Abstract
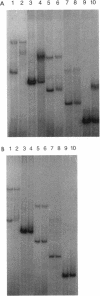
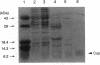
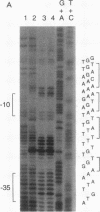
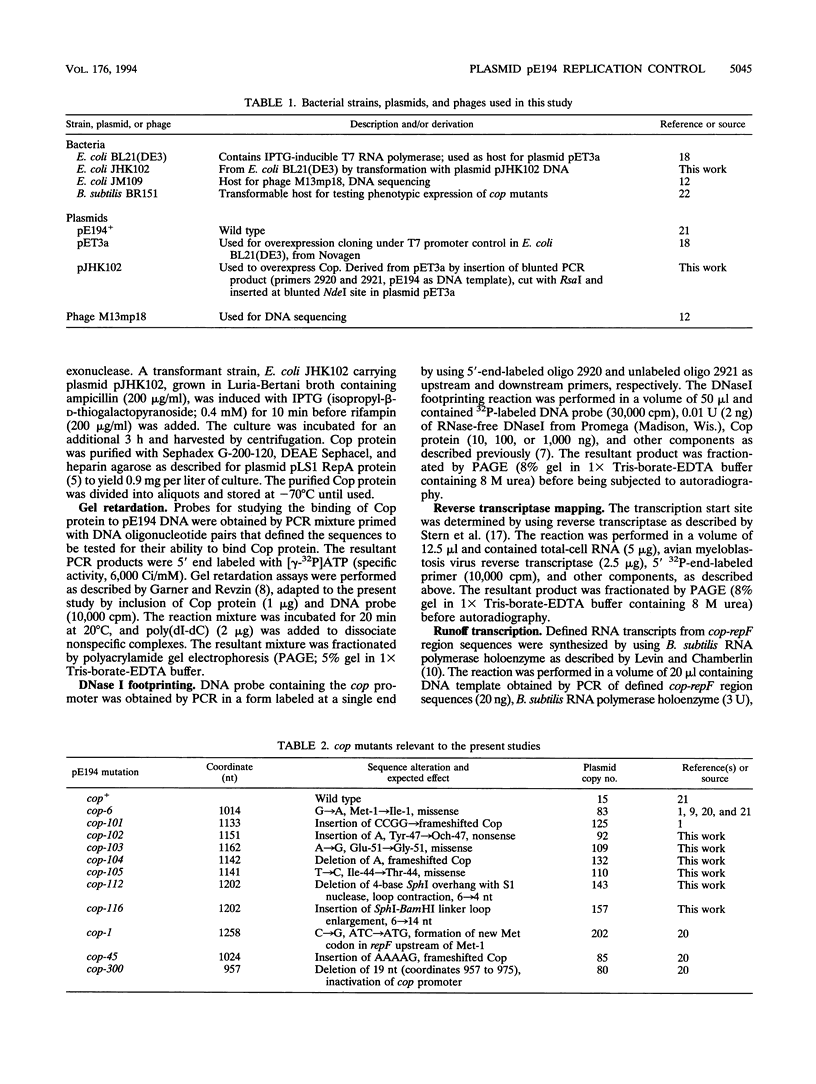
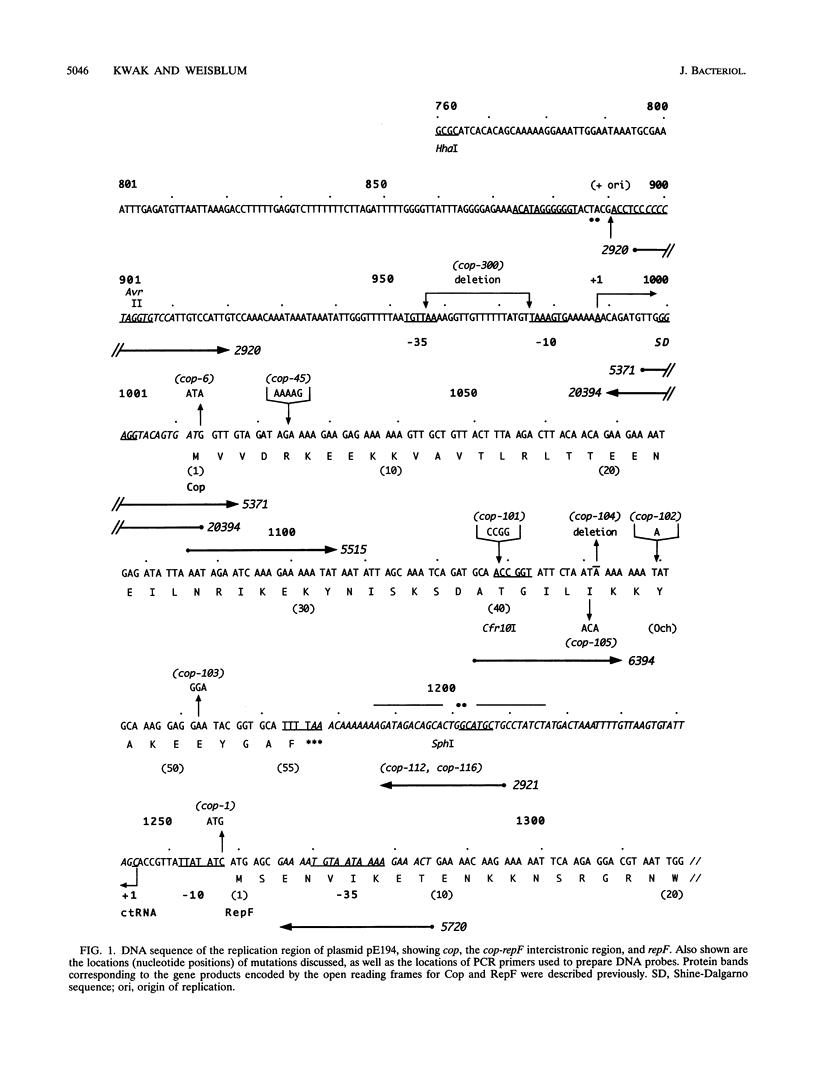
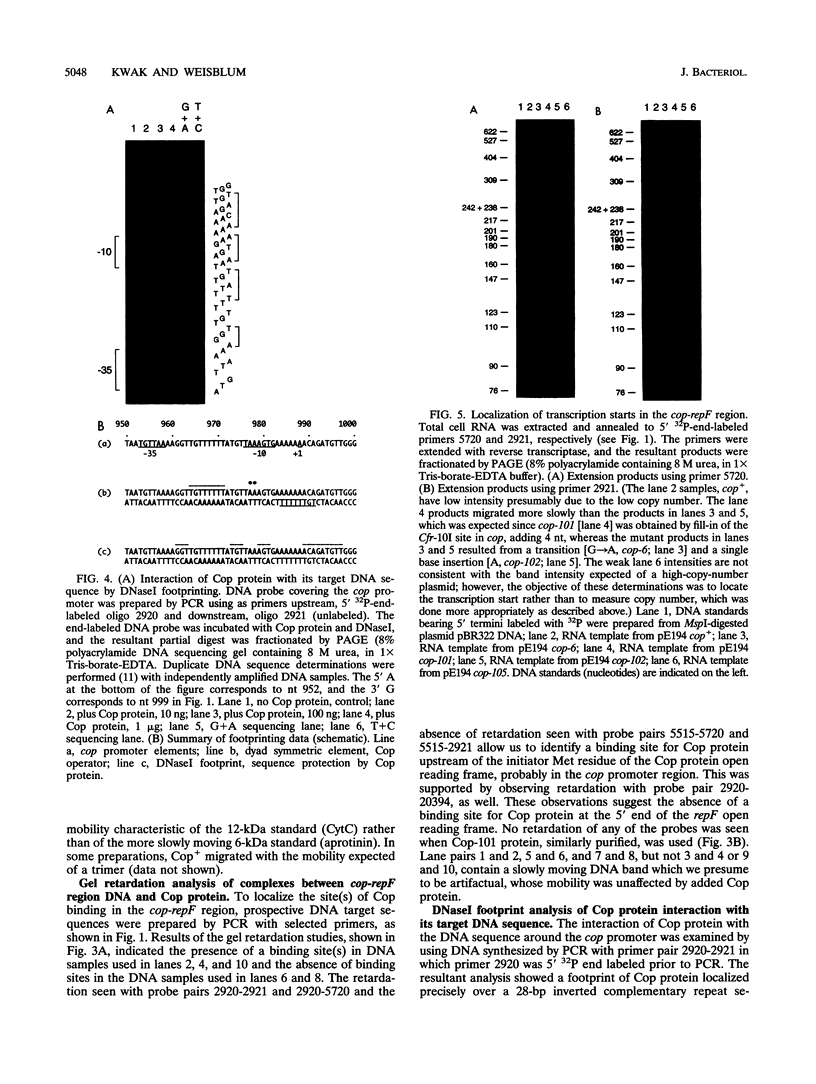
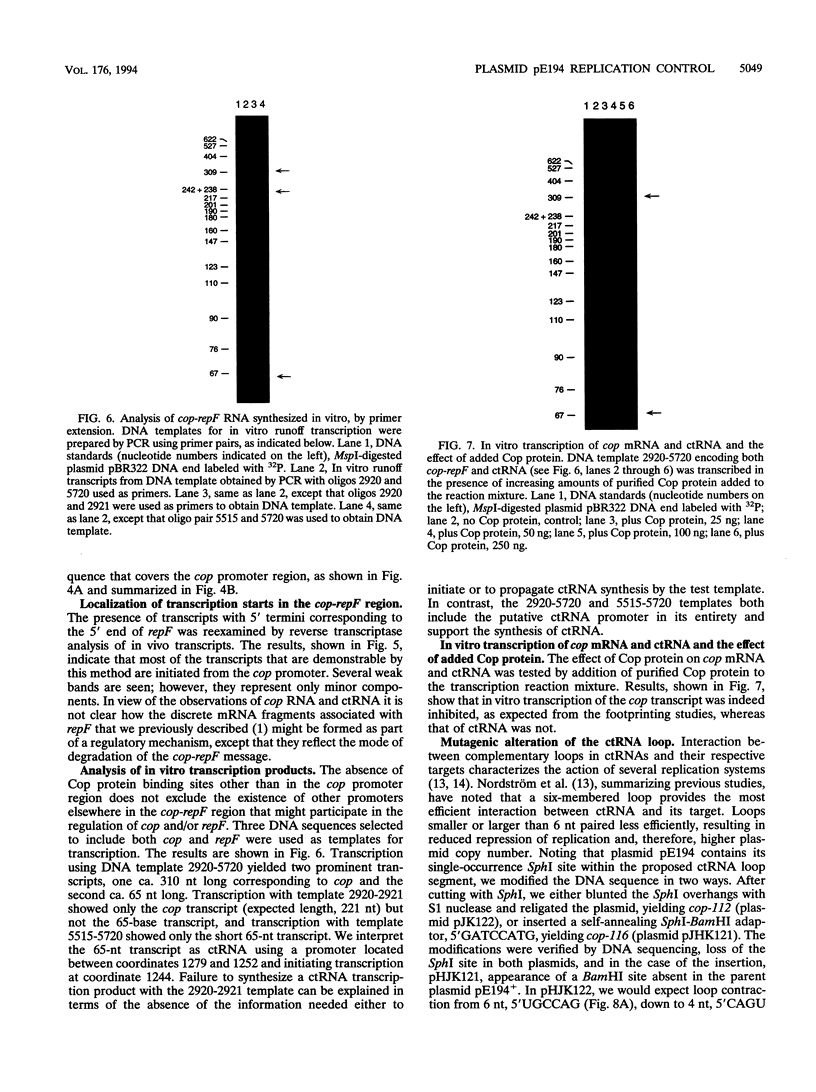
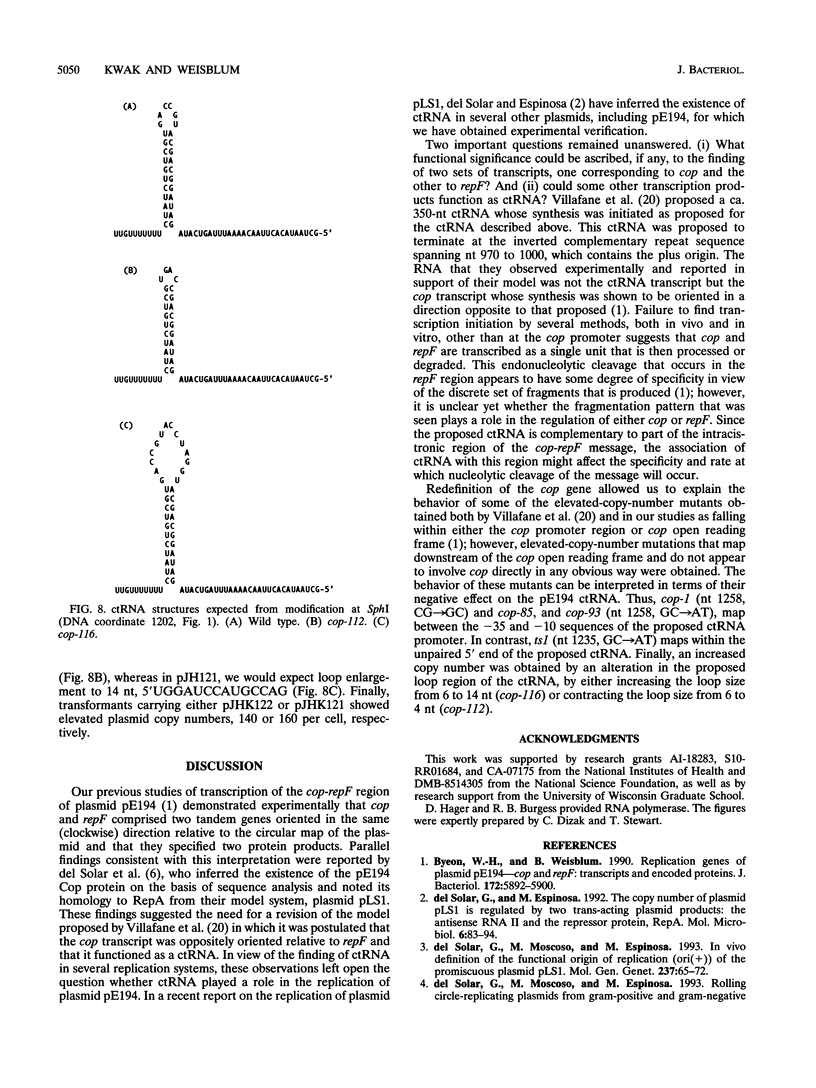
The cop-rep region of plasmid pE194 contains two tandem structural genes, cop and repF, as well as the plus and minus origins of replication. The two structural genes comprise an operon whose expression is repressed by the binding of Cop protein to a 28-bp inverted complementary repeat sequence that overlaps the cop-repF promoter. From its position relative to the promoter and the experimentally determined footprint made by the Cop protein, the 28-bp inverted complementary repeat sequence is presumed to function as the cop operator. The intercistronic region between cop and repF is 80 nucleotides (nt) long and is transcribed bidirectionally: in the forward direction as part of the synthesis of the cop-repF message (ca. 900 nt), and in the reverse direction to yield a countertranscript ca. 65 nt long. The proposed countertranscript RNA (ctRNA) can form a single stem-and-loop structure that includes the single SphI sequence of plasmid pE194 as part of the loop-forming segment. Enlargement of the proposed loop from 6 to 14 nt by insertion of a SphI-BamHI adapter at the SphI site or contraction of the proposed loop down to 4 nt, by cutting with SphI followed by blunting with S1 nuclease, yields mutants with an increased copy number. By gel retardation and DNaseI footprinting analysis, Cop protein was shown to bind to the promoter region of cop; no binding by Cop protein at the 5' end of repF was detected. Two major transcripts were synthesized in vitro by using cop-repF region DNA as a template, the tandem cop-repF transcript, and the ctRNA. Addition of purified Cop protein to an vitro transcription reaction mixture reduced only the rate of cop-repF transcription but not that of ctRNA. These observations suggest that regulations of repF occurs at two levels: (i) with Cop protein acting as a repressor of cop-repF mRNA transcription and (ii) with ctRNA acting as a repressor of RepF translation.
Full text
PDF


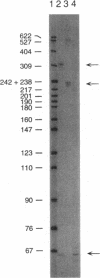
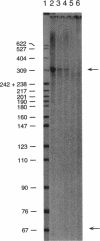
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byeon W. H., Weisblum B. Replication genes of plasmid pE194-cop and repF: transcripts and encoded proteins. J Bacteriol. 1990 Oct;172(10):5892–5900. doi: 10.1128/jb.172.10.5892-5900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. R., Chamberlin M. J. Mapping and characterization of transcriptional pause sites in the early genetic region of bacteriophage T7. J Mol Biol. 1987 Jul 5;196(1):61–84. doi: 10.1016/0022-2836(87)90511-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nordström K., Wagner E. G., Persson C., Blomberg P., Ohman M. Translational control by antisense RNA in control of plasmid replication. Gene. 1988 Dec 10;72(1-2):237–240. doi: 10.1016/0378-1119(88)90148-5. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Plasmid incompatibility. Microbiol Rev. 1987 Dec;51(4):381–395. doi: 10.1128/mr.51.4.381-395.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Sozhamannan S., Dabert P., Moretto V., Ehrlich S. D., Gruss A. Plus-origin mapping of single-stranded DNA plasmid pE194 and nick site homologies with other plasmids. J Bacteriol. 1990 Aug;172(8):4543–4548. doi: 10.1128/jb.172.8.4543-4548.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern S., Moazed D., Noller H. F. Structural analysis of RNA using chemical and enzymatic probing monitored by primer extension. Methods Enzymol. 1988;164:481–489. doi: 10.1016/s0076-6879(88)64064-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Villafane R., Bechhofer D. H., Narayanan C. S., Dubnau D. Replication control genes of plasmid pE194. J Bacteriol. 1987 Oct;169(10):4822–4829. doi: 10.1128/jb.169.10.4822-4829.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B., Graham M. Y., Gryczan T., Dubnau D. Plasmid copy number control: isolation and characterization of high-copy-number mutants of plasmid pE194. J Bacteriol. 1979 Jan;137(1):635–643. doi: 10.1128/jb.137.1.635-643.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E., Smith C., Reilly B. E. Chromosomal location of genes regulating resistance to bacteriophage in Bacillus subtilis. J Bacteriol. 1969 Jun;98(3):1087–1097. doi: 10.1128/jb.98.3.1087-1097.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Solar G. H., Pérez-Martín J., Espinosa M. Plasmid pLS1-encoded RepA protein regulates transcription from repAB promoter by binding to a DNA sequence containing a 13-base pair symmetric element. J Biol Chem. 1990 Jul 25;265(21):12569–12575. [PubMed] [Google Scholar]
- del Solar G. H., de al Campa A. G., Pérez-Martín J., Choli T., Espinosa M. Purification and characterization of RepA, a protein involved in the copy number control of plasmid pLS1. Nucleic Acids Res. 1989 Apr 11;17(7):2405–2420. doi: 10.1093/nar/17.7.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Solar G., Espinosa M. The copy number of plasmid pLS1 is regulated by two trans-acting plasmid products: the antisense RNA II and the repressor protein, RepA. Mol Microbiol. 1992 Jan;6(1):83–94. doi: 10.1111/j.1365-2958.1992.tb00840.x. [DOI] [PubMed] [Google Scholar]
- del Solar G., Moscoso M., Espinosa M. In vivo definition of the functional origin of replication (ori(+)) of the promiscuous plasmid pLS1. Mol Gen Genet. 1993 Feb;237(1-2):65–72. doi: 10.1007/BF00282785. [DOI] [PubMed] [Google Scholar]
- del Solar G., Moscoso M., Espinosa M. Rolling circle-replicating plasmids from gram-positive and gram-negative bacteria: a wall falls. Mol Microbiol. 1993 May;8(5):789–796. doi: 10.1111/j.1365-2958.1993.tb01625.x. [DOI] [PubMed] [Google Scholar]
- te Riele H., Michel B., Ehrlich S. D. Single-stranded plasmid DNA in Bacillus subtilis and Staphylococcus aureus. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2541–2545. doi: 10.1073/pnas.83.8.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]