Abstract
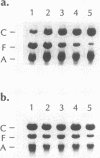
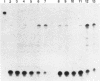
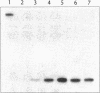
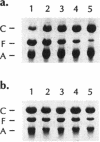
micF RNA, produced from a multicopy plasmid, was originally shown to be a major factor in negative osmoregulation of the OmpF outer membrane protein in Escherichia coli. However, subsequent experiments with a micF deletion strain suggested that chromosomal micF RNA was not a key component in this process. We report here that micF RNA is essential for the reduction in OmpF levels in cells grown in media of low-to-intermediate levels of osmolarity. Under these conditions, the amount of OmpF was reduced up to 60% in the parent strain while OmpF levels were not altered in the micF deletion mutant. In medium of higher osmolarity, OmpF synthesis was strongly inhibited in both strains. RNA measurements showed that micF RNA levels rose rapidly in cells grown in low-to-intermediate levels of osmolarity concomitant with the reduction in OmpF protein, while ompF mRNA decreased strongly only during high-osmolarity conditions. Taken together, these results strongly suggest that the negative osmoregulation of OmpF at low-to-intermediate osmolarity levels requires micF RNA and that this is masked at higher osmolarity by the known strong inhibition of OmpF transcription by OmpR. Results consistent with this model were also obtained by using procaine, a compound reported to inhibit ompF expression by a mechanism very similar to that involved in osmoregulation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Matsuyama S., Mizuno T., Mizushima S. Function of micF as an antisense RNA in osmoregulatory expression of the ompF gene in Escherichia coli. J Bacteriol. 1987 Jul;169(7):3007–3012. doi: 10.1128/jb.169.7.3007-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba H., Mizuno T. Phosphorylation of a bacterial activator protein, OmpR, by a protein kinase, EnvZ, stimulates the transcription of the ompF and ompC genes in Escherichia coli. FEBS Lett. 1990 Feb 12;261(1):19–22. doi: 10.1016/0014-5793(90)80626-t. [DOI] [PubMed] [Google Scholar]
- Aiba H., Nakasai F., Mizushima S., Mizuno T. Evidence for the physiological importance of the phosphotransfer between the two regulatory components, EnvZ and OmpR, in osmoregulation in Escherichia coli. J Biol Chem. 1989 Aug 25;264(24):14090–14094. [PubMed] [Google Scholar]
- Alphen W. V., Lugtenberg B. Influence of osmolarity of the growth medium on the outer membrane protein pattern of Escherichia coli. J Bacteriol. 1977 Aug;131(2):623–630. doi: 10.1128/jb.131.2.623-630.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J., Delihas N., Ikenaka K., Green P. J., Pines O., Ilercil O., Inouye M. The isolation and characterization of RNA coded by the micF gene in Escherichia coli. Nucleic Acids Res. 1987 Mar 11;15(5):2089–2101. doi: 10.1093/nar/15.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J., Delihas N. micF RNA binds to the 5' end of ompF mRNA and to a protein from Escherichia coli. Biochemistry. 1990 Oct 2;29(39):9249–9256. doi: 10.1021/bi00491a020. [DOI] [PubMed] [Google Scholar]
- Andersen J., Forst S. A., Zhao K., Inouye M., Delihas N. The function of micF RNA. micF RNA is a major factor in the thermal regulation of OmpF protein in Escherichia coli. J Biol Chem. 1989 Oct 25;264(30):17961–17970. [PubMed] [Google Scholar]
- Chou J. H., Greenberg J. T., Demple B. Posttranscriptional repression of Escherichia coli OmpF protein in response to redox stress: positive control of the micF antisense RNA by the soxRS locus. J Bacteriol. 1993 Feb;175(4):1026–1031. doi: 10.1128/jb.175.4.1026-1031.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., McMurry L. M., Levy S. B. marA locus causes decreased expression of OmpF porin in multiple-antibiotic-resistant (Mar) mutants of Escherichia coli. J Bacteriol. 1988 Dec;170(12):5416–5422. doi: 10.1128/jb.170.12.5416-5422.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J., Green P. J., Inouye M. The use of RNAs complementary to specific mRNAs to regulate the expression of individual bacterial genes. Cell. 1984 Jun;37(2):429–436. doi: 10.1016/0092-8674(84)90373-8. [DOI] [PubMed] [Google Scholar]
- Coyer J., Andersen J., Forst S. A., Inouye M., Delihas N. micF RNA in ompB mutants of Escherichia coli: different pathways regulate micF RNA levels in response to osmolarity and temperature change. J Bacteriol. 1990 Aug;172(8):4143–4150. doi: 10.1128/jb.172.8.4143-4150.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S., Comeau D., Norioka S., Inouye M. Localization and membrane topology of EnvZ, a protein involved in osmoregulation of OmpF and OmpC in Escherichia coli. J Biol Chem. 1987 Dec 5;262(34):16433–16438. [PubMed] [Google Scholar]
- Forst S., Delgado J., Inouye M. Phosphorylation of OmpR by the osmosensor EnvZ modulates expression of the ompF and ompC genes in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6052–6056. doi: 10.1073/pnas.86.16.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S., Delgado J., Rampersaud A., Inouye M. In vivo phosphorylation of OmpR, the transcription activator of the ompF and ompC genes in Escherichia coli. J Bacteriol. 1990 Jun;172(6):3473–3477. doi: 10.1128/jb.172.6.3473-3477.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granett S., Villarejo M. Regulation of gene expression in Escherichia coli by the local anesthetic procaine. J Mol Biol. 1982 Sep 15;160(2):363–367. doi: 10.1016/0022-2836(82)90181-4. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the ompB locus in Escherichia coli K-12. J Mol Biol. 1981 Sep 5;151(1):1–15. doi: 10.1016/0022-2836(81)90218-7. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. The ompB locus and the regulation of the major outer membrane porin proteins of Escherichia coli K12. J Mol Biol. 1981 Feb 15;146(1):23–43. doi: 10.1016/0022-2836(81)90364-8. [DOI] [PubMed] [Google Scholar]
- Heyde M., Portalier R. Regulation of major outer membrane porin proteins of Escherichia coli K 12 by pH. Mol Gen Genet. 1987 Jul;208(3):511–517. doi: 10.1007/BF00328148. [DOI] [PubMed] [Google Scholar]
- Huang L., Tsui P., Freundlich M. Integration host factor is a negative effector of in vivo and in vitro expression of ompC in Escherichia coli. J Bacteriol. 1990 Sep;172(9):5293–5298. doi: 10.1128/jb.172.9.5293-5298.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igo M. M., Ninfa A. J., Silhavy T. J. A bacterial environmental sensor that functions as a protein kinase and stimulates transcriptional activation. Genes Dev. 1989 May;3(5):598–605. doi: 10.1101/gad.3.5.598. [DOI] [PubMed] [Google Scholar]
- Igo M. M., Ninfa A. J., Stock J. B., Silhavy T. J. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 1989 Nov;3(11):1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Constitutive and conditional suppression of exogenous and endogenous genes by anti-sense RNA. Science. 1985 Jul 26;229(4711):345–352. doi: 10.1126/science.2990048. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Peters R., Bernheimer H., Berendsen W. Influence of cultural conditions and mutations on the composition of the outer membrane proteins of Escherichia coli. Mol Gen Genet. 1976 Sep 23;147(3):251–262. doi: 10.1007/BF00582876. [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Mizushima S. Construction and characterization of a deletion mutant lacking micF, a proposed regulatory gene for OmpF synthesis in Escherichia coli. J Bacteriol. 1985 Jun;162(3):1196–1202. doi: 10.1128/jb.162.3.1196-1202.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A. Injected anti-sense RNAs specifically block messenger RNA translation in vivo. Proc Natl Acad Sci U S A. 1985 Jan;82(1):144–148. doi: 10.1073/pnas.82.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra R., Reeves P. R. Role of micF in the tolC-mediated regulation of OmpF, a major outer membrane protein of Escherichia coli K-12. J Bacteriol. 1987 Oct;169(10):4722–4730. doi: 10.1128/jb.169.10.4722-4730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Chou M. Y., Inouye M. A unique mechanism regulating gene expression: translational inhibition by a complementary RNA transcript (micRNA). Proc Natl Acad Sci U S A. 1984 Apr;81(7):1966–1970. doi: 10.1073/pnas.81.7.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Kageyama M. Separation and characterization of the outer membrane of Pseudomonas aeruginosa. J Biochem. 1978 Jul;84(1):179–191. doi: 10.1093/oxfordjournals.jbchem.a132106. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Kato M., Jo Y. L., Mizushima S. Interaction of OmpR, a positive regulator, with the osmoregulated ompC and ompF genes of Escherichia coli. Studies with wild-type and mutant OmpR proteins. J Biol Chem. 1988 Jan 15;263(2):1008–1012. [PubMed] [Google Scholar]
- Norioka S., Ramakrishnan G., Ikenaka K., Inouye M. Interaction of a transcriptional activator, OmpR, with reciprocally osmoregulated genes, ompF and ompC, of Escherichia coli. J Biol Chem. 1986 Dec 25;261(36):17113–17119. [PubMed] [Google Scholar]
- Pugsley A. P., Conrard D. J., Schnaitman C. A., Gregg T. I. In vivo effects of local anesthetics on the production of major outer membrane proteins by Escherichia coli. Biochim Biophys Acta. 1980 Jun 20;599(1):1–12. doi: 10.1016/0005-2736(80)90051-6. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan G., Ikenaka K., Inouye M. Uncoupling of osmoregulation of the Escherichia coli K-12 ompF gene from ompB-dependent transcription. J Bacteriol. 1985 Jul;163(1):82–87. doi: 10.1128/jb.163.1.82-87.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampersaud A., Inouye M. Procaine, a local anesthetic, signals through the EnvZ receptor to change the DNA binding affinity of the transcriptional activator protein OmpR. J Bacteriol. 1991 Nov;173(21):6882–6888. doi: 10.1128/jb.173.21.6882-6888.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Ausubel F. M. Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell. 1987 Jun 5;49(5):579–581. doi: 10.1016/0092-8674(87)90530-7. [DOI] [PubMed] [Google Scholar]
- Rosner J. L., Chai T. J., Foulds J. Regulation of ompF porin expression by salicylate in Escherichia coli. J Bacteriol. 1991 Sep;173(18):5631–5638. doi: 10.1128/jb.173.18.5631-5638.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo F. D., Slauch J. M., Silhavy T. J. Mutations that affect separate functions of OmpR the phosphorylated regulator of porin transcription in Escherichia coli. J Mol Biol. 1993 May 20;231(2):261–273. doi: 10.1006/jmbi.1993.1281. [DOI] [PubMed] [Google Scholar]
- Sato T., Yura T. Chromosomal location and expression of the structural gene for major outer membrane protein Ia of Escherichia coli K-12 and of the homologous gene of Salmonella typhimurium. J Bacteriol. 1979 Aug;139(2):468–477. doi: 10.1128/jb.139.2.468-477.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A., McDonald G. A. Regulation of outer membrane protein synthesis in Escherichia coli K-12: deletion of ompC affects expression of the OmpF protein. J Bacteriol. 1984 Aug;159(2):555–563. doi: 10.1128/jb.159.2.555-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons R. W., Kleckner N. Biological regulation by antisense RNA in prokaryotes. Annu Rev Genet. 1988;22:567–600. doi: 10.1146/annurev.ge.22.120188.003031. [DOI] [PubMed] [Google Scholar]
- Slauch J. M., Silhavy T. J. Genetic analysis of the switch that controls porin gene expression in Escherichia coli K-12. J Mol Biol. 1989 Nov 20;210(2):281–292. doi: 10.1016/0022-2836(89)90330-6. [DOI] [PubMed] [Google Scholar]
- Taylor R. K., Hall M. N., Silhavy T. J. Isolation and characterization of mutations altering expression of the major outer membrane porin proteins using the local anaesthetic procaine. J Mol Biol. 1983 May 25;166(3):273–282. doi: 10.1016/s0022-2836(83)80085-0. [DOI] [PubMed] [Google Scholar]
- Tokishita S., Yamada H., Aiba H., Mizuno T. Transmembrane signal transduction and osmoregulation in Escherichia coli: II. The osmotic sensor, EnvZ, located in the isolated cytoplasmic membrane displays its phosphorylation and dephosphorylation abilities as to the activator protein, OmpR. J Biochem. 1990 Sep;108(3):488–493. doi: 10.1093/oxfordjournals.jbchem.a123226. [DOI] [PubMed] [Google Scholar]
- Tsung K., Brissette R. E., Inouye M. Identification of the DNA-binding domain of the OmpR protein required for transcriptional activation of the ompF and ompC genes of Escherichia coli by in vivo DNA footprinting. J Biol Chem. 1989 Jun 15;264(17):10104–10109. [PubMed] [Google Scholar]
- Villarejo M., Case C. C. envZ mediates transcriptional control by local anesthetics but is not required for osmoregulation in Escherichia coli. J Bacteriol. 1984 Sep;159(3):883–887. doi: 10.1128/jb.159.3.883-887.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Alphen L., Lugtenberg B., van Boxtel R., Hack A. M., Verhoef C., Havekes L. meoA is the structural gene for outer membrane protein c of Escherichia coli K12. Mol Gen Genet. 1979 Jan 31;169(2):147–155. doi: 10.1007/BF00271665. [DOI] [PubMed] [Google Scholar]