Abstract
Objective
Investigators have reported that weight gain attributed to clozapine is associated with its clinical response. However, weight gain is a nonspecific physiological variable that, in itself, does not explain the mechanism underlying this relation. Alternatively, other biological variables that are often associated with weight gain, such as serum lipids, may assist in explaining this observation. The primary objective of this study was to determine whether an increase in serum lipids is associated with improvement in schizophrenia symptoms during steady state treatment with clozapine.
Methods
The data for this study represent a subset of data from a randomized, double-blinded trial that evaluated subjects with schizophrenia who demonstrated a poor treatment response to clozapine. While continuing their clozapine therapy, subjects were randomly assigned to receive either risperidone 3 mg daily or placebo for 8 weeks. This course of treatment was followed by an optional (open-label) 18 weeks of augmentation with risperidone. In the present study, we included all subjects from the previously reported trial who had fasting lipid analyses and Positive and Negative Syndrome Scale (PANSS) scores from days 7 and 63 (n = 55). For the primary analyses, we used multiple regression to examine the association between serum lipid concentrations and PANSS scores, after controlling for weight.
Results
The analyses showed that the change in serum lipid concentration predicted change in symptoms over that of change in weight. Specifically, an increase in serum triglyceride concentration was associated with a decrease in the total PANSS score (p = 0.037). In addition, an increase in either serum total cholesterol concentration (p = 0.007), serum triglyceride concentration (p = 0.017) or their combined effect (p = 0.010) was associated with a decrease in PANSS negative subscale scores.
Conclusion
Elevation of serum lipids is associated with an improvement in schizophrenia symptoms in subjects treated with clozapine. Although the mechanism is unclear, serum lipids may play a role in influencing clozapine's therapeutic activity.
Medical subject headings: clozapine, triglycerides, cholesterol, lipids
Abstract
Objectif
Les chercheurs ont signalé que le gain de poids attribué à la clozapine est associé à sa réaction clinique. Toutefois, le gain de poids est une variable physiologique non spécifique qui, en soi, n'explique pas le mécanisme sous-tendant cette relation. D'autres variables biologiques qui sont souvent associées au gain de poids, comme les lipides sériques, peuvent aider à expliquer cette observation. Le principal objectif de cette étude consistait à déterminer si une augmentation des lipides sériques est associée à une amélioration des symptômes de la schizophrénie pendant le traitement en état stable à la clozapine.
Méthodes
Les données de cette étude représentent un sous-ensemble de données tirées d'une étude cliniquee randomisée, à double insu, dans le cadre de laquelle on a évalué des sujets atteints de schizophrénie qui démontraient une réaction médiocre au traitement à la clozapine. Les sujets ont été affectés au hasard au groupe recevant 3 mg de rispéridone par jour ou au groupe recevant un placebo pendant huit semaines tout en continuant leur traitement à la clozapine. Ce protocole de traitement a été suivi d'un traitement optionnel (ouvert) de 18 semaines où on augmentait la dose de rispéridone. Dans l'étude actuelle, nous avons inclus tous les sujets de l'étude clinique antérieure qui ont subi des analyses des lipides à jeun et avaient une note sur l'échelle des syndromes positifs et négatifs (Positive and Negative Syndrome Scale – PANSS) pour les jours 7 et 63 (n = 55). Pour les analyses primaires, nous avons eu recours à la régression multiple afin d'examiner le lien entre les concentrations de lipides sériques et les notes PANSS, après un contrôle du poids.
Résultats
Les analyses ont révélé que la variation de la concentration de lipides sériques a prédit un changement des symptômes par rapport à ceux d'un changement de poids. Plus particulièrement, une augmentation de la concentration de triglycéride sérique était associée à une diminution de la note totale PANSS (p = 0,037). De plus, une augmentation de la concentration totale de cholestérol sérique (p = 0,007), de la concentration de triglycéride sérique (p = 0,017) ou de leur effet combiné (p = 0,010) était associée à une diminution des notes PANSS négatives sur la sous-échelle.
Conclusion
: L'élévation des lipides sériques est associée à une amélioration des symptômes de la schizophrénie chez les sujets ayant subi un traitement à la clozapine. Bien que le mécanisme ne soit pas clair, les lipides sériques pourraient influencer l'activité thérapeutique de la clozapine.
Introduction
In 1994, the first documented account of elevated triglycerides associated with clozapine treatment was published as an isolated case report.1 The following year, Ghaeli and Dufresne published a case series that described how elevated serum triglyceride concentrations in 4 patients treated with clozapine were reduced upon switching to risperidone.2 In 2 of these patients, clozapine was reinitiated (after discontinuing risperidone) and serum triglyceride concentrations again increased. These reports are consistent with 4 larger retrospective chart reviews showing that treatment with clozapine was associated with a significant increase in mean serum triglyceride concentrations from baseline values (range 33.8%–48.1%).3–6 Similarly, in prospectively designed studies, patients treated with clozapine for 6 weeks (n = 14), 12 weeks (n = 8) and 52 weeks (n = 50) showed increases in serum triglyceride levels of 22.6%, 11.0% and 41.8%, respectively.7–9
It is possible that the above-noted increase in serum triglycerides could have influenced clozapine's pharmaco-logical activity. Rationale for this comes from studies demonstrating that interactions with plasma lipoproteins can modify the pharmacokinetics, tissue distribution and pharmacological activity of lipophilic drugs.10–13 Further, some clinical data suggest that serum triglycerides may be associated with clozapine's clinical effectiveness. First, circumstantial evidence comes from studies reporting that clozapine-associated weight gain is a good predictor of clinical response.14–18 Although serum triglycerides were not measured in these studies, there is a high probability of an increase in serum triglyceride concentrations with significant weight gain. This assumption only offers, at best, speculative evidence linking serum triglycerides to clinical response. More direct evidence, however, has been provided by the prospective study of 8 treatment-resistant individuals alluded to above.8 In this study, 6 women and 2 men with schizophrenia were tapered off their current antipsychotic medications and then started clozapine. After the 12-week study period, the investigators reported an 11% increase in serum triglyceride concentrations from baseline that coincided with a significant reduction in the Brief Psychiatry Rating Scale (BPRS) scores (i.e., baseline BPRS = 43.7, standard deviation [SD] 3.1, end point BPRS = 25.0, SD 3.9). No significant change was noted for total cholesterol, high-density lipoprotein (HDL) or low-density lipoprotein (LDL) concentrations in this study.
The above study suggests an association between an increase in serum triglyceride concentration (presumably secondary to clozapine) and treatment response. If serum triglycerides do influence the pharmacological activity of clozapine, then changes in its serum concentration by any factor (i.e., weight gain, diet, medication, illness) would also have an effect on clozapine's activity. Presumably, this would include normal biological fluctuations in serum triglycerides concentrations that occur over time.19,20 The primary hypothesis of the present study is that, during the course of steady-state clozapine treatment, an increase in serum triglyceride concentration, but not weight, is associated with improvement in the symptoms of schizophrenia. To test this hypothesis, we used data from a previously reported randomized controlled trial.21
Methods
Data for this study represent a subset of data from a multisite international randomized double-blind trial that evaluated subjects with schizophrenia who responded poorly to clozapine treatment (Fig. 1).21 In summary, subjects who were currently treated with clozapine with a stable dosage of 400 mg/d or more but who showed poor treatment response entered a 1-week phase of single-blind placebo augmentation. At day 7, subjects with an improvement in overall Positive and Negative Syndrome Scale (PANSS) score of 20% or greater were dropped from the protocol. All other subjects (n = 68) continued to take clozapine and were randomly assigned to receive 8 weeks of daily augmentation with risperidone or a placebo. Risperidone was administered as 1-mg tablets, and the dosage was increased to 3 mg per day over the first 15 days. Investigators were allowed to decrease the dose by 1 tablet per day if side effects were intolerable. Anticholinergic drugs were only allowed for the treatment of acute side effects. The primary outcome was reduction in the total score for severity of symptoms on the PANSS. Subjects with a 20% or greater reduction in total PANSS score were classified as responders. The double-blind phase was followed by an optional (open-label) 18 weeks of augmentation with risperidone.
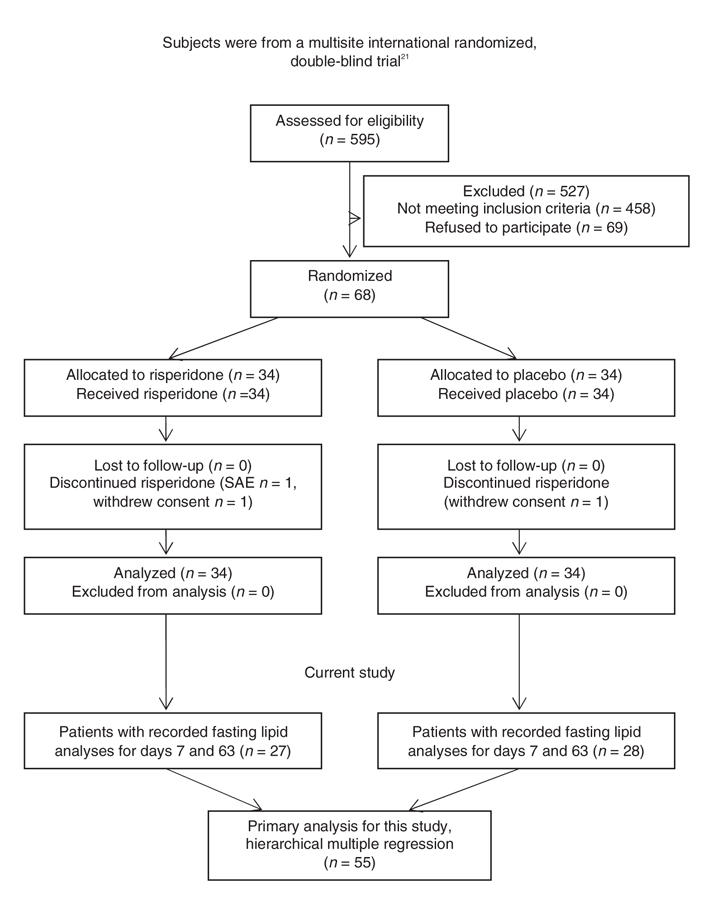
Fig. 1: Enrollment, allocation and analysis of patients. SAE = serious adverse event.
Outcome measures
In the present study, we examined the association between serum lipids, weight, severity of symptoms and serum concentrations of clozapine and norclozapine during the blinded phase of the original study. Fasting serum lipids were analyzed in our laboratory in a blinded fashion from samples taken on days 7, 63 and 189. The day 189 values were used only to calculate the biological coefficients of variation (CVb) in subjects with 3 lipid determinations (n = 31). We determined total cholesterol, triglyceride and HDL cholesterol with a previously published enzymatic assay method.22 These values were used to calculate LDL cholesterol concentrations by means of the Friedewald formula.23 Our laboratory has been approved and standardized by the Canadian Cholesterol Reference Method Laboratory Network.
Serum concentrations of clozapine and norclozapine were performed in the Department of Laboratory and Diagnostic Services at Riverview Hospital. Briefly, clozapine and norclozapine serum concentrations were determined by liquid chromatography-mass spectrometry after diluting serum with acetonitrile containing levorphanol as an internal standard. Primary ions used for quantification included 327 g/mol (clozapine), 313 g/mol (norclozapine) and 258 g/mol (levorphanol). The mobile phase used for the chromatography was 10 mmol/L aqueous ammonium acetate adjusted to pH 3.2 and acetonitrile (72:28). The column was a Zorbax SB-C8 (2.1 mm × 50 mm × 3.5 μm), (Agilent Technologies, Wilmington, Del.).
As in the original study, we measured the severity of symptoms with PANSS scores. We included all subjects from the preliminary trial with recorded fasting lipid analyses and PANSS scores from days 7 and 63 (Fig. 1). The raters were fluent in English, and standardized videotaped PANSS interviews were provided for training. Each centre was led by a university research psychiatrist. After describing the study to the subjects, we obtained written informed consent. The protocol received ethics approval from the institutional review board.
Data analysis
Owing to the small number of subjects in each augmentation subgroup (i.e., clozapine plus placebo versus clozapine plus risperidone), analyses were conducted on the entire sample. As previously reported,21 no difference in symptomatic improvement was noted between these augmentation subgroups. For the primary analyses, we used hierarchical multiple regression (SPSS version 14, Chicago, Ill.) to test the hypothesis that changes in serum lipid concentrations between days 7 and 63 would predict changes in PANSS total score and positive and negative scores after controlling or accounting for the change in weight. With the first step, the effect of weight change on PANSS scores was ascertained. Subsequently, we determined the effect of lipid change (additional to that contributed by weight). For step 2, the effects of change in serum triglyceride concentrations, change in serum total cholesterol concentrations and their combined effects were separately evaluated.
We also performed Mann–Whitney U analyses on the rankings of weight change and change in serum lipid concentrations for the responders versus the nonresponders. This nonparametric test was selected over the parametric alternative (i.e., t test) because of outlier observations, particularly in the nonresponder group. As in the original study, and consistent with current convention in trials of antipsychotic drugs, subjects with a 20% or greater reduction in total PANSS score were classified as responders. Further exploratory analyses were also performed to evaluate the associations between change in serum lipid concentrations against change in serum clozapine and norclozapine concentration.
Results
Of the 68 subjects randomized to double-blind treatment, 55 had adequate data (i.e., lipid analyses and PANSS scores for days 7 and 63) to be included in our analysis. Of these subjects, 28 were randomized to have their clozapine treatment augmented with placebo, while the remaining 27 were randomized to have their clozapine augmented with risperidone. Within each treatment group, the mean values of weight, clozapine dose, serum concentrations of triglycerides, total cholesterol, clozapine and norclozapine on day 7 and on day 63 did not differ significantly (Table 1). These measures also showed no significant differences between subjects randomized to risperidone or to placebo augmentation. We noted significant reductions in PANSS total score and positive and negative subscale scores from baseline in both the risperidone and the placebo augmentation treatment groups.
Table 1
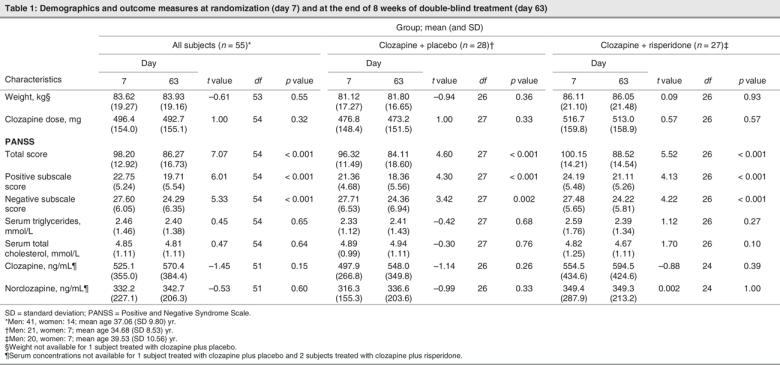
Change in lipids and symptoms
The primary analyses showed that increases in serum lipid concentration predicted antipsychotic response over that of weight change. Table 2 displays the unstandardized regression coefficients (B) and standardized regression coefficients (β) as well as the R2 and the ΔR2 for each step in the regression analyses. Weight change (step 1 in each analysis) was not associated with improvement in total, positive subscale or negative subscale PANSS scores (all p values > 0.100). Nonetheless, after entering weight change, an increase in serum lipid concentration (steps 2 a, b, c) predicted response. Specifically, an increase in serum triglyceride concentration accounted for approximately 8% of the variance of the decrease in the total PANSS score (p = 0.037). Conversely, change in serum total cholesterol (p = 0.162) or the overall change of serum lipid concentrations (p = 0.101; serum triglycerides and total serum cholesterol combined) failed to predict response according to total PANSS score. In the case of positive symptoms, lipid changes were not associated with outcome (all ps > 0.250). However, increases in either serum total cholesterol concentrations (p = 0.007), serum triglyceride concentrations (p = 0.017) or their combined effects (p = 0.010) were associated with decreases in PANSS negative subscale scores. Indeed, these lipid changes accounted for between 11% and 16% of the variance of the change in negative symptoms.
Table 2
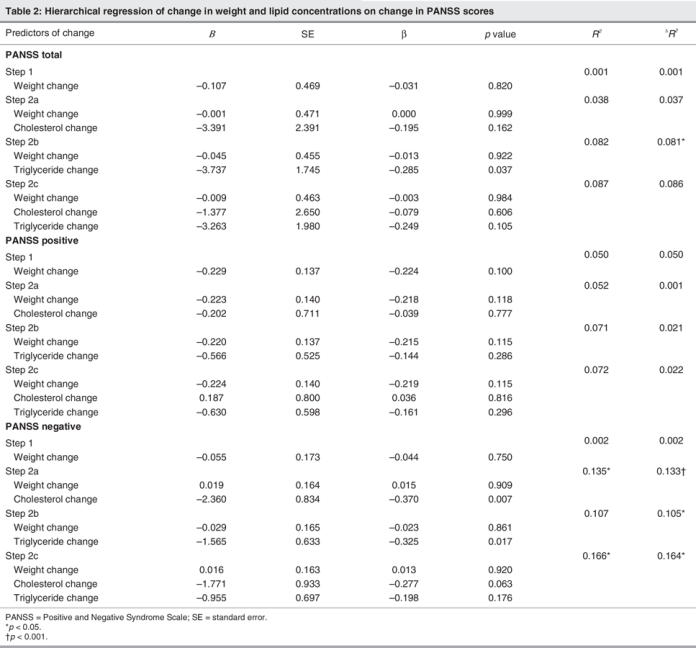
The regression coefficients further summarize these findings by providing estimates relevant to clinical response. Although none of the regression coefficients were significant in their prediction of change in positive PANSS scores, significant and important observations were evident for change in both negative and total PANSS scores (after controlling for weight change). Specifically, each 1 mmol/L increase in serum triglycerides predicted a 3.74 point decrease in total PANSS score (Table 2, see B top panel, Step 2b). Further, a 1 mmol/L increase in serum triglycerides predicted approximately a 1.60-point decrease in negative PANSS score (Table 2, see B bottom panel, Step 2b). Finally, each 1 mmol/L increase in serum total cholesterol predicted approximately a 2.36 reduction in negative PANSS score (Table 2, see B bottom panel, Step 2a). Despite being entered on step 1 in predication of the PANSS scores, the regression coefficients for weight change itself were all small and nonsignificant (see Table 2).
No correlations were noted between the change in serum triglyceride concentration and the change in clozapine (r = 0.21; t50 = 1.54, p = 0.13) or norclozapine (r = 0.13; t50 = 0.91, p = 0.37) concentration. Conversely, an increase in serum total cholesterol concentration correlated with an increase in norclozapine concentration (r = 0.35, t50 = 2.67, p = 0.01) but not with an increase in clozapine concentration (r = 0.24; t50 = 1.73, p = 0.09).
Of the 55 subjects in our primary analyses, 31 had blood drawn on day 189 during the optional (open-label) 18 weeks of augmentation with risperidone. Thus, the mean biological coefficients of variation for serum total cholesterol and triglyceride concentrations were calculated with 3 samples from each of the 31 subjects taken on days 7, 63 and 189. The mean CVb for serum total cholesterol and triglycerides were 8.3% and 20.8%, respectively.
Responders versus nonresponders
Responders and nonresponders differed in their ranking of change in serum triglyceride concentrations (U = 126.0, p = 0.004). Responders showed higher rankings of change in serum triglyceride concentrations (average increase of 21%; day 7: 2.56 mmol/L, SD 1.89, v. day 63: 3.11 mmol/L, SD 1.53), relative to nonresponders (average decrease of 10%; day 7: 2.43 mmol/L, SD 1.54, v. day 63: 2.18 mmol/L, SD 1.26). For change in serum total cholesterol concentration, a similar difference emerged (U = 139.5, p = 0.008), with responders showing higher rankings of change (average increase of 7%; day 7: 4.93 mmol/L, SD 0.72, v. day 63: 5.27 mmol/L, SD 0.86) versus nonresponders (average decrease of 4%; day 7: 4.83 mmol/L, SD 1.22, v. day 63: 4.66 mmol/L, SD 1.15). No difference in the ranking of weight change was observed between the groups (U = 247, p = 0.917).
Discussion
These results are the first to demonstrate that an increase in serum lipids, independent of weight, is associated with an improvement in schizophrenia symptoms during a randomized controlled trial with a stable dosage of clozapine. Consistent with our a priori hypothesis, our data show that an increase in serum triglyceride concentration is associated with an improvement in symptoms, as measured by a significant reduction in total PANSS score and the negative subscale score. In addition, our data also suggest that an increase in serum total cholesterol concentrations is associated with an improvement in negative symptoms.
Upon initial reflection, it would appear that our data do not support previous findings linking weight gain and clozapine14–18 (or, for that matter, olanzapine)24,25 to clinical response. However, when we take into account the temporal relation of when the data were collected to when clozapine treatment was initiated, all the reported findings are complementary. For example, the previous studies collected data before initiating clozapine and thus examined the relation of treatment response pre-and postclozapine. As expected, the subjects gained weight. An improvement in symptoms was also noted; thus the investigators made a valid association between weight gain attributed to clozapine and clinical response. However, weight gain is a nonspecific physiological variable that does not in itself enable one to explain the mechanism underlying this relation. Alternatively, weight gain may result in elevated serum lipids, which can independently modify the pharmacokinetics, tissue distribution and pharmacological activity of lipophilic drugs.10–13 We propose that an increase in serum lipids, which likely emerged secondary to the weight gain, was the variable responsible for the clinical improvements observed in these subjects treated with clozapine.
In the present study, our subjects had already received clozapine for an average of 161, SD 201, weeks before entering the trial. This being the case, it would be difficult to attribute any metabolic change observed in this study to clozapine alone. The fact that we did not observe a significant change in weight (Table 1) is consistent with this premise and was not unforeseen, because the preponderance of weight gain associated with clozapine treatment would have already occurred. Conversely, we did note intraindividual variations in serum lipids (presumably biologically mediated) among the subjects. In this case, the increase in serum lipid concentrations was associated with symptom improvement.
Our data also complement a case in which the change in serum lipid concentration had an impact on clozapine's clinical effectiveness.26 In this case, the initial improvement in the patient's symptoms coincided with an increase in serum lipids, presumably secondary to clozapine. Treating the dyslipidemia with atorvastatin normalized the serum lipids; however, the patient relapsed. Upon discontinuing the atorvastatin, a significant improvement in symptoms once again coincided with an increase in serum lipids. Taken together, this case and the data from the current study suggest that fluctuations in the serum lipid concentration (from whatever cause) could be responsible for some of the variations in symptoms over time in schizophrenia patients who are treated with clozapine.
We believe that the changes in serum lipid concentrations seen in this study reflect naturally occurring intraindividual biological variations. It is well known that serum lipid concentrations fluctuate considerably within individuals over relatively short periods of time. Attributed variables include intrinsic factors (i.e., hormonal variation and illness),27,28 extrinsic factors (i.e., diet)29 and biological factors.19,20 The mechanism explaining this biological variation in serum lipid concentrations lies in intrinsic factors related to their biosynthesis and tissue use, as regulated by genetic factors and their interactions with extrinsic factors.30 According to a metaanalysis of 30 studies published between 1970 and 1992,31 the CVb for serum total cholesterol and triglyceride concentrations are 6.1% and 22.6%, respectively. Our calculated CVb for serum total cholesterol (8.3%) and triglyceride (20.8%) concentrations are in-line with these values.
To explain the association between serum lipid concentration and symptoms, we consider 3 possibilities. First, it is possible that serum lipids may have a direct effect on symptoms independent of clozapine. In this regard, there is evidence that lowering serum cholesterol levels may decrease central serotonin receptor function, possibly through a membrane effect.32 This finding led to the hypothesis that low serum cholesterol levels might contribute to treatment resistance in patients with schizophrenia, conceivably by decreasing central serotonin function.33–35 Second, it may be that serum lipids are a marker of the effectiveness of clozapine but have no effect themselves. This could be due to the rapid change in insulin sensitivity and lipids that can occur from a metabolic change in intraabdominal fat, independent of other adipose tissue stores. This is, however, unlikely, because insulin resistance occurs early in therapy, and our subjects had been on clozapine for some time at the beginning of the study. A third possibility is that serum lipids enhance the therapeutic efficacy of clozapine.
We propose 3 possible mechanisms by which serum lipids could influence the effectiveness of clozapine. The first mechanism hypothesizes that serum lipids create a physiological depot for clozapine within the lipoprotein fraction of serum. We suggest this based on data from our previous in vitro study that examined the effects of dyslipidemia on the serum distribution of clozapine.36 In this study, we incubated [3H]clozapine plus cold clozapine in serum samples with varying total cholesterol, lipoprotein cholesterol and triglyceride concentrations. After incubation, the serum was separated into its lipoprotein and lipoprotein-deficient fractions by density gradient ultracentrifugation, and clozapine distribution was determined. Our findings revealed a positive correlation (r22 = 0.98, p < 0.05) between serum triglyceride concentrations and clozapine recovery within the very low-density lipoprotein (VLDL) fraction. That is, clozapine redistributed itself from the lipoprotein deficient fraction (where it is primarily bound to albumin and α1-acid glycoprotein) to the VLDL fraction as serum triglyceride concentrations increased. This partitioning of clozapine into the VLDL fraction of serum would almost certainly modify its pharmacokinetic profile and potentially its efficacy. One could therefore speculate that a “physiological depot” for clozapine is created in vivo by lipids and that clozapine is being released from the triglyceride-rich VLDL fraction of serum in a sustained manner. In turn, this release of clozapine could improve its efficacy in a manner that is comparable with other depot antipsychotic medications.37
The second mechanism hypothesizes that a partitioning of clozapine into the lipoprotein fraction of serum results in an increase in the free unbound (active) concentration of clozapine, norclozapine or both. We have shown that an increase in serum total cholesterol concentration correlates with an increase in norclozapine concentration (r = 0.35; t50 = 2.67; p = 0.01). We cannot confirm that this positive correlation represents an increase in free unbound norclozapine because our reported drug concentrations represented total (bound and unbound) concentration. Even if there is an increase in the concentration of free clozapine, norclozapine or both, this would only represent peripheral concentrations. Further, we did not find a correlation between changes in clozapine or norclozapine concentrations and changes in symptoms, as measured by PANSS scores (data not shown).
A third mechanism hypothesizes that the redistribution of clozapine into the lipoprotein fraction improves its ability to cross the blood–brain barrier. The blood–brain barrier is made of lipophilic endothelial cells and modulates the passage of compounds from the blood into the brain's interstitial tissues. The rate of transport of compounds across this physiological barrier is dependent on molecular size, density and the lipophilic nature of the agent.38 It is conceivable that clozapine's association with the lipoprotein fraction of human serum facilitates its penetration across the blood–brain barrier either by passive diffusion or by a receptor-mediated process. Alternatively, the mechanism by which serum lipids influence clozapine's therapeutic response may be a combination of the above hypotheses.
In conclusion, these are the first results to demonstrate that an increase in serum lipid concentration, independent of weight, is associated with symptom improvement in people with schizophrenia who are treated with clozapine. A limitation of our study is that not all subjects were taking clozapine monotherapy (27 of our 55 subjects were treated with a combination of clozapine and risperidone). This raises the possibility that risperidone might have contributed to our finding. However, we performed exploratory analyses, including repeated measures, on the subgroups and ruled out this possibility (data are on file). Clearly, the present findings are from a randomized clinical trial designed for purposes other than testing hypotheses related to the effects of lipids on clinical response to clozapine. Larger naturalistic studies will help confirm these observations. Another potential limitation is that multiple comparisons used to test our null hypotheses increase the potential for type I errors. Nonetheless, this study highlights the possibility that clozapine's pharmacological and thus therapeutic activity is associated with serum lipids. Recognizing the health consequences associated with elevated serum lipids, the implications of this study will almost certainly evoke debate regarding the clinical management of patients. As such, the relation between serum lipids and clozapine, as well as other antipsychotic drugs, requires further investigation.
Acknowledgments
Supported by a grant from the Stanley Medical Research Institute. Risperidone and placebo were provided by Janssen-Ortho, Canada. Additional support was provided by the British Columbia Mental Health and Addictions Services and the Michael Smith Foundation for Health Research. We thank Dr. Jiri Frolich, MD, University of British Columbia, for his consultation and review of the manuscript and Dr. Raymond Koopman, Simon Fraser University, for his stastical consultation. The following institutions and investigators participated in the Clozapine and Risperidone Enhancement Study Group: Castle Peak Hospital, Hong Kong, China: S.P. Leung and J.O.Y Wong; Centre de Recherche Fernand-Seguin, Montréal: J.P. Rodriguez, P. Lalonde and E. Stip; Fulbourn Hosptial, Cambridge, UK: E. Pomarol-Clotet and P.J. McKenna; Peace Arch Hospital, White Rock, B.C; G.W. MacEwan; Queen Mary Hospital, Hong Kong, China: E.Y.H. Chen and R.C.K. Chan; Riverview Hospital, Port Coquitlam, BC: S.W. Flynn, S. Altman, W.G. Honer, A.E. Thornton, R. M. Procyshyn, S. Huckin, T. Au, V. Boudreau and B. Humphries; Royal Jubilee Hospital, Victoria, BC: R. Williams; University of British Columbia, Vancouver, BC: K. Wasan; University Clinic, Hamburg, Germany: A. Bergmann, P. Falkai, T. Wobrock and H. Wollny.
Footnotes
Contributors: Drs. Procyshyn, Wasan, Thornton, Chen, Stip, Williams and Honer designed the study. Drs. Wasan, Chen, Pomarol-Clotet, Stip, Williams, MacEwan and Honer acquired the data, which Drs. Procyshyn, Wasan, Thornton, Barr, Stip, Birmingham and Honer analyzed. Drs. Procyshyn, Thornton, Stip, Birmingham and Honer wrote the article, and Drs. Procyshyn, Wasan, Thornton, Barr, Chen, Pomarol-Clotet, Stip, Willia ms, MacEwan and Honer revised it. All authors gave final approval for the article to be published.
Competing interests: Dr. Procyshyn has received consulting or advisory board fees and lecture fees from AstraZeneca, GlaxoSmithKline, Janssen and Eil Lilly. Dr. Chen has recieved grant funding from AstraZeneca. Dr. Stip has recieved consulting or advisory board fees from AstraZeneca, Janssen and Eli Lilly; Pfizer lecture fees from AstraZeneca, Janssen and Eli Lilly; and grant funding from AstraZeneca. Dr. Williams has received consulting or advisory board fees from AstraZeneca, Genpharm, Janssen, Eli Lilly and Prestwick Pharmaceuticals; lecture fees from AstraZeneca, Genpharm, Janssen, Eli Lilly, Novartis, Pfizer and Prestwick Pharmaceuticals; and grant funding from AstraZeneca, Janssen and Pfizer. Dr. MacEwan has received consulting or advisory board fees from AstraZeneca, Janssen, Eli Lilly and Novartis; lecture fees from GlaxoSmithKline; and grant support from AstraZeneca. Dr. Honer has received consulting or advisory board fees from In Silico Biosciences, Janssen, and Wyeth and lecture fees from AstraZeneca.
Correspondence to: Dr. Ric M. Procyshyn, 128 Administration Building, Riverview Hospital, 2601 Lougheed Highway, Coquitlam BC V3C 4J2; fax 604 524-7800; rprocyshyn@bcmhs.bc.ca
References
- 1.Vampini C, Steinmayr M, Bilone F. The increase of plasma levels of triglyceride during clozapine treatment: a case report [abstract]. Neuropsychopharmacology 1994;10:249s.
- 2.Ghaeli P, Dufresne RI. Elevated serum triglycerides on clozapine resolve with risperidone [abstract]. Pharmacotherapy 1995;15:382-3. [DOI] [PubMed]
- 3.Gaulin BD, Markowitz JS, Caley DF, et al. Clozapine-associated elevation in serum triglycerides. Am J Psychiatry 1999;156:1270-2. [DOI] [PubMed]
- 4.Spivak B, Lamschtein C, Talmon Y, et al. The impact of clozapine treatment on serum lipids in chronic schizophrenic patients. Clin Neuropharmacol 1999;22:98-101. [DOI] [PubMed]
- 5.Henderson DC, Cagliero E, Gray C, et al. Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: a five-year naturalistic study. Am J Psychiatry 2000;157:975-81. [DOI] [PubMed]
- 6.Wirshing DA, Boyd JA, Meng LR, et al. The effects of novel antipsychotics on glucose and lipid levels. J Clin Psychiatry 2002; 63: 856-65. [DOI] [PubMed]
- 7.Atmaca M, Kuloglu M, Tezcan E, et al. Serum leptin and triglyceride levels in patients on treatment with atypical antipsychotics. J Clin Psychiatry 2003;64:598-604. [DOI] [PubMed]
- 8.Dursun SM, Szemis A, Andrews H, et al. The effects of clozapine on levels of total cholesterol and related lipids in serum of patients with schizophrenia: a prospective study. J Psychiatry Neurosci 1999; 24: 453-5. [PMC free article] [PubMed]
- 9.Baymiller SP, Ball P, McMahon RP, et al. Serum glucose and lipid changes during the course of clozapine treatment: the effect of concurrent beta-adrenergic antagonist treatment. Schizophr Res 2003; 59:49-57. [DOI] [PubMed]
- 10.Lemaire M, Tillement JP. Role of lipoproteins and erythrocytes in the in vitro binding and distribution of cyclosporin A in the blood. J Pharm Pharmacol 1982;34:715-8. [DOI] [PubMed]
- 11.Brajtburg J, Elberg S, Boland J, et al. Interaction of plasma proteins and lipoproteins with amphotericin B. J Infect Dis 1984;149:986-92. [DOI] [PubMed]
- 12.Brunner LJ, Vadiei K, Luke DR. Cyclosporine disposition in the hyperlipidemic rat model. Res Commun Chem Pathol Pharmacol 1988; 59:339-48. [PubMed]
- 13.Wasan KM. Modifications in plasma lipoprotein concentration and lipid composition regulate the biological activity of hydrophobic drugs. J Pharmacol Toxicol Methods 1996;36:1-11. [DOI] [PubMed]
- 14.Leadbetter R, Shutty M, Pavalonis D, et al. Clozapine-induced weight gain: prevalence and clinical relevance. Am J Psychiatry 1992; 149:68-72. [DOI] [PubMed]
- 15.Lamberti JS, Bellnier T, Schwarzkopf SB. Weight gain among schizophrenic patients treated with clozapine. Am J Psychiatry 1992; 149:689-90. [DOI] [PubMed]
- 16.Bai YM, Lin CC, Chen JY, et al. Weight gain among patients on clozapine [letter]. Psychiatr Serv 1999;50:704-5. [DOI] [PubMed]
- 17.Czobor P, Volavka J, Sheitman B, et al. Antipsychotic-induced weight gain and therapeutic response: a differential association. J Clin Psychopharmacol 2002;22:244-51. [DOI] [PubMed]
- 18.Meltzer HY, Perry E, Jayathilake K. Clozapine-induced weight gain predicts improvement in psychopathology. Schizophr Res 2003; 59:19-27. [DOI] [PubMed]
- 19.Durrington PN. Biological variation in serum lipid concentrations. Scand J Clin Lab Invest Suppl 1990;198:86-91. [PubMed]
- 20.Marcovina SM, Gaur VP, Albers JJ. Biological variability of cholesterol, triglyceride, low-and high-density lipoprotein cholesterol, lipoprotein(a), and apolipoproteins A-1 and B. Clin Chem 1994; 40:574-8. [PubMed]
- 21.Honer WG, Thornton AE, Chen EYH, et al. Clozapine alone versus clozapine and risperidone with refractory schizophrenia. N Engl J Med 2006;354:472-82. [DOI] [PubMed]
- 22.Wasan KM, Ramaswamy M, Haley J, et al. Administration of long-term tamoxifen therapy modifies the plasma lipoprotein-lipid concentration and lipid transfer protein I activity in postmenopausal women with breast cancer. J Pharm Sci 1997;86:876-9. [DOI] [PubMed]
- 23.Demacker PN, Hijmans AG, Brenninkmeijer BJ, et al. Five methods for determining low-density lipoprotein cholesterol compared. Clin Chem 1984;30:1797-800. [PubMed]
- 24. Ascher-Svanum H, Stensland MD, Kinon BJ, et al. Weight gain as a prognostic indicator of therapeutic improvement during acute treatment of schizophrenia with placebo or active antipsychotic. J Psychopharmacol 2005;19(6 Suppl);110-7. [DOI] [PubMed]
- 25.Ascher-Svanum H, Stensland M, Zhao Z, et al. Acute weight gain, gender, and therapeutic response to antipsychotics in the treatment of patients with schizophrenia. BMC Psychiatry 2005;5:3. Available: www.biomedcentral.com/1471-244X/5/3 (accessed 2006 March 29). [DOI] [PMC free article] [PubMed]
- 26.Pande S, Procyshyn RM, Nazerali M, et al. Do triglycerides modulate the effectiveness of clozapine? Int Clin Psychopharmacol 2002; 17: 197-9. [DOI] [PubMed]
- 27.Alvarez C, Ramos A. Lipids, lipoproteins, and apoproteins in serum during infection. Clin Chem 1986;32:142-5. [PubMed]
- 28.Tonolo G, Ciccarese M, Brizzi P, et al. Cyclical variation of plasma lipids, apolipoproteins, and lipoprotein(a) during menstrual cycle of normal women. Am J Physiol 1995;269:E1101-5. [DOI] [PubMed]
- 29.O'Hanesian MA, Rosner B, Bishop LM, et al. Effects of inherent responsiveness to diet and day-to-day diet variation on plasma lipoprotein concentrations. Am J Clin Nutr 1996;64:53-9. [DOI] [PubMed]
- 30.Pereira MA, Weggemans RM, Jacobs DR, et al. Within-person variation in serum lipids: implications for clinical trials. Int J Epidemiol 2004; 33: 534-41. [DOI] [PubMed]
- 31.Smith SJ, Cooper GR, Myers GL, et al. Biological variability in concentrations of serum lipids: sources of variation among results from published studies and composite predicted values. Clin Chem 1993;39:1012-22. [PubMed]
- 32.Hawthon K, Cowen P, Owens D, et al. Low serum cholesterol and suicide. Br J Psychiatry 1993;162:818-25. [DOI] [PubMed]
- 33.Dursun SM, Reveley MA. Low serum cholesterol and serotonin receptor subtypes. [Letter]. Br J Psychiatry 1993;163:417-8. [DOI] [PubMed]
- 34.Boston PF, Dursun SM, Zafar R, et al. Serum cholesterol and treatment-resistance in schizophrenia. Biol Psychiatry 1996;40:542-3. [DOI] [PubMed]
- 35.Boston PF, Dursun SM, Reveley MA. Cholesterol and mental disorder. Br J Psychiatry 1996;169:682-9. [DOI] [PubMed]
- 36.Procyshyn RM, Kennedy NB, Marriage S, et al. Plasma protein and lipoprotein distribution of clozapine. Am J Psychiatry 2001;158:949-51. [DOI] [PubMed]
- 37.Davis JM, Matalon L, Watanabe MD, et al. Depot antipsychotic drugs: place in therapy. Drugs 1994;47:741-73. [DOI] [PubMed]
- 38.Machard B, Missline P, Lemaire M. Influence of plasma protein binding on the brain re-uptake of an antifungal agent, terbinafine, in rats. J Pharm Pharmacol 1989;41:700-4. [DOI] [PubMed]


