Abstract
Flos Lonicerae is a medically useful traditional Chinese medicine herb. However, little is known about the antioxidant properties of Flos Lonicerae extracts. Here the antioxidant capacity of water, methanolic and ethanolic extracts prepared from Flos Lonicerae to scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical and reduce Fe3+ to Fe2+ is examined. Chlorogenic acid, a major component of Flos Lonicerae, is identified and further purified from 70% ethanolic extract with high performance liquid chromatography (HPLC) and its antioxidant capacity is characterized. The total phenolic compounds and chlorogenic acid contents in Flos Lonicerae are determined. The present results demonstrate that the Flos Lonicerae extracts exhibit antioxidant activity and chlorogenic acid is a major contributor to this activity.
Keywords: Flos Lonicerae, Chlorogenic acid, Antioxidant
INTRODUCTION
Flos Lonicerae, also called Jinyinhua, is one of the widely used herbs prescribed in many Chinese formulas. It has latent-heat-clearing, antipyretic, detoxicant and anti-inflammatory actions. It has been, therefore, prescribed to treat fever due to common cold, febrile disease, dysentery, carbuncles, and virulent swellings (Chang and But, 1986; MPHPRC, 2000). In many previous reports, chlorogenic acid, one of the major components in Flos Lonicerae, has been widely adopted to control the quality of Flos Lonicerae owing to its high content and antibiotic property. Several kinds of the other components, such as flavonoids, iridoid glucosides and saponins have been also discovered and characterized using advanced analytical techniques due to their effective activities (Chai et al., 2005; Chang and But, 1986; Chen et al., 2007; Li et al., 2003; Song et al., 2006; Zhang et al., 2006). Therefore great progress has been made in the studies and clinical applications of Flos Lonicerae in the last couple of years (Chang and But, 1986; MPHPRC, 2000; Shi et al., 2007).
Free radicals are highly reactive species produced in the body during normal metabolic functions or introduced from the environment. Free radicals have been reported to play an important role in more than sixty different health conditions, including the aging process, cancer, atherosclerosis, and so on (Ames et al., 1993; Harman, 1984). Therefore, it is beneficial for our health to scavenge these harmfully free radicals. Nowadays, scientists have been exploring the biosensors to evaluate their antioxidant status to prevent some diseases and control the quality of foods (Mello and Kubota, 2007), which has lead to a revolution that is promising a new paradigm of healthcare. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) is one of the stable nitrogen centered free radicals and a free radical representative to be effectively scavenged by antioxidants (Villaño et al., 2007). Ion reducing ability is also one of the important properties possessed by antioxidants, although the metal chelation has often been considered as a minor mechanism in the antioxidant action. Antioxidants can bind to and reduce Fe3+ to Fe2+ and form an inactive Fe2+-anti-oxidant complex to protect Fe2+ from auto-oxidation (Yoshino and Murakami, 1998). The examination of the abilities for an antioxidant to scavenge DPPH radical and ferric reduction has been traditionally and widely accepted to justify the antioxidant capacity.
Although Flos Lonicerae has been extensively studied for many years, no investigation on the antioxidant activity of the extracts prepared from Flos Lonicerae has been reported so far. In this study, we examined the antioxidant activity of extracts differently prepared from Flos Lonicerae with two different assays and found that all three extracts exhibit antioxidant activity. The contribution of the major component of Flos Lonicerae, chlorogenic acid, to the antioxidant activity of Flos Lonicerae has been characterized.
MATERIALS AND METHODS
Materials
The Flos Lonicerae sample was collected from He’nan Province of China. High performance liquid chromatography (HPLC) apparatus was purchased from American Lab Alliance Corporation. Beckman DU-640 spectrophotometer equipped with a thermostatized auto-cell-holder was purchased from Beckman Corporation (USA). The chlorogenic acid reference, DPPH and Folin-Ciocalteu reagent were purchased from Sigma Corporation (USA). All reagents used in HPLC analyses were HPLC grade. The other reagents were of analytical grade.
Methods
1. Preparation of Flos Lonicerae extracts
Flos Lonicerae sample was dried at 50 °C to constant weight. Approximately 10 g of pulverized sample was added to a round-bottomed flask containing 250 ml of different solvents including distilled water, 70% ethanol and methanol, respectively. The mixture was heated under reflux for 4 h. The extracts were filtered and the residue was re-extracted under the same conditions. The extracts were combined, concentrated and evaporated to dryness with rotary evaporator under reduced pressure. The residue was dissolved with 30 ml of water into a 100 ml flask and the product was stored at 4 °C for 24 h followed by centrifugation at 10 000 r/min for 30 min. The supernatant fraction was designated as Flos Lonicerae crude extract and lyophilized to fine powder for long term storage and dissolved with methanol for identification and quantification analysis with HPLC.
2. HPLC analysis
The dissolved extracts were filtered through 0.22 μm membrane filter before loading into the HPLC system. Chromatography was carried out on C18 analytical/preparative column at 25 °C. The sample loop of 20 μl or 1 ml was used for sample injection. The mobile phase consisted of two solvent components: water (solvent A) and acetonitrile-citrate acidwater (20:40:40, v/v, solvent B). In the gradient elution solvent B was increased from 5%~100% within 30 min. The flow rate was 0.2 ml/min for analytical and 0.8 ml/min for preparative purpose respectively. The wavelength used to detect chlorogenic acid was 324 nm. Twenty microlitres of different concentrations (0.02~0.50 mg/ml) of chlorogenic acid reference was loaded into HPLC system for construction of the calibration curve by plotting the peak areas (Y) versus the concentrations (X). Each extract powder was dissolved with methanol to a concentration of 0.5 mg/ml and 20 μl of the crude extract was loaded into HPLC system for identification and quantification of chlorogenic acid in Flos Lonicerae respectively. The content of chlorogenic acid in Flos Lonicerae was determined from the corresponding calibration curve and the content was expressed as milligram of chlorogenic acid per gram of Flos Lonicerae. The peak area of chlorogenic acid in each extract was mean of three parallel measurements for chlorogenic acid quantification. The chromatography peak was identified by comparing the retention time with that of chlorogenic acid reference. The chlorogenic acid used for antioxidant activity assay was produced through preparative column separation and manually collected according to the retention time and chromatography peak of chlorogenic acid under the same chromatography conditions.
3. Determination of total phenolic compounds content
Total phenolic compound content in each extract was spectrophotometrically determined according to the Folin-Ciocalteu procedure by reading the absorbance at 760 nm against a methanol blank (Singleton and Rossi, 1965). Briefly, samples (150 μl, three replicates) were introduced into test tubes and then 750 μl of Folin-Ciocalteu reagent and 600 μl of sodium carbonate (7.5%) were added. The tubes’ contents were mixed and incubated at 50 °C for 10 min. Absorption at 760 nm was measured. The total phenolic content was expressed as milligram of gallic acid equivalents (GAE) per gram of Flos Lonicerae.
4. Scavenging DPPH radical assay
The capacity of each sample to scavenge DPPH radical was spectrophotometrically measured according to Sreejayan-Rao procedure (Sreejayan and Rao, 1996). Briefly, the freshly prepared stock solution of each extract was diluted into different concentrations of solutions (50, 100, 250, 500, 1 000 and 2 000 μg/ml) with methanol. The preparative chlorogenic acid used in this assay was quantified and also dissolved with methanol. One hundred microlitres of each solution was added into methanolic solution of DPPH radical (100 μmol, 2.90 ml). The mixture was shaken vigorously for 30 s and left to stand for 20 min at room temperature in a dark room. Absorbance was recorded at 517 nm. The effect of DPPH radical scavenging of the sample was calculated according to the formula:
| Scavenging effect (%)=[(Ac−Aa)/Ac]×100, |
where A c is the absorbance of the control and A a is the absorbance of the sample. DPPH radical scavenging reaction kinetics of the samples, including 1 000 μg/ml of preparative chlorogenic acid, chlorogenic acid reference and 70% ethanolic extract, was also observed. Absorbance was recorded at regular intervals of 15 s for total 2 min after shaking the mixture vigorously for 3 s. Ascorbic acid and methanol served as positive and negative control respectively. The results are mean±SD of three parallel measurements.
5. Ferric reducing antioxidant power (FRAP) assay
FRAP assay is a simple and reliable colorimetric method commonly used for measuring the total antioxidant capacity (Benzie and Strain, 1996). Briefly, 900 μl of FRAP reagent, prepared freshly and warmed at 37 °C, was mixed with 90 μl of distilled water and 30 μl of test sample (100 μg). The FRAP reagent contained 2.5 ml of a 10 mmol/L 2,4,6-tripyridy-striazine (TPTZ) solution in 40 mmol/L HCl plus 2.5 ml of 20 mmol/L FeCl3·6H2O and 25 ml of 0.3 mol/L acetate buffer, pH 3.6. The mixture was shaken vigorously for 3 s and left to stand for 30 min at room temperature and the readings at the absorption maximum (595 nm) were recorded. The ferric reducing reaction kinetics of the samples, including 100 μg of preparative chlorogenic acid, chlorogenic acid reference and 70% ethanolic extract, was also observed. The readings were taken every 30 s using a Beckman DU-640 spectrophotometer at 37 °C for up to 30 min. Ascorbic acid and methanol served as positive and negative control respectively. Results are mean±SD of three parallel measurements.
RESULTS AND DISCUSSION
Identification and quantification of chlorogenic acid
It is well known that the pharmacological and biological activities of Flos Lonicerae are closely linked with its functional components, of which chlorogenic acid has been extensively studied mainly due to its high content and bioactive functions. In this study, we prepared three Flos Lonicerae extracts with different solvents and the chlorogenic acid component was identified by comparing the retention time with that of chlorogenic acid reference (Figs.1a, 1b, 1c and 1e) and quantified with HPLC (Table 1). The linear regression equation was calculated as:
| Y=1.05×108X+8.53×106, r2=0.9985, |
which showed good linear regression within the test ranges (0.02~0.50 mg/ml). The chlorogenic acid content in Flos Lonicerae extracts was calculated as shown in Table 1. The content (0.51%~1.44%) of chlorogenic acid in He’nan Flos Lonicerae is lower than the content (3.36%~3.83%) of chlorogenic acid in Hanzhong Flos Lonicerae (Hai et al., 2006), but similar to the criterion (1.5%) provided in China Pharmacopoeia (2000 Ed.). Moreover, the total phenolic compounds contents in Flos Lonicerae and its extracts were also quantified to explore the correlation between the phenolic compounds content and the antioxidant activity (Table 1).
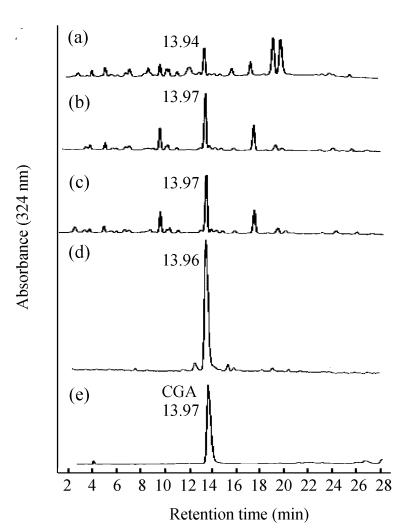
Fig. 1.
HPLC analysis. The HPLC profiles (a), (b), and (c) represent the identification of chlorogenic acid in water, methanolic and 70% ethanolic extracts from Flos Lonicerae respectively. The HPLC profile (d) represents purified chlorogenic acid from 70% ethanolic extract with HPLC. There are several contaminant constituents in the prepared chlorogenic acid fraction around chlorogenic acid peak which are caused by manual collection during purification. The peaks labelled with CGA are chlorogenic acid because the retention time was identical with that of chlorogenic acid standard (e) (13.97 min). Ten micrograms the samples (a), (b), (c), (d) were respectively loaded into the HPLC system except chlorogenic acid standard (5 μg)
Table 1.
Chlorogenic acid and total phenolic compounds contents in Flos Lonicerae and its extracts
| Solvents | Chlorogenic acid (mg/g) |
Total phenolic compounds (mg GAE/g) |
||
| Flos Lonicerae | Extracts | Flos Lonicerae | Extracts | |
| H2O | 5.09±0.64 | 134.54±6.34 | 8.60±0.83 | 205.43±9.76 |
| Methanol | 13.89±1.18 | 338.45±13.36 | 2.32±1.94 | 428.93±16.39 |
| 70% ethanol | 14.38±1.21 | 345.36±13.45 | 2.54±1.76 | 432.08±16.54 |
Results are mean±SD of three measurements
Determination of antioxidant activity of Flos Lonicerae extracts
Antioxidants are widely applied to foods and medicines because they can counteract cellular free radicals and reduce metal ion to interrupt the oxidizing chain reaction before they cause damages. Antioxidants, therefore, can protect our bodies against many health problems (Ames et al., 1993; Halliwel and Gutteridge, 1999; Harman, 1984; Venkat Ratnam et al., 2007). It was reported that blood lipid was markedly decreased by intravenous injection combined with concomitant oral administration of Flos Lonicerae extract for cases of hyperlipidemia (Chang and But, 1986). We suspect that the activity of Flos Lonicerae extract may be linked with its antioxidant components, because antioxidants have been thought to be involved in reducing production of free radicals and arterial deposition of cholesterol as well as blood lipid (Chang and But, 1986; Chenni et al., 2007; Durak et al., 2004; Fernández-Checa, 2003; Joshi et al., 2001; Ramkumar et al., 2003; Yang et al., 2006; Zhang et al., 2001).
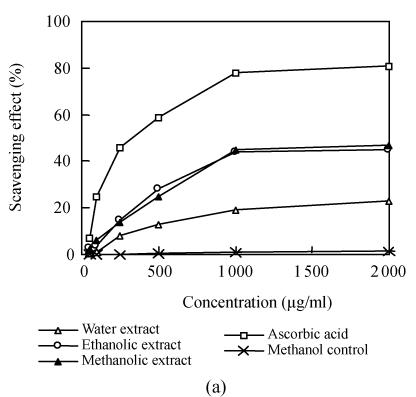
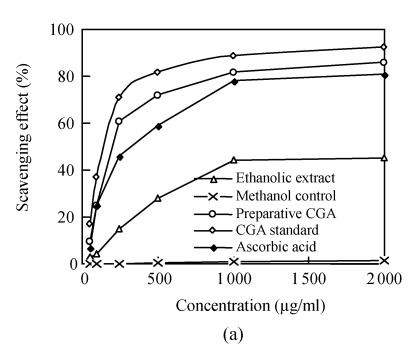
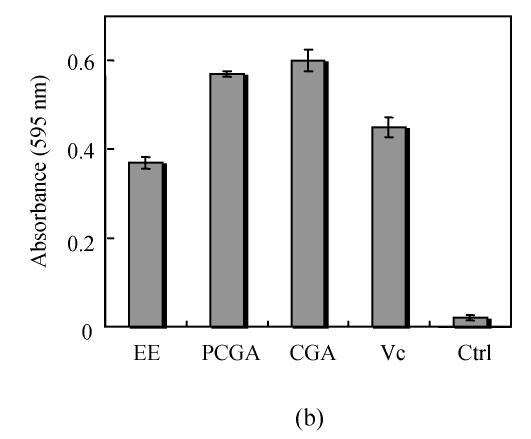
To determine whether the Flos Lonicerae extracts have the antioxidant activity, two classic assays, scavenging DPPH radical assay and ferric reducing assay, were carried out. The scavenging DPPH radical assay results indicated that all Flos Lonicerae crude extracts examined here could scavenge DPPH free radical. Twenty-three percent to forty-seven percent of DPPH radicals were scavenged within 20 min when the concentration of each extract was over 1000 μg/ml, which is, however, lower than that of ascorbic acid (81%), a potent antioxidant to scavenge radicals (Fig.2a). The ferric reducing assay results also showed that all crude extracts of Flos Lonicerae exhibit antioxidant activity to reduce Fe3+ to Fe2+, which is, however, still lower than that of ascorbic acid, a potent ferric-reducing-ability antioxidant (Fig.2b). Among three Flos Lonicerae extracts the antioxidant activity of water extract is the lowest and that of the other two extracts is higher and similar, which is linked with the total phenolic compounds contents in the extracts (Table 1) and consistent with previous reports about the correlation between antioxidant activity and total phenolic compounds contents in the extracts (Katalinic et al., 2004; Sun and Ho, 2005).
Fig. 2.
Determination of the antioxidant capacity of Flos Lonicerae crude extracts. (a) Determination of the antioxidant capacity of Flos Lonicerae extracts to scavenge DPPH radical; (b) Determination of the antioxidant capacity of Flos Lonicerae extracts to reduce Fe3+ to Fe2+
WE, EE, ME, Vc and Ctrl stand for Flos Lonicerae water extract, 70% ethanolic extract, methanolic extract, ascorbic acid and methanol control, respectively. Results are mean of three parallel measurements and calculated with MS Excel software
Contribution of chlorogenic acid to antioxidant activity of Flos Lonicerae extract
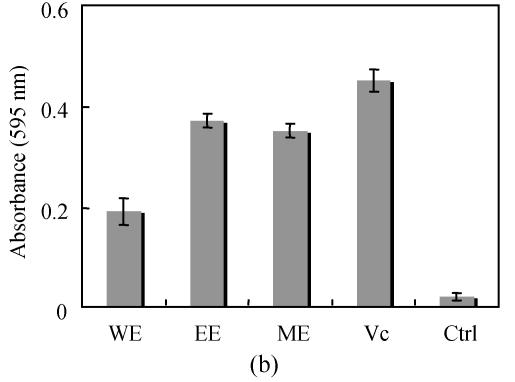
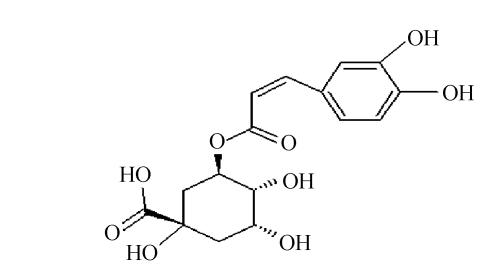
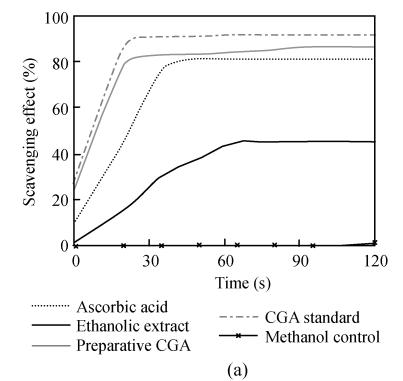
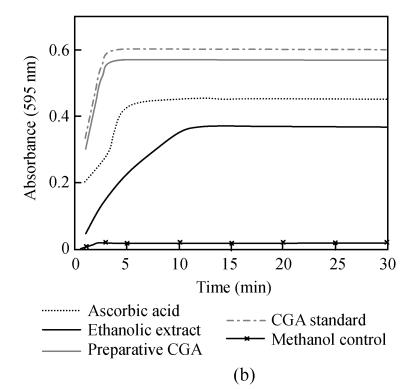
Chlorogenic acid, an ester formed between caffeic acid and quinic acid (Fig.3), is one of the most naturally existing phenolic compounds found in numerous plant species and also a major component of Flos Lonicerae that has long been regarded as one of the most important chemical standards for quality evaluation of Flos Lonicerae (Chai et al., 2005). It was widely reported that chlorogenic acid and related compounds are well known to be antioxidants (Kono et al., 1997; Ohnishi et al., 1994). Therefore, we wonder whether chlorogenic acid is a major contributor to the antioxidant activity of Flos Lonicerae extract. To test this hypothesis, we firstly identified and further purified chlorogenic acid from 70% ethanolic extract, due to its relatively high efficiency of chlorogenic acid extraction, low cost and little toxicity, with HPLC (Figs.1d and 1e) and examined its antioxidant activity compared with that of 70% ethanolic extract and ascorbic acid as shown in Fig.4. Preparative chlorogenic acid exhibits very strong antioxidant activity, which is much higher than that of 70% ethanolic extract and even higher than that of ascorbic acid, a well-known potent antioxidant; Secondly, we determined chlorogenic acid content in the extracts (Table 1). The chlorogenic acid content in the water extract is lower than that of ethanolic and methanolic extracts by over 50%. The chlorogenic acid content reflects the antioxidant activity of the Flos Lonicerae extracts to a certain extent (Table 1, Figs.1a~1c and Fig.2); Thirdly, the reaction kinetics of the samples including preparative chlorogenic acid, 70% ethanolic extract, ascorbic acid and chlorogenic acid reference to scavenge DPPH radical and reduce Fe3+ to Fe2+ was observed respectively (Fig.5). The present results demonstrated that higher content of chlorogenic acid exhibits higher efficiency to scavenge DPPH radical and reduce Fe3+ to Fe2+. As shown in the Fig.5, preparative chlorogenic acid can scavenge 80% DPPH radicals within 20 s and then keep stable, while 70% ethanolic extract and ascorbic acid can only scavenge 15% and 45% under the same conditions, and finally keep stable after 65 s and 35 s respectively. The antioxidant activity of preparative chlorogenic acid is slightly lower than that of chlorogenic acid standard, which is caused by impurity of chlorogenic acid manually collected (Figs.1d and 1e). According to the above analyses, we concluded that chlorogenic acid, a major component of Flos Lonicerae, is also a major contributor to the antioxidant activity of its extracts.
Fig. 3.
The structure of chlorogenic acid
Fig. 4.
Determination of the antioxidant capacity of preparative chlorogenic acid from Flos Lonicerae extract. (a) The antioxidant capacity of the samples to scavenge DPPH radical; (b) The antioxidant capacity of the samples to reduce Fe3+ to Fe2+
EE, PCGA, CGA, Vc and Ctrl stand for Flos Lonicerae 70% ethanolic extract, preparative chlorogenic acid, chlorogenic acid standard, ascorbic acid and methanol control. Results are mean of three parallel measurements and calculated with MS Excel software
Fig. 5.
Reaction kinetics of DPPH radical scavenging (a) and ferric reducing (b)
Results are mean of three parallel measurements and calculated with MS Excel software
CONCLUSION
In this study, for the first time we characterized the antioxidant activity of extracts from Flos Lonicerae with two different ways: DPPH free radical scavenging assay and FRAP assay. The results demonstrated that the extracts of Flos Lonicerae bear antioxidant activity which is correlated with the total phenolic compounds content and mainly contributed by the presence of chlorogenic acid.
Footnotes
Project (No. 20039902) supported by the Education Committee of Tianjin, China
References
- 1.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proceedings of the National Academy of Science USA. 1993;90(17):7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 3.Chai XY, Li SL, Li P. Quality evaluation of Flos Lonicerae through a simultaneous determination of seven saponins by HPLC with ELSD. Journal of Chromatography A. 2005;1070(1-2):43–48. doi: 10.1016/j.chroma.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 4.Chang HM, But PPH. Pharmacology and Applications of Chinese Material Medica (II) Singapore: World Scientific; 1986. pp. 787–792. [Google Scholar]
- 5.Chen J, Song Y, Li P. Capillary high-performance liquid chromatography with mass spectrometry for simultaneous determination of major flavonoids, iridoid glucosides and saponins. Journal of Chromatography A. 2007;1157(1-2):217–226. doi: 10.1016/j.chroma.2007.05.063. [DOI] [PubMed] [Google Scholar]
- 6.Chenni A, Yahia DA, Boukortt FO, Prost J, Lacaille-Dubois MA, Bouchenak M. Effect of aqueous extract of Ajuga iva supplementation on plasma lipid profile and tissue antioxidant status in rats fed a high-cholesterol diet. Journal of Ethnopharmacology. 2007;109(2):207–213. doi: 10.1016/j.jep.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 7.Durak I, Kavutcu M, Aytaç B, Avci A, Devrim E, Özbek H, Öztürk HS. Effects of garlic extract consumption on blood lipid and oxidant/antioxidant parameters in humans with high blood cholesterol. The Journal of Nutritional Biochemistry. 2004;15(6):373–377. doi: 10.1016/j.jnutbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Fernández-Checa JC. Redox regulation and signaling lipids in mitochondrial apoptosis. Biochemical and Biophysical Research Communications. 2003;304(3):471–479. doi: 10.1016/S0006-291X(03)00619-3. [DOI] [PubMed] [Google Scholar]
- 9.Hai J, Wei W, Ding R. Determination of chlorogenic acid in Flos Lonicerae of Hanzhong by HPLC. Shanxi Li Gong Xue Yuan Xue Bao. 2006;22(1):87–89. (in Chinese) [Google Scholar]
- 10.Halliwel B, Gutteridge JMC. Free Radical in Biology and Medicine. UK: Oxford University Press; 1999. p. 617. [Google Scholar]
- 11.Harman D. Free radical theory of aging: the “free radical” diseases. Age. 1984;7(4):111–131. doi: 10.1007/BF02431866. [DOI] [Google Scholar]
- 12.Joshi R, Adhikari S, Patro BS, Chattopadhyay S, Mukherjee T. Free radical scavenging behavior of folic acid: evidence for possible antioxidant activity. Free Radical Biology and Medicine. 2001;30(12):1390–1399. doi: 10.1016/S0891-5849(01)00543-3. [DOI] [PubMed] [Google Scholar]
- 13.Katalinic V, Milos M, Modun D, Musi I, Boban M. Antioxidant effectiveness of selected wines in comparison with (+) catechin. Food Chemistry. 2004;86(4):593–600. doi: 10.1016/j.foodchem.2003.10.007. [DOI] [Google Scholar]
- 14.Kono Y, Kobayashi K, Tagawa S, Adachi K, Ueda A, Sawa Y, Shibata H. Antioxidant activity of polyphenolics in diets: rate constants of reactions of chlorogenic acid and caffeic acid with reactive species of oxygen and nitrogen. Biochimica et Biophysica Acta (BBA)-General Subjects. 1997;1335(3):335–342. doi: 10.1016/S0304-4165(96)00151-1. [DOI] [PubMed] [Google Scholar]
- 15.Li HJ, Li P, Ye WC. Determination of five major iridoid glucosides in Flos Lonicerae by high-performance liquid chromatography coupled with evaporative light scattering detection. Journal of Chromatography A. 2003;1008(2):167–172. doi: 10.1016/S0021-9673(03)00978-6. [DOI] [PubMed] [Google Scholar]
- 16.Mello LD, Kubota LT. Biosensors as a tool for the antioxidant status evaluation. Talanta. 2007;72(2):335–348. doi: 10.1016/j.talanta.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 17.MPHPRC (Ministry of Public Health of the People’s Republic of China) Pharmacopoeia of the People’s Republic of China. Beijing: Chemical Industry Press; 2000. p. 177. (in Chinese) [Google Scholar]
- 18.Ohnishi M, Morishita H, Iwahashi H, Toda S, Shirataki Y, Kimura M, Kido R. Inhibitory effects of chlorogenic acids on linoleic acid peroxidation and haemolysis. Phytochemistry. 1994;36(3):579–583. doi: 10.1016/S0031-9422(00)89778-2. [DOI] [Google Scholar]
- 19.Ramkumar KM, Rajesh R, Anuradha CV. Food restriction attenuates blood lipid peroxidation in carbon tetrachloride-intoxicated rats. Nutrition. 2003;19(4):358–362. doi: 10.1016/S0899-9007(02)00961-9. [DOI] [PubMed] [Google Scholar]
- 20.Shi GR, Rao LQ, Yu HZ, Xiang H, Pen GP, Long S, Yang C. Yeast-cell-based microencapsulation of chlorogenic acid as a water-soluble antioxidant. Journal of Food Engineering. 2007;80(4):1060–1067. doi: 10.1016/j.jfoodeng.2006.06.038. [DOI] [Google Scholar]
- 21.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture. 1965;16(3):144–158. [Google Scholar]
- 22.Song Y, Li SL, Wu MH, Li HJ, Li P. Qualitative and quantitative analysis of iridoid glycosides in the flower buds of Lonicera species by capillary high performance liquid chromatography coupled with mass spectrometric detector. Analytica Chimica Acta. 2006;564(2):211–218. doi: 10.1016/j.aca.2006.01.068. [DOI] [Google Scholar]
- 23.Sreejayan N, Rao MNA. Free radical scavenging activity of curcuminoids. Drug Res. 1996;46(22):169–171. [PubMed] [Google Scholar]
- 24.Sun T, Ho CT. Antioxidant activities of buckwheat extracts. Food Chemistry. 2005;90(4):743–749. doi: 10.1016/j.foodchem.2004.04.035. [DOI] [Google Scholar]
- 25.Venkat Ratnam D, Ankolaa D, Bhardwaja V, Sahanaa DK, Ravi Kumar MNV. Role of antioxidants in prophylaxis and therapy: a pharmaceutical perspective. Journal of Controlled Release. 2007;113(3):189–207. doi: 10.1016/j.jconrel.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Villaño D, Fernández-Pachóna MS, Moyáb ML, Troncosoa AM, García-Parrilla MC. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta. 2007;71(1):230–235. doi: 10.1016/j.talanta.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 27.Yang R, Le G, Li A, Zheng J, Shi Y. Effect of antioxidant capacity on blood lipid metabolism and lipoprotein lipase activity of rats fed a high-fat diet. Nutrition. 2006;22(11-12):1185–1191. doi: 10.1016/j.nut.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 28.Yoshino M, Murakami K. Interaction of iron with polyphenolic compounds: application to antioxidant characterization. Analytical Biochemistry. 1998;257(1):40–44. doi: 10.1006/abio.1997.2522. [DOI] [PubMed] [Google Scholar]
- 29.Zhang ZS, Chang Q, Zhu M, Huang Y, Walter K, Ho K, Chen ZY. Characterization of antioxidants present in hawthorn fruits. Journal of Nutritional Biochemistry. 2001;12(3):144–152. doi: 10.1016/S0955-2863(00)00137-6. [DOI] [PubMed] [Google Scholar]
- 30.Zhang HY, Hu P, Luo G. Screening and identification of multi-component in Qingkailing injection using combination of liquid chromatography/time-of-flight mass spectrometry and liquid chromatography/ion trap mass spectrometry. Analytica Chimica Acta. 2006;577(2):190–200. doi: 10.1016/j.aca.2006.06.053. [DOI] [PubMed] [Google Scholar]