Abstract
β-Glucan elicitor (GE), released from the cell wall of the phytopathogenic fungus Phytophthora megasperma by soybean glucanases, causes defense reactions in soybean. A GE-binding protein (GEBP) was purified from the membrane fraction of soybean root cells, and its cDNA was isolated. Expression of the cDNA clone in tobacco suspension cultured cells and in Escherichia coli conferred GE-binding activity to both. An antibody against the recombinant protein was found to inhibit the GE binding with the soybean cotyledon membrane fraction as well as the resulting accumulation of phytoalexin. Immunolocalization assays indicated that the GEBPs are located in the plasma membrane of root cells. These results suggest that the cDNA encodes a GE receptor and may mediate the signaling of the elicitor.
Plants defend themselves from infection by invasive phytopathogenic fungi by a combination of constitutive as well as induced defenses such as phytoalexin accumulation; the hypersensitive reaction; and the production of chitinase, glucanase, and polygalacturonase inhibitor (1). Certain defense reactions are elicited by compounds referred to as elicitors, such as oligosaccharides, proteins, and glycoproteins released from fungal and plant cell walls (reviewed in refs. 2–4). Specific gene activation is involved in many of these defense reactions (5–7). The molecular mechanism of the elicitor signal transduction pathway in plants is not yet understood. However, it is likely that elicitors bind to targets, presumably receptors on the plant plasma (microsomal) membranes, and trigger the signaling events necessary for the onset of the defense response (8–12).
The interaction between the soybean Glycine max L. and the β-glucan elicitor (GE) from the phytopathogenic fungus Phytophthora megasperma f. sp. glycinea is one of the best-characterized. The first event between the soybean and fungus is an attack of the fungal cell wall by soybean β-1,3-glucanase, resulting in the release of active GEs, which then initiate phytoalexin accumulation in plants (13–15). The structure of the GE was proposed to be a β-1,6-linked glucan backbone of varying length with frequent side branches composed of one or two β-1,3-linked glucose moieties, as determined from 1H and 13C NMR analysis, methylation analysis, and degradation studies employing endo- and exoglucanases (16). The smallest elicitor-active β-glucan has been determined (hepta-β-glucoside) (17), and the chemically synthesized elicitor was reported not only to induce phytoalexin accumulation but also to have a high affinity for the plasma membrane fraction (8, 18, 19). These reports strongly suggested that these GEs bind to a receptor(s) on the plasma membrane of soybean, resulting in the accumulation of phytoalexins. The purification of hepta-β-glucoside-binding proteins from soybean root plasma membrane has been reported, but as yet no amino acid sequences have been described (20, 21). This paper describes the purification, cloning, and characterization of a GE-binding protein (GEBP) that may recognize the fungal elicitor and act as a signal transducer.
MATERIALS AND METHODS
Plant Material.
Soybean (G. max cv. Green Homer) seeds were purchased from Takayama Seed Co. (Kyoto). The tobacco suspension cell BY-2 was kindly provided by Japan Tobacco Inc. (Tokyo).
Preparation of the GE.
Race 1 of P. megasperma f. sp. glycinea was grown at 25°C for 7 days (22), and the mycelial wall was isolated (14). The cell wall was digested with β-1,3-glucanase to obtain β-glucan polymer mixtures (16, 23). They were further fractionated and purified by gel filtration (Sephadex G-75). The elicitor fraction, whose average molecular mas was 10,000 Da, was rechromatographed by G-75. The GE tyramine conjugate was prepared by reductive amination (24, 25). A total of 42.5 μg of 125I-labeled GE tyramine conjugate (1.6 × 105 Ci/mmol; 1 Ci = 37 GBq) was obtained.
Elicitor Activity.
Elicitor activity was defined as accumulation of most phytoalexins in the soybean cotyledon (22, 26). The cotyledons from 8-day-old seedlings were wounded by cutting away the lower surface (about 1 mm thick). Twenty cotyledons were divided into four groups of five, and were arranged cut-side up on moist filter papers. A 50-μl aliquot of GE (200 ng), containing rifampicin (10 ppm) and ampicillin (500 ppm), was placed onto the wounded areas of the cotyledons, which were then incubated at 25°C for 20 hr. Each group of five cotyledons was transferred to 10 ml of distilled water, and the absorbance at 285 nm of a 10-fold diluted solution of cotyledon was measured. The original GE and its tyramine conjugate produced an increase in absorbance of 0.27 and 0.25 per cotyledon, respectively. For the inhibition assay, antibiotics and purified antibody were applied to the cotyledon 1 hr before application of GE.
Binding Activity of the GE.
Solubilized samples were incubated in 50 mM Tris·HCl, pH 7.4/0.1 M sucrose/5 mM MgCl2/1 mM phenylmethylsulfonyl fluoride (PMSF)/5 mM EDTA for 2 hr at 4°C in the absence and presence of a 100-fold molar excess of unlabeled GE. 125I-labeled GE (only 53.2 nM; sample in 500 μl) was added to these reaction mixtures and incubated for an additional 2 hr at 4°C. The reaction mixtures were filtered through a 0.3% polyethylenimine-treated glass fiber filter GF/B (Whatman) (27) and washed three times with 10 mM Tris·HCl, pH 7.0/1 M NaCl/10 mM MgCl2. The radioactivity trapped on the filters was measured. The binding of labeled GE in the presence of an excess of unlabeled GE was defined as nonspecific binding, and this value was subtracted from the total binding to obtain the specific binding value. Kd was calculated by nonlinear squares method (28).
Purification and Amino Acid-Sequencing of a GEBP.
All steps for purification were performed at 4°C unless mentioned otherwise. Soybean roots (40 kg, wet weight), grown in soil for 7 days followed by growth in a hydroponic apparatus (TS farm system, Q. P. Co., Tokyo) for 15 days, were homogenized in a Waring Blender in 25 mM Tris·HCl, pH 7.0/30 mM MgCl2/2 mM DTT/2.5 mM sodium metabisulfite/1 mM PMSF for 2 min. The slurry was recovered at 10,000 × g for 15 min and centrifuged at 100,000 × g for 20 min, and the pellet (membrane fraction) was resuspended in 25 mM Tris·HCl, pH 7.4/0.1 M sucrose/5 mM MgCl2/1 mM PMSF/5 mM EDTA, 0.25% (wt/vol) Zwittergent 3–12 (ZW; Boehringer Mannheim) and stirred for 30 min at 8°C. Centrifuged at 100,000 × g for 20 min, the supernatant (solubilized fraction) was dialyzed against 50 mM Tris·HCl, pH 8.0/0.2% ZW/5 mM EDTA and applied to a protrap column (Takara Shuzo, Kyoto) to remove serine proteinases. The sample was loaded onto a Q Sepharose HP 26/10 column. A salt gradient, 0–1 M NaCl in column buffer (CB; 50 mM Tris·HCl, pH 8.0/0.1 M sucrose/5 mM MgCl2/1 mM PMSF/5 mM EDTA/0.2% ZW) was applied, and the GEBP was eluted at 0.45 M NaCl. Dialyzed against CB, the GEBP was loaded onto a Mono Q column and eluted at 0.25 M NaCl. Gel attached with maltose (60 mg per 6 g of resin prepared as in ref. 24) was added and mixed slowly for 1 hr. The supernatant was recovered by centrifugation at 1,000 × g for 2 min and then stirred gently for 16 hr with GE-binding gel (34 mg per 490 mg of resin prepared as in ref. 24). From the gel washed three times with CB by centrifugation, the sample was eluted with 4 M guanidine/10 mM Tris·HCl, pH 8.0, and dialyzed against CB (purified protein fraction; see Fig. 1B). After separation by SDS/PAGE (10–20% gradient gel), a GEBP was electroblotted onto poly(vinylidene difluoride) membrane, first digested with Achromobacter protease I (lysylendopeptidase) and then subdigested with endoproteinase Asp-N (29). The digested peptides were chromatographed by reverse-phase HPLC, and the amino acid sequences were determined with a gas-phase sequencer (model PSQ-2, Shimadzu) (30, 31). Protein was determined by DC protein assay kit (Bio-Rad), and purified GEBP was estimated by the density of the silver-stained SDS/PAGE band.
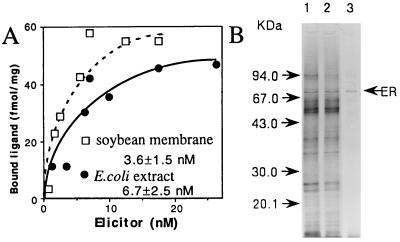
Figure 1.
(A) Concentration dependence of 125I-labeled elicitor binding. Detergent-solubilized soybean root membrane proteins (290 μg) or E. coli crude extracts (496 μg) were incubated with the indicated concentrations of labeled elicitor. (B) Purification of the GEBP. Lanes: 1, Mono Q fraction 9 μg; 2, after passage through a maltose-binding gel, 8 μg; 3, purified protein fraction, 0.05 μg, after dilution in loading buffer and placement in a boiling water bath for 3 min. The proteins were silver-stained.
Screening of the cDNA Clone.
Poly(A)+ RNAs from the seedling roots grown hydroponically for 15 days were prepared (32) and used for a cDNA library (Pharmacia cDNA Synthesis Kit) based on lambda gt10 DNA (Takara Shuzo). A 47-bp cDNA with KYKPGAYSIVQDFLNL was amplified by PCR using the set of mixed primers (5′-GTNAAYAARATNCARAC-3′) and (5′-ARRTTNAGRAARTCYTC-3′), in which Y indicates C/T, R indicates A/G, and N indicates A/G/C/T. A 40-bp cDNA for KSIDGDLVGVVGDS was amplified using primers 5′-AARAGYATHGAYGGNGA-3′ and 5′-WRTCNCCNACNAC-3′, in which H indicates A/C/T, and W indicates A/T. PCR was carried out under the standard thermal program (Perkin–Elmer GeneAmp 9600) against 0.5-μg cDNAs. Based on the two cDNA sequences, primers were designed again, and a 516-bp fragment was amplified by PCR. Using the 32P-labeled fragment, approximately 2 × 105 plaques were screened by hybridization [2× standard saline citrate (SSC)/5× Denhardt’s solution/1% SDS/100 μg/ml salmon sperm DNA at 65°C for 24 hr; washed twice in 0.1× SSC/0.1% SDS at 65°C]. The DNAs isolated from 20 plaques indicating strong signal were derived from the same cDNA, and the longest clone was subcloned into pBluescript (Stratagene; pER23) and sequenced for both strands.
Escherichia coli Expression of the GEBP.
PCR was carried out against pER23 DNA using an appropriate pair of primers (see the positions in Fig. 3). Amplified DNAs were inserted into pMAL-c2 (New England Biolabs) to obtain a set of clones that contained gene fragments fused to the malB gene regulated by the tac promoter. After induction with 0.3 mM of isopropyl β-d-thiogalactoside for 4 hr, the cells were sonicated and solubilized by 0.25% ZW. A recombinant protein containing amino acids 239–442 was further purified on an amylose resin column, cleaved with Factor Xa, chromatographed by gel filtration (Superdex 200, Pharmacia), and used to immunize mice as in ref. 33. The antibody from mouse ascites fluid was purified by Protein-A Sepharose (Pharmacia).
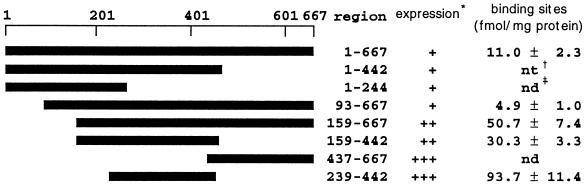
Figure 3.
Crude extracts from E. coli cells expressing proteins fused with the maltose-binding protein were used in the GB-binding assay. The solid bars and residue numbers shown in the region column indicate the region expressed. ∗, relative levels of expression were scored on the basis of the results of immunoblot analysis using anti-maltose binding protein serum; †, not tested; ‡, not detected.
Expression of the GEBP in Tobacco Suspension Cells.
The expression vector pKV1 contains the kanamycin resistance cassette and the cauliflower mosaic virus 35S promoter of pCaP35J (34). The pER23 insert was ligated into pKV1 to produce pKV1-ER. Transient expression for tobacco BY-2 cells was carried out as described (35).
The protoplasts were embedded in Murashige and Skoog medium (36) containing 1% melted agarose and 0.4 M mannitol and allowed to solidify as beads over a solid flat surface of the medium containing 0.4 M mannitol. After 1 week, transformants were selected by the addition of kanamycin (100 ppm). Stable transformants (C2-1 and C2-4 for pKV1, I1 and I6 for pKV1-ER) were maintained as a suspension culture.
Tobacco cells were suspended in 25 mM Tris·HCl, pH 7.0/30 mM MgCl2/2 mM DTT/2.5 mM sodium metabisulfite/1 mM PMSF on ice, sonicated for 2 min, and solubilized by 0.35% ZW.
Immunocytochemistry.
Soybean roots were cut (≈1 mm3) and fixed in 4% (vol/vol) glutaraldehyde, 0.1% paraformaldehyde, and 0.1 M NaPi (pH 7.4) overnight on ice. The fixed roots were dehydrated in a graded ethanol series, and infiltrated in LR White acrylic resin (London Resin Co., Basingstoke, U.K.). Ultrathin sections were mounted on formvar- and carbon-coated stainless grids, incubated in 5% skim milk for 1 hr at room temperature, and washed three times with 0.1 M Tris·HCl, pH 7.2/0.2% (vol/vol) Tween 20/1% BSA/0.5 M NaCl (TTBS). The sections were incubated with a serum against the GEBP or preimmune serum overnight, washed in TTBS, and incubated for 1 hr in goat anti-mouse IgG conjugated to 15-nm gold particles. The grids were washed in TTBS and poststained in 5% (wt/vol) aqueous uranyl acetate for 15 min and lead citrate for 10 min. The thin sections were viewed with an electron microscope (Hitachi, Tokyo; model 7100F) at 75 kV.
RESULTS
Purification of the GEBP.
To better understand the mechanism of elicitor recognition, a GE was radiolabeled with 125I (18) to help identify the cellular components that interact directly with the GE. The labeled GE was confirmed to have a high affinity for the membrane fraction of soybean roots (Fig. 1A; Kd = 3.6 ± 1.5 nM), displaying the same level of elicitor activity (93%) as the original elicitor (see Materials and Methods). The 125I-labeled tyramine-GE was used as a probe to purify a GEBP from the membrane fraction of soybean roots using a combination of conventional and affinity column chromatography. Approximately 4 μg of purified protein, consisting mainly of a 70-kDa protein, was obtained from 40 kg of soybean roots (Fig. 1B and Table 1). The protein was separated by SDS/PAGE, the blotted protein band was digested with proteinases, and peptide fragments were sequenced to obtain sequences, including the N-terminal sequence (Fig. 2A).
Table 1.
Purification of GEBP from soybean roots
| Fraction | Protein, mg | Binding activity, pmol | Recovery, % | Binding sites, pmol/mg |
|---|---|---|---|---|
| Membrane fraction | 17900 | 537 | 100 | 0.030 |
| Solubilized fraction | 2000 | 214 | 40 | 0.107 |
| Q Sepharose | 190 | 205 | 38 | 1.07 |
| Mono Q | 49 | 233 | 43 | 4.76 |
| β-Glucan affinity | 0.004 | 45 | 8.4 | 11300 |
Figure 2.
(A) Primary structure of the GEBP. The amino acid sequences determined experimentally from the purified protein are underlined, with the N terminus being marked with an asterisk. The predicted glycosylation sites (dots) are indicated. (B) Two portions of the predicted GEBP (Soybean) and the yeast gene products (S.p., SPAC23D3.10c; S.c., YRL144c) are aligned. Colons and dots represent identity and similarity of amino acid residues between GEBP and yeast gene products, respectively. Numbers refer to amino acid residue positions for each sequence.
Cloning of the GEBP cDNA.
A set of PCRs based on the amino acid sequence data were used to obtain a partial cDNA fragment (see Materials and Methods). Using the fragment to screen a soybean root cDNA library, the longest DNA clone was obtained and the nucleotide sequence was determined. The deduced amino acid sequence contains the determined N-terminal amino acid sequence plus an additional methionine start (Fig. 2A). The upstream sequence does not encode features common to signal sequences, suggesting that the methionine codon (position −1 in Fig. 2A) is probably the translational start codon and that the protein has no cleavable signal sequence. This sequence encodes a polypeptide of 667 aa with a calculated molecular mass of 74,424 Da. When compared with sequences in the current data bases, the polypeptide shows homology to three unknown proteins, SPAC23D3.10c (24.2% identity in positions 251–638; GenBank accession no. Z64354Z64354), YLR144c (25.7% identity in positions 247–624; GenBank accession no. Z73316Z73316), and YNR067c (26.9% identity in positions 330–656; GenBank accession no. Z71682Z71682), which are predicted from genomic DNAs of yeasts. In particular, two regions shown in Fig. 2B have great homology among these proteins.
The predicted amino acid sequence has seven potential N-glycosylation sites with the consensus sequence Asn-X-Ser/Thr, but according to the results of amino acid sequence determination, two of these sites were not modified (positions 6 and 635 in Fig. 2A). Recently, receptor-like protein kinases and disease resistance genes isolated from plants demonstrated that the motifs of a serine-threonine kinase and a leucine-rich repeat were expected to be important for cellular signaling processes mediating self-incompatibility response and disease resistance (37, 38). It has been reported that there is significant homology among β-glucan-binding proteins and glucanases (39). These motifs appear to be absent in the cloned GEBP.
Expression of the GEBP in E. coli and Tobacco Suspension Cells, and Mapping of the GE-Binding Region.
A full-length as well as a partial cDNA fusion with the malE gene of E. coli were constructed, as shown in Fig. 3. Although fusion proteins containing a full-length or N-terminal region were expressed at a low level in E. coli, fusion proteins consisting of the central and/or the C-terminal region of the GEBP were highly expressed. The labeled GE bound to fusion proteins containing the central region of the GEBP but not to fusion proteins containing only the N- or C-terminal region (Fig. 3). This result shows that a GE-binding domain(s) is present in the central one-third of the cDNA product. Crude extracts from E. coli expressing the fusion protein with the central one-third cDNA product were used to determine the dissociation constant (Fig. 1A; Kd = 6.7 ± 2.5 nM). The GE-binding region was purified and used an antigen for the production of antibody.
The solubilized fraction of tobacco cells did not exhibit any binding activity with the GE (Table 2). A set of tobacco cell transformants expressing the GEBP cDNA either transiently or stably were obtained by electroporation. The labeled GE was found to bind exclusively to the transformants containing the insert but not to transformants carrying vector DNA alone (Table 2).
Table 2.
Binding activity of 125I-labeled GE with solubilized fraction from soybean root and tobacco suspension cells
| Samples | Transformation vector* (stable clone) | Binding sites, fmol/mg |
|---|---|---|
| Soybean root (soil) | − | 26.6 ± 2.8 |
| Soybean root (water) | − | 107 ± 11.6 |
| Tobacco suspension cells | − | ND |
| Tobacco suspension cells | Vector (transient) | ND |
| Tobacco suspension cells | GEBP (transient) | 90.5 ± 7.7 |
| Tobacco suspension cells | Vector (C2-1) | ND |
| Tobacco suspension cells | Vector (C2-4) | ND |
| Tobacco suspension cells | GEBP (I1) | 150 ± 17.2 |
| Tobacco suspension cells | GEBP (I6) | 196 ± 17.8 |
ND, Not detected.
No vector (−), control vector (Vector), or GEBP-expressed vector (GEBP) was used to transform tobacco cells.
Demonstration of Receptor Activities.
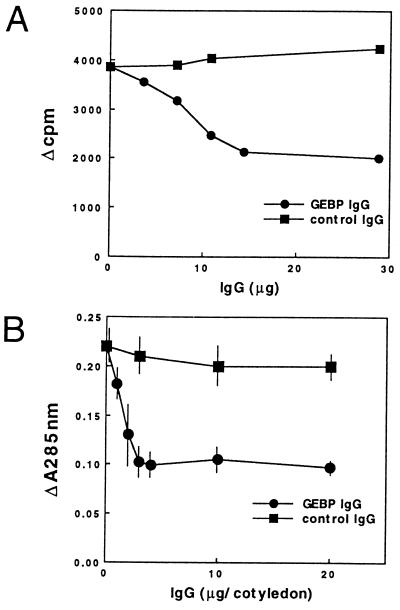
An antibody was produced by immunization with the recombinant protein synthesized in E. coli containing an GE-binding domain(s) (the amino acid sequence from position 239 to position 442; see Fig. 3). The antibody inhibited the binding of the labeled GE to the cotyledon membrane fraction in a dose-dependent manner (51% decrease with 28.8 μg of IgG) as shown in Fig. 4A. A control mouse antibody, against yeast double-stranded RNase (pac1 protein), did not show any detectable inhibition (Fig. 4A). No binding activity was detected by incubating the labeled GE with the antibody, confirming that the antibody itself does not bind to the GE. These results indicate that the cloned cDNA encodes a GEBP from soybean cotyledon and that the antibody can inhibit the interaction between the GE and the GEBP.
Figure 4.
(A) Inhibition of GB-binding activity by the antibody against GEBP. An antibody was incubated in the binding assay reaction mixture with detergent-solubilized cotyledon membrane proteins (8.2 mg) for 2 hr before the labeled elicitor was added. (B) Inhibition of phytoalexin accumulation activity by the antibody against GEBP.
To examine whether the binding protein is a GE receptor involved in the signal transduction of the defense reaction, we attempted to inhibit the signal pathway with the same antibody. As shown in Fig. 4B, the antibody inhibited the induction of phytoalexin accumulation in soybean cotyledons in a dose dependent-manner (53% decrease with 20.0 μg of IgG). On the other hand, the control antibody did not show any inhibition of elicitor activity (Fig. 4B). Neither the antibody against the GE-binding region nor the control antibody were found to have any detectable elicitor activity under these experimental conditions (data not shown). Moreover, the addition of the GE-binding region protein synthesized in E. coli and used as an antigen also inhibited the induction of phytoalexin accumulation in soybean cotyledons (27% decrease with 100 μg of the protein per cotyledon) by 200 ng of GE per cotyledon. On the other hand, the addition of the same amount of a control protein (BSA) did not show any inhibition of elicitor activity (data not shown). These results strongly suggest that the cloned cDNA encodes a genuine GE receptor that transduces the defense reaction in soybeans.
Expression and Immunolocalization of the GEBP.
A major transcript of about 2.4 kb was detected in poly(A)+ RNAs isolated from soybean roots and leaves (Fig. 5). The level of expression was much higher in roots obtained by hydroponic culture than in soil-cultured roots, and leaves. Also, the GE-binding activity of the crude fraction obtained from soybean roots grown hydroponically was about 4-fold higher than that of roots grown in soil (Table 2).
Figure 5.
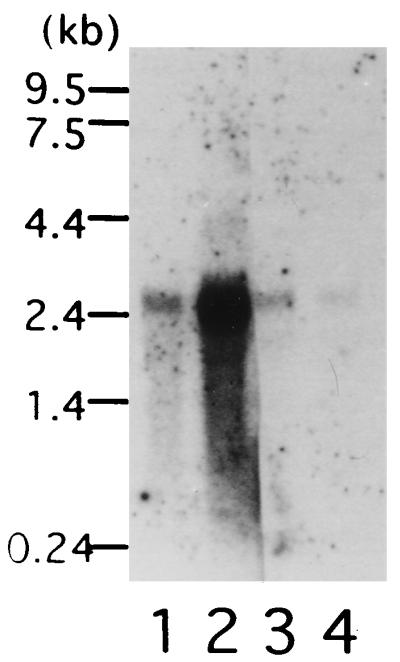
Northern blot analysis. Ten micrograms of total RNA from roots obtained by hydroponic culture (lane 1), and 5 μg of poly(A)+ RNA from roots obtained by hydroponic culture and soil culture (lanes 2 and 3) and from leaves (lane 4) were separated in 2.2% formaldehyde gels, blotted, and hybridized with full-length cDNA.
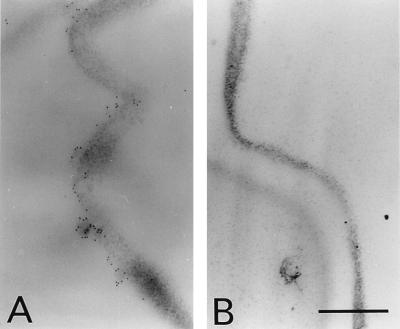
Localization of the GEBP at the plasma membrane of root cells can be demonstrated by post-embedding immunogold labeling using the above serum (Fig. 6A) and not using the preimmune serum (Fig. 6B). Gold label is sparse in the thin sections, rarely present as single gold particles, but more frequently occurring in small groups (Fig. 6A). Another serum that we had obtained to immunize another mouse with the GE-binding region also indicated similar localization (data not shown).
Figure 6.
Transmission electron micrographs of soil-grown-root sections incubated with anti-GEBP serum (A; 1:20 dilution) or preimmune serum (B), followed with and 15 nm gold-conjugated anti-mouse antibody. (A and B, ×20,000; Bar = 0.5 μm.)
DISCUSSION
The GEBP deduced from the cDNA has a molecular mass of 74,474 Da, which is in good agreement with the value of the purified protein, suggesting that the protein may not be extensively modified in plant cells, although the protein was found to have five possible glycosylation sites. The membrane fraction of soybean roots and the recombinant protein containing the GE-binding region(s) were shown to have similar affinities for the GE, indicating that the cloned cDNA encodes the GEBP in roots.
GEs released from the cell wall of the fungus P. megasperma initiate the accumulation of phytoalexins in soybean (40). Two groups have previously reported apparent dissociation constants of 1 nM (20) and 3 nM (41) for the hepta-β-glucoside elicitor and a fraction derived from soybean root membrane, values which are of the same order of magnitude as those of the present study. Recently, a photoaffinity labeling technique and affinity chromatography have been used to demonstrate that 70-, 100-, and 170-kDa polypeptides have binding activity (41, 42). We obtained preliminary data indicating that hepta-β-glucoside could partially inhibit glucan-binding activity against the recombinant protein by 51% (data not shown). Since the GEBP was isolated from the Green Homer cultivar, which is readily available for purification in Japan, and because we later found that in the cultivar phytoalexins were induced well by GE and less so by hepta-β-glucoside, we decided not to control the induction of phytoalexins by hepta-β-glucoside. Our results, however, suggest that the cloned GEBP should be similar to the protein binding hepta-β-glucoside, but we wonder whether or not they are exactly the same. Indeed, we have found different sequences homologous to the GEBP cDNA in the soybean genome and in cDNAs from a root cDNA library, indicating that the GEBP belongs to a group of similar proteins (data not shown). Further analysis of the binding specificity of the GEBP against different glucan variants will be needed to answer this question.
We could obtain an antibody against the GE-binding region synthesized in E. coli which specifically recognized the 70-kDa GEBP from soybean tissues as detected by immunoblot experiments (data not shown). This antibody inhibited phytoalexin accumulation by 50% (Fig. 4B), strongly suggesting that the cloned cDNA encodes a genuine receptor protein for the GE. The inhibition value is similar to the value obtained for antibody binding to the 125I-labeled GE (Fig. 4A). This incomplete inhibition may be due to low affinity of the antibody and/or induction of GE receptor protein during incubation with GE and antibody of cotyledons (data not shown). However, we cannot exclude the possibility that other GEBPs may exist in the same tissue as described above. Purified GE-binding region also partially inhibited phytoalexin accumulation by 27%. The GE-binding region to the left of a fused protein is unstable so that proteases may attack it easily during the incubation.
The predicted amino acid sequence of the cloned GE receptor is relatively hydrophilic and has no clear membrane-spanning domains. This property does not appear to fit our results since this protein originates from the root membrane fraction and since it was found to be located in the plasma membrane of root cells by immunocytochemistry (Fig. 6). However, the receptor protein might undergo modification at the posttranslational level, which may allow it to interact with the membrane. Also, the receptor might require other component(s) for localization in the membrane as well as for correct transmission of the elicitor response signal. Computer searches of the current data bases revealed that three yeast gene products have significant and restricted homology to the receptor (Fig. 2B). Yeast has not been known to generate any defense reactions similar to those in plants. These proteins may therefore share novel motifs conserved in yeast and plant, without necessarily having similar functions. These findings may help predict which regions are important for function of the protein.
Stably transformed tobacco cell lines did not respond to the GE to generate defense reactions. This observation may not be decisive because the original cell line seems unable to generate the reactions even by an authentic elicitor elicitin, probably because of cultivation. To further explore this point, whole transformed plants rather than cell lines should be studied.
Acknowledgments
We thank I. Furusawa for cooperation in immunoelectron microscopy. We also thank T. Ohtani and S. Ferri for valuable discussion, and T. Ogawa for the gift of antiserum against pac1 protein. We thank T. Mashiko, R. Kaneko, M. Takahashi, Y. Hachimura, K. Sato, and T. Hori for experimental assistance.
Footnotes
References
- 1.Dixon R A, Lamb C J. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:339–367. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- 2.Ebel J, Scheel D. In: Genes Involved in Plant Defense. Boller T, Meins F, editors. Vienna: Springer; 1992. pp. 183–205. [Google Scholar]
- 3.Keen N T, Dawson W O. In: Genes Involved in Plant Defense. Boller T, Meins F, editors. Vienna: Springer; 1992. pp. 85–114. [Google Scholar]
- 4.Yoshikawa M, Yamaoka N, Takeuchi Y. Plant Cell Physiol. 1993;34:1163–1173. [Google Scholar]
- 5.Wingender R, Röhrig H, Höricke C, Wing D, Schell J. Mol Gen Genet. 1989;218:315–322. doi: 10.1007/BF00331284. [DOI] [PubMed] [Google Scholar]
- 6.Bolwell G P, Coulson V, Rodgers M W, Murphy D L, Jones D. Phytochemistry. 1991;30:397–405. [Google Scholar]
- 7.Volpin H, Elkind Y, Okon Y, Kapulnik Y. Plant Physiol. 1994;104:683–689. doi: 10.1104/pp.104.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt W E, Ebel J. Proc Natl Acad Sci USA. 1987;84:4117–4121. doi: 10.1073/pnas.84.12.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kogel G, Beissmann B, Reisener H J, Kogel K. Planta. 1991;183:164–169. doi: 10.1007/BF00197784. [DOI] [PubMed] [Google Scholar]
- 10.Shibuya N, Kaku H, Kuchitsu K, Maliarik M J. FEBS Lett. 1993;329:75–78. doi: 10.1016/0014-5793(93)80197-3. [DOI] [PubMed] [Google Scholar]
- 11.Basse C W, Fath A, Boller T. J Biol Chem. 1993;268:14724–14731. [PubMed] [Google Scholar]
- 12.Nürnberger T, Nennstiel D, Hahlbrock K, Scheel D. Proc Natl Acad Sci USA. 1995;92:2338–2342. doi: 10.1073/pnas.92.6.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshikawa M, Matama M, Masago H. Plant Physiol. 1981;67:1032–1035. doi: 10.1104/pp.67.5.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keen N T, Yoshikawa M, Wang M C. Plant Physiol. 1983;71:466–471. doi: 10.1104/pp.71.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeuchi Y, Yoshikawa M, Takeba G, Tanaka K, Shibata D, Horino O. Plant Physiol. 1990;93:673–682. doi: 10.1104/pp.93.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okinaka Y, Mimori K, Takeo K, Kitamura S, Takeuchi Y, Yamaoka N, Yoshikawa M. Plant Physiol. 1995;109:839–845. doi: 10.1104/pp.109.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharp J K, Valent B, Albersheim P. J Biol Chem. 1984;259:11312–11320. [PubMed] [Google Scholar]
- 18.Cheong J-J, Birberg W, Fügedi P, Pilotti Å, Garegg P J, Hong N, Ogawa T, Hahn M G. Plant Cell. 1991;3:127–136. doi: 10.1105/tpc.3.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshikawa M, Sugimoto K. Plant Cell Physiol. 1993;34:1229–1237. [Google Scholar]
- 20.Cheong J-J, Alba R, Côté F, Enkerli J, Hahn M G. Plant Physiol. 1993;103:1173–1182. doi: 10.1104/pp.103.4.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mithöfer A, Lottspeich F, Ebel J. FEBS Lett. 1996;381:203–207. doi: 10.1016/0014-5793(96)00126-3. [DOI] [PubMed] [Google Scholar]
- 22.Yoshikawa M, Yamauchi K, Masago H. Physiol Plant Pathol. 1978;12:73–82. doi: 10.1104/pp.61.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeuchi Y, Yoshikawa M, Horino O. Ann Phytopathol Soc Jpn. 1990;56:523–531. [Google Scholar]
- 24.Jeffrey A M, Zopf D A, Ginsburg V. Biochem Biophys Res Commun. 1975;62:608–613. doi: 10.1016/0006-291x(75)90442-8. [DOI] [PubMed] [Google Scholar]
- 25.Cosio E G, Pöpperl H, Schnidt W, Ebel J. Eur J Biochem. 1988;175:309–315. doi: 10.1111/j.1432-1033.1988.tb14198.x. [DOI] [PubMed] [Google Scholar]
- 26.Hahn M G, Darvill A, Albersheim P, Bergmann C, Cheong J-J, Koller A, Lo V-M. In: Molecular Plant Pathology. Gurr S J, McPherson M J, Bowles D J, editors. Vol. 2. Oxford: IRL; 1992. pp. 103–147. [Google Scholar]
- 27.Bruns R F, Lawson-Wendling K, Pugsley T A. Anal Biochem. 1983;132:74–81. doi: 10.1016/0003-2697(83)90427-x. [DOI] [PubMed] [Google Scholar]
- 28.Sakoda M, Hiromi K. J Biochem. 1976;80:547–555. doi: 10.1093/oxfordjournals.jbchem.a131310. [DOI] [PubMed] [Google Scholar]
- 29.Iwamatsu A. Electrophoresis. 1992;13:142–147. doi: 10.1002/elps.1150130129. [DOI] [PubMed] [Google Scholar]
- 30.Aoyama H, Iwamatsu A, Dibo G, Tsunasawa S, Sakiyama F. Protein Chem. 1988;7:191. [Google Scholar]
- 31.Iwamatsu A, Yoshida-Kubomura N. J Biochem. 1996;120:29–34. doi: 10.1093/oxfordjournals.jbchem.a021389. [DOI] [PubMed] [Google Scholar]
- 32.Toguri T, Umemoto N, Kobayashi O, Ohtani T. Plant Mol Biol. 1993;23:933–946. doi: 10.1007/BF00021810. [DOI] [PubMed] [Google Scholar]
- 33.Harlow E, Lane D. Antibodies. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. pp. 53–137. [Google Scholar]
- 34.Yamaya J, Yoshioka M, Meshi T, Okada Y, Ohno T. Mol Gen Genet. 1988;211:520–525. doi: 10.1007/BF00425710. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe Y, Meshi T, Okada Y. FEBS Lett. 1987;219:65–69. [Google Scholar]
- 36.Murashige T, Skoog F. Physiol Plant. 1962;15:473–497. [Google Scholar]
- 37.Braun D M, Walker J C. Trends Biochem Sci. 1996;21:70–73. [PubMed] [Google Scholar]
- 38.Staskawicz B J, Ausubel F M, Baker B, Ellis J G, Jones J D G. Science. 1995;268:661–667. doi: 10.1126/science.7732374. [DOI] [PubMed] [Google Scholar]
- 39.Seki N, Muta T, Oda T, Iwaki D, Kuma K, Miyata T, Iwanaga S. J Biol Chem. 1994;269:1370–1374. [PubMed] [Google Scholar]
- 40.Ayers A R, Ebel J, Finelli F, Berger N, Albersheim P. Plant Physiol. 1976;57:751–759. doi: 10.1104/pp.57.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cosio E G, Frey T, Ebel J. Eur J Biochem. 1992;204:1115–1123. doi: 10.1111/j.1432-1033.1992.tb16736.x. [DOI] [PubMed] [Google Scholar]
- 42.Frey T, Cosio E G, Ebel J. Phytochemistry. 1993;32:543–550. [Google Scholar]