Abstract
Several models of experimental ulcerative colitis have been reported previously. However, none of these models showed the optimum characteristics. Although dextran sulfate sodium-induced colitis results in inflammation resembling ulcerative colitis, an obvious obstacle is that dextran sulfate sodium is very expensive. The aim of this study was to develop an inexpensive model of colitis in rats. Sprague-Dawley rats were treated with 2% dextran sulfate sodium in drinking water for 3 d followed by an intracolonic administration of 30% ethanol. The administration of 2% dextran sulfate sodium followed by 30% ethanol induced significant weight loss, diarrhea and hematochezia in rats. Severe ulceration and inflammation of the distal part of rat colon were developed rapidly. Histological examination showed increased infiltration of polymorphonuclear leukocytes, lymphocytes and existence of cryptic abscesses and dysplasia. The model induced by dextran sulfate sodium at lower concentration followed by 30% ethanol is characterized by a clinical course, localization of the lesions and histopathological features similar to human ulcerative colitis and fulfills the criteria set out at the beginning of this study.
Keywords: Model, Colitis, Dextran sulfate sodium, Ethanol
INTRODUCTION
Inflammatory bowel diseases, which include ulcerative colitis (UC) and Crohn’s disease (CD), are multifactorial diseases of unknown etiology (Sands, 2007). In China, UC has been thought uncommon. However, an analysis (Jiang and Cui, 2002) indicated that a sharp rise of the UC incidence has been observed over the last decade. Research on the etiopathogensis and therapeutic agents of UC has been hampered by the absence of adequate animal model of UC. Ideally, animal model should resemble the clinical course and histopathology, not be cumbersome to induce and have a predictable time course of inflammation (Strober, 1985). An optimum model should also not be too expensive by using widely available animal and chemical substances.
Several models of experimental colitis resembling UC have been reported previously. The most widely used models are induced by administering toxic chemical such as dextran sulfate sodium (DSS) (Gaudio et al., 1999) or trinitrobenzene sulfonic acid (TNBS) (Morris et al., 1989). However, none of these fulfilled the optimum characteristics as mentioned above.
In this study, we developed an inexpensive model of UC in Sprague-Dawley (SD) rats. We found that the administration of ethanol following a lower dose of DSS was able to induce a severe inflammation of distal colon.
MATERIALS AND METHODS
Animals
Virgin female SD rats (180~200 g), obtained from Zhejiang Animal Center (Hangzhou, China), were used in this study. They were maintained in a restricted access room with controlled temperature (23 °C) and light/dark (14 h/10 h) cycle. The animals were housed in rack-mounted, wire cages with a maximum of 6 animals per cage. Standard laboratory pelleted formula and tap water were provided ad libitum.
Induction of colitis
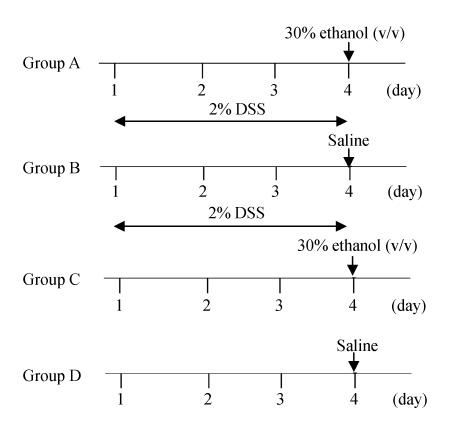
Rats were randomized into four groups. In groups A and B rats received 2% DSS (MW 5000 Da, Sigma, USA) in the drinking water for 3 d. On day 4, rats in groups A and B received 0.5 ml 30% (v/v) ethanol or saline intracolonically respectively. As a control, rats in groups C and D only received intracolonic 30% (v/v) ethanol or saline respectively (Fig.1). Before enema, the rats were lightly anesthetized with ether. A rubber catheter (OD, 2 mm) was inserted rectally into the colon such that the tip was 8 cm proximal to the anus, approximately at the splenic flexure. Thirty percent ethanol (v/v) or saline was instilled into the lumen of the colon through a rubber catheter.
Fig. 1.
Experimental protocols of the study
Assessment of disease activity
To quantify the clinical evolution of the disease we used the disease activity index (DAI) described by Cooper et al.(1993) with little modification (Table 1). The changes in growth rate, stool consistency and presence of gross bleeding or occult blood in feces were scored daily from 0~4 for each animal, as shown in Table 1. The presence of occult blood in feces was determined using a benzidine test. The total score was the sum of the 3 subscores.
Table 1.
Scoring of the disease activity index (DAI)
| Score | Decrease in growth (%) | Stool consistency | Occult/gross rectal bleeding |
| 0 | 0 | Normal | Normal |
| 1 | 1~5 | Normal | Occult blood + |
| 2 | 5~10 | Loose stools | Occult blood ++ |
| 3 | 10~15 | Loose stools | Occult blood +++ |
| 4 | >15 | Diarrhea | Gross bleeding |
Assessment of colonic inflammation and damage
At various time (day 1, day 3, day 7, day 14 and day 21) after intracolonic administration of 30% ethanol or control saline, 5 or more rats from each treatment group were randomly selected and killed after deep anesthesia by intraperitoneal administration of chloral hydrate (400 mg/kg). The distal colon was removed, opened by a longitudinal incision and was immediately examined under a stereomicroscope and visible damage was scored on a 0~5 scale (Table 2). We used a modification of criteria described by Morris et al.(1989).
Table 2.
Criteria for scoring of gross morphologic damage
| Score | Gross morphology |
| 0 | No damage |
| 1 | Localized hyperemia, but no ulcers or erosions |
| 2 | Ulcers or erosions with no significant inflammation |
| 3 | Ulcers or erosions with inflammation at one site |
| 4 | Two or more sites of ulceration and/or inflammation |
| 5 | Two or more major sites of inflammation and ulceration or one major site of inflammation and ulceration extending >1 cm along the length of the colon |
“Inflammation” was defined as regions of hyperemia and bowel wall thickening. After scoring, samples (10 mm×20 mm) with grossly visible ulceration or inflammation were excised from each colon. When no grossly visible inflammation was present, the samples were taken from the regions 3 cm proximal to the anus. To minimize physical artifacts, the removed colon was put on a thick filter paper without stretching. The colon was exposed inside out by cutting longitudinally. When the tissue fluid in the filter paper was dried after 2 min, the colonic wall adhered to the filter paper, securing a stable fixation. The tissue samples were fixed in 10% formalin and embedded in paraffin.
Histological analysis
Sections (10 µm) were processed for Hematoxylin and Eosin staining (H & E) and slides were observed using a blinded protocol. We used the parameters scored on a 0~3 described by Gaudio et al.(1999) with little modification: (1) Destruction of epithelium and glands: 0=morphologically normal, 1=focal destruction of the epithelial surface and/or focal crypt dropout, 2=zonal destruction of the epithelial surface and/or zonal crypt loss, 3=diffuse and/or mucosal ulceration involving submucosa and/or diffuse crypt loss; (2) Dilation of glandular crypts: 0=normal aspect, 1=focal dilation, 2=zonal dilation, 3=diffusely dilated crypts; (3) Depletion and loss of goblet cells: 0=normal aspect, 1=slightly depleted goblet cells, 2=zonal or moderately depleted goblet cells, 3=diffusely or complete depletion of goblet cells; (4) Inflammatory cells infiltration: 0=absence of infiltration, 1=infiltrate at the subepithelial and lamina propria level or crypt bases, 2=infiltration reaching muscularis mucosae, 3=severe and extensive infiltration reaching submucosa and/or involving muscularis propria; (5) Edema: 0=absent, 1=focal, 2=zonal and/or moderately diffuse, 3=extensive and severe; (6) Hemorrhagic mucosa: 0=absent, 1=focal, 2=zonal, 3=diffuse; (7) Crypt abscesses: 0=absent, 1=focal, 2=zonal, 3=diffuse; (8) Dysplasia: 0=absent, 1=focal, 2=zonal, 3=diffuse.
The colitis score of individual rats represents the sum of the subscores of the different histological parameters.
Statistical methods
Unless otherwise stated, data are expressed as mean±SEM. Nonparametric data were analyzed with Mann-Whitney U test. With all statistical analyses, an associated probability (P value) of ≤5% was considered significant.
RESULTS
Twenty-four hours after the single intracolonic administration of 30% ethanol, all the animals that had received DSS developed gross bleeding and diarrhea, which is main symptom of UC. Gross blood adhered to the anus in most rats. Diarrhea or loose stools was observed in 100% of the rats taking DSS killed 1 or 3 d after ethanol.
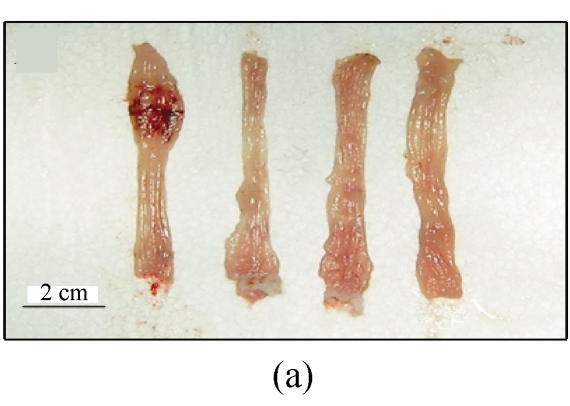
Extensive macroscopic damage of the colon was observed. Ulcerated and hemorrhagic mucosae were found. The sites of inflammation and ulceration varied from the perirectal region to 8 cm proximal to the anus. There were often two or more separate sites of ulcers in the distal colon. No damage was detected proximal to the splenic flexure (Fig.2a).
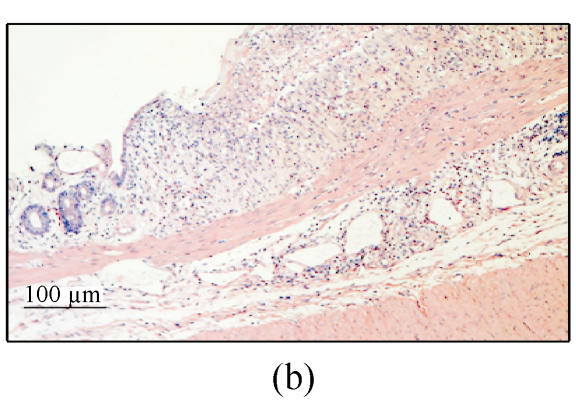
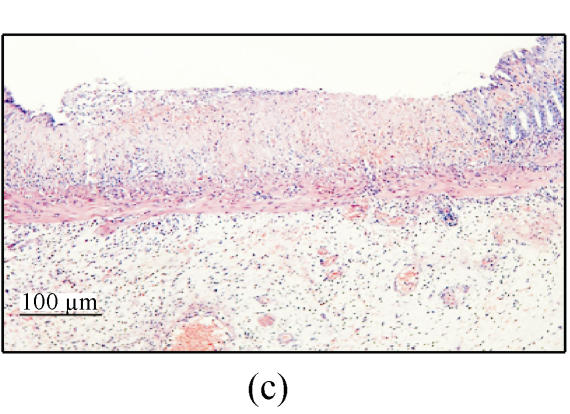
Fig. 2.
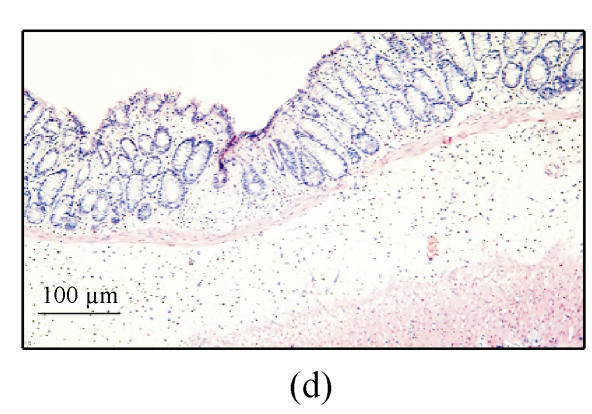
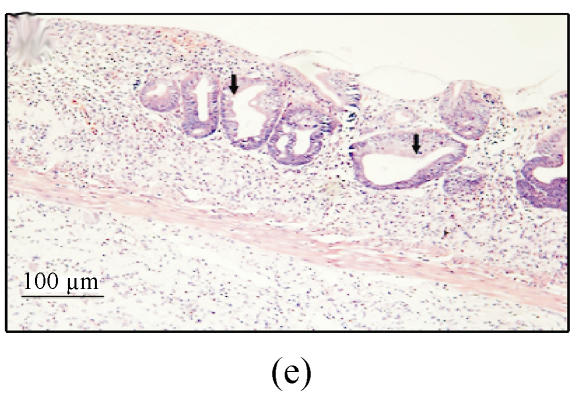
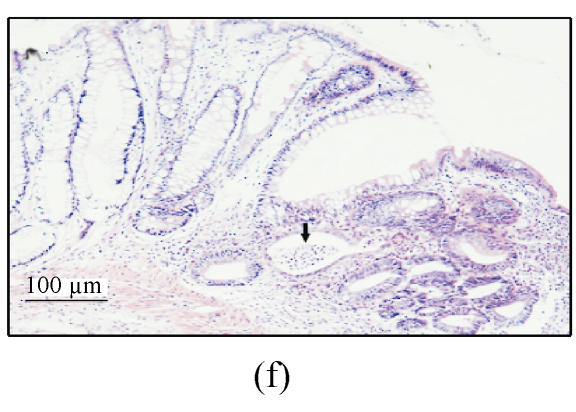
(a) Ulcerated and hemorrhagic mucosa was found in 3 d after ethanol administration in group A while only localized hyperemia was identified in groups B and C and no damage in group D; (b) Ulceration and inflammation of the colonic mucosa of rats from group A 3 d after intracolonic administration of 30% ethanol; (c) Twenty-four hours after 30% ethanol. Ulceration can be found in group C; (d) Three days after 30% ethanol administration in group C. The damage was resolved obviously; (e) Colonic mucosa of rats in group A 7 d after intracolonic administration of 30% ethanol. Dysplasia and dilation of crypt (arrow) are visible; (f) Colonic mucosa of rats in group A 7 d after intracolonic administration of 30% ethanol. Note the crypt abscess (arrow), dysplasia and dilation of crypt
Histological analysis showed that extensive diffuse coagulative necrosis and multiple hemorrhagic lesions of the entire colonic mucosa were present 24 h and 3 d (Fig.2b) after ethanol administration. The submucosa was diffusely edematous and contained multifocal areas of ulceration and inflammation. Extensive infiltration by polymorphonuclear leukocytes, eosinophils and lymphocytes was apparent. The mucosa adjacent to ulcer showed crypt abscesses or/and dysplasia or crypt distortion was found.
Three days after DSS/ethanol administration, all three of the employed indications of damage and inflammation of group A were significantly (P<0.05) elevated above the levels observed in animals that received one of the three control treatments (group B, C or D) (Table 3).
Table 3.
Time course study of disease activity index (DAI), gross morphology and histological score
| Group | Time | DAI | Gross morphology | Histological score |
| A | Day 1 | 9.6±0.5* | 3.8±0.4Δ | 13.4±3.0Δ |
| Day 3 | 6.6±0.9* | 4.2±0.4* | 15.6±3.8* | |
| Day 7 | 2.8±1.1* | 3.6±0.5* | 13.1±2.1* | |
| Day 14 | 0±0 | 1.8±0.4* | 6.4±1.1* | |
| B | Day 1 | 3.6±0.9# | 0.8±0.5* | 3.2±0.4* |
| Day 3 | 0±0# | 0±0* | 2.6±0.8* | |
| Day 7 | 0±0# | 0±0# | 1.4±0.5* | |
| C | Day 1 | 1.6±0.7 # | 2.4±0.5* | 11.4±1.8Δ |
| Day 3 | 0±0# | 1.4±0.5* | 5.6±1.8* |
P<0.05 compared with other groups at the same time point;
P<0.05 compared with group B at the same time point;
P<0.05 compared with group A at the same time point
Only loose stools were observed in rats with DSS administration alone. Gross appearance showed localized hyperemia. However, no ulcers or erosions was observed in rats of group B. Histological finding showed dilation of glandular crypts or/and zonal or moderately depleted goblet cells, but no crypt abscess or dysplasia was found.
Diarrhea was not observed in rats only treated with 30% ethanol. Mucosal ulceration and hemorrhage were observed 24 h after ethanol administration. However, this damage was resolved obviously at 3 d after ethanol administration. Histological finding showed administration of 30% ethanol destroyed the intestinal epithelium and caused extensive mucosal damage 24 h after ethanol administration (Fig.2c). This change was resolved obviously at 3 d after ethanol administration (Fig.2d). No crypt abscess or dysplasia was found in group C.
In group A, ulcers or erosions still existed 7 d after the ethanol administration. The lesions consisting of extensive defects in the mucosa were replaced by areas of fibroplasis and angiogenesis and the luminal surface was coated with a layer of fibrin and neutrophils. The submucosa contained prominent infiltration of eosinophils and neutrophils. The mucosa adjacent to ulcer showed extensive crypt distortion, crypt abscesses and dysplasia (Figs.2e and 2f).
Seven days after the ethanol administration, in groups B and C the damage was completed resolved macroscopically and only focal dilation of glandular crypts could be found in group B. Two weeks after the ethanol administration, focal defects of the mucosal surface were observed in rats of group A. Polymorphonuclear leukocytes were primarily located within the superficial regions of the defects. Not any change was identified in rats of group B or C. Three weeks after challenge, no macroscopic or histological damage was noted in any of the groups. No macroscopic or histological damage was found at any time in the rats in group D.
DISCUSSION AND CONCLUSION
The aim of the study was to establish an inexpensive model of experimental colitis resembling UC in SD rats. Although several models of experimental colitis have been reported previously, none of these showed the optimum characteristics. In recent years, some kinds of knockout (KO) mice have been reported (Hibi et al., 2002). Unfortunately, these strains of mice are not widely available, thus limiting their usefulness. The most widely used models are induced by administering toxic chemical such as TNBS or DSS.
The inflammation of TNBS-induced colitis is transmural and includes the formation of granulomas and Langhan’s type giant cells (Morris et al., 1989). The mucosa frequently has a “cobble-stone”-like appearance. This evidence suggests that this model is histopathologically relevant to the features of CD, not UC. The DSS-induced colitis results in inflammation mainly in the distal colonic mucosa and the histopathology of this model showed some characteristics resembling UC. But this colitis can only be induced by administration of DSS at a high concentration for several days (Faure et al., 2003; Gaudio et al., 1999). Thus the amount of DSS used for rats will be very large, which limited its wide use in many regions because DSS is very expensive.
In this study we showed that intracolonic administration of the “barrier breaker” (30% ethanol) after 2% DSS treatment resulted in a rapid development of severe ulceration and inflammation of the distal part of rat colon. The DSS/ethanol treatment induced significant weight loss, diarrhea and hematochezia. These features are similar to what happen in human UC where the major symptoms include diarrhea, rectal bleeding and weight loss. Macroscopic finding showed the damage was characterized by marked ulceration and hemorrhage and that the diseased site was limited to the distal colon. Histological finding showed increased infiltration of polymorphonuclear leukocytes, lymphocyte and existence of cryptic abscesses, which is a hallmark of human UC. Such result was not induced by administration of ethanol or DSS alone. Moreover, dysplasia was commonly found in DSS/ethanol group. The most important clinical issue in the management of patients with IBD is an increased risk for development of dysplasia and neoplasia. Taken together, these features indicate that DSS/ethanol induced colitis and human UC shares many similar clinical and morphological aspects.
DSS can induce reproducible acute colitis in rodents when given at a concentration of 4% for more than 6 d (Faure et al., 2003; Gaudio et al., 1999; Okayasu et al., 1990). The exact mechanism by which DSS causes inflammation is not fully elucidated. It appears that the potential roles of DSS in induction of colitis may be: (a) direct cytotoxicity; (b) interference with the normal interaction between intestinal lymphocytes and epithelial cells (Ni et al., 1996); (c) DSS causes a change in the intestinal microflora, and particularly an increase in the number of Gram-negative anaerobes (Okayasu et al., 1990). Administration of DSS also activates the immune response (Ni et al., 1996; Vicario et al., 2005) and stimulates chemokine production by epithelial cells (Ohtsuka and Sanderson, 2003). DSS at a lower concentration upregulates cytokines, although it does not cause bloody diarrhea and ulceration in colon (Egger et al., 2000; Vicario et al., 2005). We also found aggregated lymphocytes in the colonic mucosa after the administration of DSS for 3 d. This fact indicates the administration of lower concentration of DSS may also activate the immune response.
Acting as a barrier breaker, ethanol is a very commonly used vehicle for models by increasing mucosal permeability. In our study we found that 30% ethanol cannot cause any change of stool in SD rats. However, it does destroy the intestinal epithelium, which is considered to be a part of the innate immune system. The intestinal epithelium forms a tight, highly selective barrier between the body and the intraluminal microenvironment. It plays an active role in the maintenance of mucosal homeostasis (Yu et al., 2004). Failure of this barrier may result in intestinal inflammation, most likely through exposure to fecal antigens (Bamias et al., 2005).
The administration of 2% DSS for 3 d could only cause loose stool in rats and no obvious macroscopic damage was observed at any time. Thirty percent ethanol treatment induces acute injury that could be observed at 24 h and 3 d, but with no obvious change at 1 week. For rats treated with DSS/ethanol, the mucosal injury was still present at 1 week and 2 weeks. Our data confirmed that the combination of DSS and ethanol produced a more severely acute injury in the distal colon than that induced by DSS or ethanol alone. Therefore, it seems the activated immune response after administration of DSS and the disruption of the superficial epithelium by 30% ethanol are essential to enable the consequent induction of a more severe inflammatory reaction. The possible mechanism is that ethanol causes dysfunction of barrier which leads to exposure to fecal antigens and this effect was exaggerated when the immune response was activated by administration of DSS. Colitis may be a result from a dysregulated response of the mucosal immune system toward intraluminal antigens of bacterial origin (Duchmann et al., 1995; 1996; Lu et al., 2003).
Several advantages of this model make it a useful one for the study of the pathophysiology and therapy of UC, especially in developing countries. First, the animal used is the SD rat, which is inexpensive and widely available. Second, the concentration of DSS is 2% and the duration of administration is 3 d, thus the model is relatively inexpensive since the most commonly used DSS-induced colitis can only be induced after the administration of 4% DSS for 6 d. Third, this model is relevant in that it has several features of human UC. Finally, the inflammation is easy to be induced and very reproducible.
In conclusion, the DSS/ethanol model is characterized by a clinical course, localization of the lesions and histopathological features similar to human UC. The model fulfills the criteria set out at the beginning of study and can serve as a useful model in the assessment of future novel drugs for the therapy of UC.
References
- 1.Bamias G, Nyce MR, de la Rue SA, Cominelli F. New concepts in the pathophysiology of inflammatory bowel disease. Ann Intern Med. 2005;143(12):895–904. doi: 10.7326/0003-4819-143-12-200512200-00007. [DOI] [PubMed] [Google Scholar]
- 2.Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69(2):238–249. [PubMed] [Google Scholar]
- 3.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer BK. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102(3):448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duchmann R, Schmitt E, Knolle P, Meyer KH, Neurath MT. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol. 1996;26(4):934–938. doi: 10.1002/eji.1830260432. [DOI] [PubMed] [Google Scholar]
- 5.Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Buchler MW. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000;62(4):240–248. doi: 10.1159/000007822. [DOI] [PubMed] [Google Scholar]
- 6.Faure M, Moennoz D, Montigon F, Mettraux C, Mercier S, Schiffrin EJ. Mucin production and composition is altered in dextran sulfate sodium-induced colitis in rats. Dig Dis Sci. 2003;48(7):1366–1373. doi: 10.1023/A:1024175629909. [DOI] [PubMed] [Google Scholar]
- 7.Gaudio E, Taddei G, Vetuschi A, Sferra R, Frieri G, Ricciardi G. Dextran sulfate sodium (DSS) colitis in rats: clinical, structural, and ultrastructural aspects. Dig Dis Sci. 1999;44(7):1458–1475. doi: 10.1023/A:1026620322859. [DOI] [PubMed] [Google Scholar]
- 8.Hibi T, Ogata H, Sakuraba A. Animal models of inflammatory bowel disease. J Gastroenterol. 2002;37(6):409–417. doi: 10.1007/s005350200060. [DOI] [PubMed] [Google Scholar]
- 9.Jiang XL, Cui HF. An analysis of 10218 ulcerative colitis cases in China. World J Gastroenterol. 2002;8(1):158–161. doi: 10.3748/wjg.v8.i1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu J, Wang A, Ansari S, Hershberg RM, McKay DM. Colonic bacterial superantigens evoke an inflammatory response and exaggerate disease in mice recovering from colitis. Gastroenterology. 2003;125(6):1785–1795. doi: 10.1053/j.gastro.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96(3):795–803. [PubMed] [Google Scholar]
- 12.Ni J, Chen SF, Hollander D. Effects of dextran sulphate sodium on intestinal epithelial cells and intestinal lymphocytes. Gut. 1996;39(2):234–241. doi: 10.1136/gut.39.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohtsuka Y, Sanderson IR. Dextran sulfate sodium-induced inflammation is enhanced by intestinal epithelial cell chemokine expression in mice. Pediatr Res. 2003;53(1):143–147. doi: 10.1203/00006450-20030100000024. [DOI] [PubMed] [Google Scholar]
- 14.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98(3):694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 15.Sands BE. Inflammatory bowel disease: past, present, and future. J Gastroenterol. 2007;42(1):16–25. doi: 10.1007/s00535-006-1995-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strober W. Animal models of inflammatory bowel disease—an overview. Dig Dis Sci. 1985;30(12 Suppl.):3S–10S. doi: 10.1007/BF01296964. [DOI] [PubMed] [Google Scholar]
- 17.Vicario M, Crespi M, Franch A, Amat C, Pelegri C, Moreto M. Induction of colitis in young rats by dextran sulfate sodium. Dig Dis Sci. 2005;50(1):143–150. doi: 10.1007/s10620-005-1292-y. [DOI] [PubMed] [Google Scholar]
- 18.Yu Y, Sitaraman S, Gewirtz AT. Intestinal epithelial cell regulation of mucosal inflammation. Immunol Res. 2004;29(1-3):55–68. doi: 10.1385/IR:29:1-3:055. [DOI] [PubMed] [Google Scholar]