carriers who have been abused tend to display increased dramatic–erratic or borderline-type psychopathology.
If the preceding is correct, then it remains to be explained why parallel effects were not obtained on scales measuring affective instability or impulsivity, both of which have a conceptual bearing on the dramatic–erratic personality construct. We presume that differences may be attributable to relatively low statistical power provided by our sample size and differential sensitivities of the scales involved. Indeed, the pattern of results obtained on affective instability and impulsivity (see Table 1), and a p < 0.08 trend toward gene–abuse interaction observed on affective instability (using a biallelic model), may yet (under conditions of increased power) be consistent with elevated psychopathology in S-allele carriers who had been abused. Thus, although the phenomenology implicated needs to be specified, our results (in keeping with previous reports25–27,29) suggest a special susceptibility to maladjustment in S-allele carriers who have been maltreatment.
What processes might explain increased psychopathological manifestations in S-allele carriers who have been abused? We and other investigators have linked behavioural dysregulation in a population with bulimic syndromes to underlying 5-HT disturbances.5,6 Further, we have documented tendencies for previously abused women with bulimia to display more pronounced serotonergic anomalies than those without a history of abuse.5 Based on the preceding, and on findings suggesting that childhood trauma corresponds to abnormal 5-HT functioning in adults with other disorders,39 we postulated that dysregulated variants of BN may often implicate amplification, by effects of developmental stressors, of latent genetic (5-HT mediated) propensities toward a behaviourally, interpersonally and affectively unstable phenotype. That is, trauma sequelae might activate or amplify latent propensities toward dysregulation associated with the 5HTTLPR S allele.14,15,19
Although observed gene–environment interactions could reflect the activation, by abuse, of potentials associated with the 5HTTLPR S allele, it remains possible that the effect actually reflects the opposite direction of causality — the S allele increasing the risk of abuse — through such possible correlates as heightened impulsivity (in potentially abusive, genetically disposed parents), or heightened precociousness or risk-taking (in genetically disposed children). Such effects could account for an observed interaction between a genetic propensity (associated with 5HTTLPR) and exposure to abuse.
Gene–environment interactions of the type we observe in this study have various clinical and theoretical potentials, worthy of further investigation: 1) Such effects may help explain inconsistencies in the available literature on candidate-gene effects in BN.40 Indeed, with only rare exceptions, previous studies in this literature ignore environmental effects and, in so doing, may overlook important influences on gene expression; 2) Findings indicate a striking lack of influence from genetic (i.e., 5HTTLPR) or environmental (i.e., abuse) factors on eating-symptom expression. Such results are compatible with findings documenting the absence of effects of serotonin-linked genotypes14 or exposure to childhood abuse22,24 on bulimic symptom severity. From a theoretical perspective, the apparent absence of a genetic influence on eating symptoms is interesting because it suggests that develpmental factors (e.g., childhood abuse) and genetic factors (e.g., being an S-allele carrier) may more closely predict psychopathology than does severity of eating symptoms, in people with EDs; 3) These findings point to genetic and psychosocial substrates for a distinction, proposed by various clinician–theorists, between people with BN who show prominent dysregulation and those who do not.3,4,6 In line with previous studies in other populations,15,19,25,27 our findings localize phenotypic differences to differences between S and no-S variants of 5HTTLPR genotypes. This necessitates a final note: some recent findings have suggested that clinically relevant differences occur between carriers of a presumably high-function 5HTTLPR LA allele and of low-function S and LG alleles.29 However, gene or gene–abuse effects studied here were nonsignificant when patients were classified according to such a triallelic (S and LG v. LA) model. We assume that discrepant results obtained using biallelic or triallelic models may be artifacts of limited statistical power, related to our sample size. If so, then the actual functional and clinical significances of 5HTTLPR alleles and the relative merits of biallelic or triallelic models will need further study.
Clinical implications
The present findings corroborate the view that genetic variations and developmental factors are relevant predictors of subphenotypic variations occurring in a population of people with bulimic syndromes.3,6 Such findings raise the hope that a fuller understanding of trait variations (and their apparent constitutional and developmental correlates) may facilitate the development of individualized treatments. For example, a “high functioning” (less genetically and developmentally compromised) group of people with bulimia might have relatively focal treatment needs, for example, nutritionally oriented therapies proving adequate to treat disturbances that result more from prolonged caloric restraint than from a fundamental and pervasive psychopathology. Alternatively, a more unstable subgroup (with greater novelty seeking and interpersonal insecurity) might require a different type and intensity of intervention. If influenced by serotonergic problems (related to hereditary factors, or consequences of severe developmental stressors), nutritionally focused therapies might prove inadequate. Pharmacological support, or more elaborate psychotherapeutic interventions aimed at core trait pathologies or posttraumatic sequelae might, in such cases, be more appropriate.
Acknowledgments
This research was supported by grant no. SR-4306, awarded to Drs. Steiger and Joober by the Quebec government's Joint CQRS-FRSQ-MSSS Program in Mental Health. Preliminary results from this study were presented at the annual meetings of the Eating Disorders Research Society, Toronto, Sept. 30, 2005 and the Academy for Eating Disorders, Barcelona, Spain, June 9, 2006.
Footnotes
Contributors: Drs. Steiger, Joober, Gauvin, Israel, Bruce, Kin and Young designed the study. Drs. Steiger, Joober, Israel and Howard and Ms. Richardson acquired the data, which Drs. Steiger, Joober, Gauvin, Israel, Bruce, Kin, Young and Ms. Richardson analyzed. Drs. Steiger and Gauvin wrote the article, and all authors revised it. All authors gave final approval for the article to be published.
We thank Annelie Anestin, Catherine Dandurand, Melanie Aubut, Marie Grassia and Sandra Mansour for their contributions.
Competing interests: None declared.
Correspondence to: Dr. Howard Steiger, Eating Disorders Program, Douglas Hospital, 6875 LaSalle Blvd., Montréal QC H4H 1R3; fax 514 888-4085; stehow@douglas.mcgill.ca
References
- 1.Cassin SE, von Ranson KM. Personality and eating disorders: a decade in review. Clin Psychol Rev 2005;25:895-916. [DOI] [PubMed]
- 2.Grilo CM. Recent research of relationships among eating disorders and personality disorders. Curr Psychiatry Rep 2002;4:18-24. [DOI] [PubMed]
- 3.Westen D, Harnden-Fischer J. Personality profiles in eating disorders: rethinking the distinction between axis I and axis II. Am J Psychiatry 2001;158:547-62. [DOI] [PubMed]
- 4.Wonderlich SA, Crosby RD, Joiner T, et al. Personality subtyping and bulimia nervosa: psychopathological and genetic correlates. Psychol Med 2005;35:649-57. [DOI] [PubMed]
- 5.Steiger H, Jabalpurwala S, Champagne J. Axis-II comorbidity and developmental adversity in bulimia nervosa. J Nerv Ment Dis 1996; 184: 555-60. [DOI] [PubMed]
- 6.Steiger H. Eating disorders and the serotonin connection: state, trait and developmental effects. J Psychiatry Neurosci 2004;29:20-9. [PMC free article] [PubMed]
- 7.Tauscher J, Pirker W, Willeit M, et al. Beta-CIT and single photon emission computer tomography reveal reduced brain serotonin transporter availability in bulimia nervosa. Biol Psychiatry 2001; 49: 326-32. [DOI] [PubMed]
- 8.Kuikka JT, Tammela L, Karhunen AR, et al. Reduced serotonin transporter binding in binge eating women. Psychopharmacology (Berl) 2001;155:310-4. [DOI] [PubMed]
- 9.Steiger H, Richardson J, Israel M, et al. Reduced density of platelet-binding sites for 3H-paroxetine in remitted bulimic women. Neuropsychopharmacology 2005;30:1028-32. [DOI] [PubMed]
- 10.Steiger H, Gauvin L, Joober R, et al. Intrafamilial correspondences on platelet [3H-] paroxetine-binding indices in bulimic probands and their unaffected first-degree relatives. Neuropsychopharmacology 2006;31:1785-92. [DOI] [PubMed]
- 11.Di Bella DD, Catalano M, Cavallini MC, et al. Serotonin transporter linked polymorphic region in anorexia nervosa and bulimia nervosa. Mol Psychiatry 2000;5:233-41. [DOI] [PubMed]
- 12.Lauzurica N, Hurtado A, Escarti A, et al. Polymorphisms within the promoter and the intron 2 of the serotonin trasnporter gene in a population of bulimic patients. Neurosci Lett 2003;352:226-30. [DOI] [PubMed]
- 13.Monteleone P, Santonastaso P, Mauri M, et al. Investigation of the serotonin transporter regulatory region polymorphism in bulimia nervosa: relationships to harm avoidance, nutritional parameters and psychiatric comorbidity. Psychosom Med 2006;68:99-103. [DOI] [PubMed]
- 14.Steiger H, Joober R, Israël M, et al. The 5HTTLPR polymorphism, psychopathological symptoms, and platelet [3H-] paroxetine binding in bulimic syndromes. Int J Eat Disord 2005;37:57-60. [DOI] [PubMed]
- 15.Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996;274:1527-30. [DOI] [PubMed]
- 16.Courtet P, Picot M, Bellivier F, et al. Serotonin transporter gene may be involved in short-term risk of subsequent suicide attempts. Biol Psychiatry 2004;55:46-51. [DOI] [PubMed]
- 17.Sander T, Harms H, Dufeu P, et al. Serotonin transporter gene variants in alcohol-dependent subjects with dissocial personality disorder. Biol Psychiatry 1998;43:908-12. [DOI] [PubMed]
- 18.Collier DA, Stober G, Li T, et al. A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Mol Psychiatry 1996; 1: 453-60. [PubMed]
- 19.Anguelova M, Benkelfat C, Turecki G. A systematic review of association studies investigating genes coding for serotonin receptors and the serotonin transporter: II. Suicidal behavior. Mol Psychiatry 2003;8:646-53. [DOI] [PubMed]
- 20.Ni X, Chan K, Bulgin N, et al. Association between serotonin transporter gene and borderline personality disorder. J Psychiatr Res 2006;40:448-53. [DOI] [PubMed]
- 21.Jacobi C, Hayward C, de Zwaan M, et al. Coming to terms with risk factors for eating disorders: application of risk terminology and suggestions for a general taxonomy. Psychol Bull 2004;130:19-65. [DOI] [PubMed]
- 22.Wonderlich SA, Brewerton TD, Jocic Z, et al. Relationship of childhood sexual abuse and eating disorders. J Am Acad Child Adolesc Psychiatry 1997;36:1107-15. [DOI] [PubMed]
- 23.Moreno JK, Selby MJ, Neal S. Psychopathology in sexually abused and non-sexually abused eating disordered women. Psychother priv pract 1998;17:1-9.
- 24.Steiger H, Gauvin L, Israël M, et al. Association of serotonin and cortisol indices with childhood abuse in bulimia nervosa. Arch Gen Psychiatry 2001;58:837-43. [DOI] [PubMed]
- 25.Caspi A, McClay J, Moffitt TE, et al. Role of genotype in the cycle of violence in maltreated children. Science 2002;297:851-4. [DOI] [PubMed]
- 26.Eley TC, Sugden K, Corsico A, et al. Gene-environment interaction analysis or serotonin system markers with adolescent depression. Mol Psychiatry 2004;9:908-15. [DOI] [PubMed]
- 27.Kaufman J, Yang BZ, Douglas-Palumberi H, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A 2004;101:17316-21. [DOI] [PMC free article] [PubMed]
- 28.Hu X-Z, Lipsky RH, Zhu G, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet 2006;78:815-26. [DOI] [PMC free article] [PubMed]
- 29.Zalsman G, Huang Y-y, Oquendo MA, et al. Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am J Psychiatry 2006;163:1588-93. [DOI] [PubMed]
- 30.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994.
- 31.Fairburn CG, Harrison PJ. Eating disorders. Lancet 2003;361:407-16. [DOI] [PubMed]
- 32.Fairburn CG, Cooper P. The eating disorders examination. In: Fairburn CG, Wilson GT, editors. Binge eating: nature, assessment and treatment. 12th ed. New York: Guilford; 1993. p. 317-60.
- 33.Garner DM, Olmsted M, Bohr Y, et al. The eating attitudes test: psychometric features and clinical correlates. Psychol Med 1982; 12: 871-8. [DOI] [PubMed]
- 34.Livesley WJ, Jackson DN, Schroeder ML. Factorial structure of traits delineating personality disorders in clinical and general population samples. J Abnorm Psychol 1992;101:432-40. [DOI] [PubMed]
- 35.Patton JH, Stanford MS, Barrat E. Factor structure of the Barrat Impulsiveness Scale. J Clin Psychol 1995;51:768-74. [DOI] [PubMed]
- 36.Weissman MM, Sholomskas D, Pottenger M, et al. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol 1977;106:203-14. [DOI] [PubMed]
- 37.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV axis-II personality disorders (SCID-II), version 2.0. New York: Biometrics Research Department, New York State Psychiatric Institute; 1996.
- 38.Fink LA, Bernstein D, Handelsman L, et al. Initial reliability and validity of the Childhood Trauma Interview. Am J Psychiatry 1995; 152: 1329-35. [DOI] [PubMed]
- 39.Rinne T, Westenberg HGM, den Boer JA, et al. Serotonergic blunting to meta-chlorophenylpiperazine (m-CPP) highly correlates with sustained childhood abuse in impulsive and autoaggressive female borderline patients. Biol Psychiatry 2000;47:548-56. [DOI] [PubMed]
- 40.Bulik CM, Tozzi F. Genetics in eating disorders: state of the science. CNS Spectr 2004;9:511-5. [DOI] [PubMed]