Abstract
This study examined the effects of dietary α-tocopheryl acetate supplementation on antioxidant enzyme activities and fillet quality in commercial-size Sparus macrocephalus. Three hundred fish [main initial weight (350±12) g] were divided into three groups (E250, E500 and E1000) and reared in 9 cages. The fish were fed for 8 weeks with three diets containing different levels of dietary α-tocopheryl acetate (289, 553, 1 069 mg/kg). Over the experimental period, fish were fed to satiation and reached a final mean weight of (465±28) g without significant body weight difference and proximate composition difference. Fillet α-tocopherol was significantly (P<0.05) different between groups, reaching levels of 14.2, 22.1, 30.9 μg/mg fillet for groups E250, E500 and E1000, respectively. Total serum superoxide dismutase (SOD) activity increased significantly (P<0.05) in fish fed the diets high in α-tocopheryl acetate, but serum glutathione peroxidase (GPX) activity was unaffected. In storage on ice, fillets of fish fed the diets high in α-tocopheryl acetate exhibited significantly lower (P<0.05) levels of oxidation. These results suggested that increased dietary α-tocopheryl acetate could increase its flesh deposition, increase the activity of SOD and prevent lipid peroxidation of Sparus macrocephalus fillets in retail storage on ice.
Keywords: Fillet quality, Lipid peroxidation, Sparus macrocephalus, α-Tocopherol
INTRODUCTION
Lipid peroxidation is a serious problem for biological materials containing unsaturated fatty acids. This is particularly important for aquatic animals since they normally contain greater amount of polyunsaturated fatty acids (PUFA) than other species. Frequently, high value seafood products are stored for retail purposes on ice under refrigerated conditions, often without any further protection from oxidation. As a consequence, lipid peroxidation phenomena, which have a large influence on flesh quality along with autolytic and bacterial spoilage (Watanabe et al., 1996), may easily occur.
Among natural antioxidants, α-tocopherol is a potent biological antioxidant that can protect biomembranes and lipid components containing unsaturated fatty acids against attack from oxygen free radicals. Decreased lipid peroxidation has been found in fish when supplemented with α-tocopheryl acetate (a stable ester of α-tocopherol) at elevated levels in the diet (Huang and Huang, 2004; Kiron et al., 2004; Kolkovski et al., 2000; Tocher et al., 2002). To date, the influence of α-tocopherol on seafood quality has been studied for several species such as Atlantic salmon (Salmo salar) (Onibi et al., 1996; Scaife et al., 2000), rainbow trout (Oncorhynchus mykiss) (Jensen et al., 1998a), sea bass (Dicentrarchus labrax) (Pirini et al., 2000; Stéphan et al., 1995) and turbot (Scophthalmus maximus) (Frigg et al., 1990; Ruff et al., 2003), but many of these studies have been carried out on sub commercial-size fish and few studies exist for Sparus macrocephalus.
In fish, the relationship between dietary α-tocopheryl acetate and antioxidant enzyme activities has been reported in several studies. However, the reported effects of α-tocopherol on the levels of antioxidant enzymes in fish are not uniform (Lygren et al., 2000; Mourente et al., 2002).
The purpose of the present study was to evaluate the effect of α-tocopheryl acetate levels in preslaughter diet on tissue α-tocopherol levels, serum antioxidant enzymes activity and the susceptibility of flesh fillets (stored on ice) to lipid oxidation.
MATERIALS AND METHODS
Diets
The components of a basal diets presented in Table 1 should provide adequate nutrient supply to Sparus macrocephalus. White fishmeal, soybean meal and protein powder of blood corpuscles were used for sources of protein; fish oil and α-starch for non-protein energy. α-Tocopherol content in basal diet (E250) was 289 mg/kg. Additional α-tocopheryl acetate (Mingo Tech-Bank Co., Ltd., China) was added in fish oil and data recorded after analysis 553 and 1069 mg/kg for diets E500 and E1000, respectively.
Table 1.
Formulation and proximate composition of the experimental diet (on dry matter basis)
| Basal composition | |
| Ingredients (%) | |
| White fishmeal | 65.8 |
| Soybean meal | 5 |
| α-Starch | 14 |
| Protein power of blood powder | 2 |
| Vitamin pre-mixture* | 1 |
| Mineral pre-mixture* | 2 |
| Cellulose | 0.2 |
| Fish oil |
10 |
| α-Tocopherol content (mg/kg) | |
| Diet E250 (basal diet) | 289 |
| Diet E500 | 553 |
| Diet E1000 | 1 069 |
Vitamin and mineral pre-mixtures were provided by the Mingo Tech-Bank Co., Ltd., China;
Total energy is 15.59 kJ/kg
To avoid lipid peroxidation, diets were stored at −20 °C until used. Every morning, before feeding fish, the quantities needed for the day were taken out from the freezer and left to reach room temperature.
Fish and rearing conditions
Sparus macrocephalus weighting (350±12) g were purchased from net-cage culture in a private fish farm (Fenghuangjiao Port, Zhejiang Province, China). Three hundred fish collected from a single net-cage, containing a homogeneous lot, were placed in 9 cages of 3 m3 (1.5 m×1 m×2 m) size and divided into 3 groups. After two weeks of adaptation (basal diet fed), the fish were fed twice daily until satiety for 8 weeks. During the experimental period, water temperature and salinity were 22~29 °C and 38 g/L respectively.
Sampling and storage conditions
Ten Sparus macrocephalus per diet were sampled at the end of the experiment. Blood was drawn from the caudal vein of the individual fish. The whole blood was collected in a syringe, allowed to clot for 1 h in microtubes at room temperature, followed by 5 h at 4 °C and then serum was harvested by centrifuging at 1500×g for 5 min at 4 °C. All serum samples were preserved at −20 °C prior to analysis.
The fish were filleted in a box containing marine water and ice after blood extraction. Four fillet pieces per fish were sampled. One fillet was stored at liquid nitrogen for proximate composition, α-tocopherol content and induced thiobarbituric acid reactive substances (TBARs). Further fillets were stored on and covered with ice in boxes for up to 9 d after slaughter. On days 0, 3, 6 and 9, fillet samples for the determination of induced TBARs were taken from each fillet. Each sample was stored in liquid nitrogen until analysis.
Proximate composition analysis
The proximate composition analysis of fillets was carried out according to the AOAC International (1999) and reported on a wet weight basis.
Detection of superoxide dismutase
The activities of serum antioxidant enzymes were determined with commercial kits purchased from Jiancheng Institute of Biotechnology (Nanjing, China). The assay for total superoxide dismutase (SOD) was based on its ability to inhibit the oxidation of oxymine by the xanthine-xanthineoxidase system. The red product (nitrite) produced by the oxidation of oxymine had absorbance at 550 nm. One unit of SOD activity was defined as the amount that reduced the absorbance at 550 nm by 50%.
Detection of glutathione peroxidase
The activity of glutathione peroxidase (GPX) was determined by quantifying the rate of H2O2-induced oxidation of reduced glutathione (GSH) to oxidized glutathione (GSSH). A yellow product which had absorbance at 412 nm could be formed as GSH reacted with dithiobisnitrobenzoic acid. One unit of GPX was defined as the amount that reduced the level of GSH by 1 μmol/L in 1 min per ml.
Detection of α-tocopherol
The samples of fillet tissue were removed from liquid nitrogen for weighing, and a 1:10 (w:v) tissue homogenate was made with normal saline. After centrifugation, the supernatant was collected. α-Tocopherol content was measured with phenanthroline colorimetry (Jiancheng Institute of Biotechnology, Nanjing, China). α-Tocopherol has the ability to deoxidize ferric iron to ferrous iron, which chelates phenanthroline to form an orange red complex. The orange red complex (ferrous-phenanthroline) had absorbance at 533 nm. α-Tocopherol content was calculated in μg/g by calibration curve of standard addition method. The standards were prepared from kits.
Detection of lipid oxidation
Fillet samples were treated in the same way as for the detection of α-tocopherol content. Levels of TBARs were expressed as equivalents malondialdehyde (MDA). MDA content was determined by the thiobarbituric acid method (Jiancheng Institute of Biotechnology, Nanjing, China). MDA forms a red adduct with thiobarbituric acid, which had absorbance at 532 nm. MDA content was calculated in nmol/mg by calibration curve of standard addition method. The standards were prepared from kits.
Statistical analysis
All data were analyzed using SPSS 10.0 (SPSS Inc., Chicago, IL, USA). Regression analysis was used to determine the relationship between dietary α-tocopheryl acetate and its fillet deposition. One-way analysis of variance (ANOVA) was used to determine dietary difference on wet weight, proximate composition, enzyme activities and TBARs levels followed by a multiple comparison test (Student-Neuwman-Keuls). The relationship between fillets TBARs levels and conservation time was analyzed by the same way.
RESULTS
Growth performance and proximate composition
No difference in growth was observed among groups. After two months of feeding, the fish had reached the mean weight of (465±28) g. The proximate composition of the fish (Table 2) was not affected by dietary α-tocopheryl acetate over the experimental period.
Table 2.
Fillet proximate composition (g/kg) (mean±SD)
| Groups | Moisture | Crude lipid | Crude protein | Ash |
| E250 | 788.4±6.0 | 28.4±2.4 | 177.6±3.4 | 12.8±0.5 |
| E500 | 782.5±6.1 | 29.3±2.2 | 180.5±2.6 | 12.8±0.4 |
| E1000 | 781.2±7.3 | 28.1±1.7 | 181.1±2.7 | 12.4±0.6 |
Activities of SOD and GPX in serum of Sparus macrocephalus
The serum antioxidant enzyme activities showed some notable differences among the dietary treatments. The serum SOD activities were significantly higher in Sparus macrocephalus with diets E500 and E1000 compared to E250 (Table 3). No difference was found between E500 and E1000. The serum GPX activities (Table 3) did not change significantly among groups.
Table 3.
Effect of treatment on SOD and GPX activities in serum (mean±SD)
| Groups | Activity |
|
| SOD (U/ml) | GPX (μmol/L) | |
| E250 | 123.1±8.3a | 394.5±14.5 |
| E500 | 188.4±11.4b | 399.0±19.1 |
| E1000 | 192.5±10.9b | 400.9±16.6 |
Values with different superscript letters are significantly different (P<0.05) within the same column; n=10
Tissue α-tocopherol levels in Sparus macrocephalus
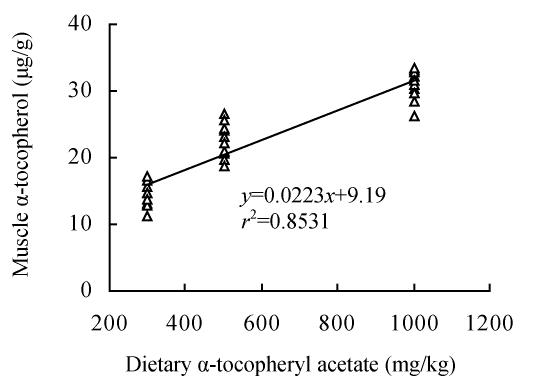
Tissue α-tocopherol levels are presented in Table 4. Diet significantly (P<0.05) affected α-tocopherol incorporation in fillet in a dose-dependent manner, with fish fed the diet high in α-tocopheryl acetate incorporating (E500 and E1000) more than those receiving low level of α-tocopheryl acetate (E250). Regression analysis (Fig.1) indicated a linear relationship between dietary and fillet concentration of α-tocopherol:
| y=9.19+0.0223x, r2=0.81, |
where y is the fillet α-tocopherol content (μg/g), and x is the dietary α-tocopheryl acetate content (mg/kg).
Table 4.
Effect of treatment on α-tocopherol (mean±SD) fillet content
| Groups | α-Tocopherol content (μg/g) |
| E250 | 14.2±1.8a |
| E500 | 22.6±2.6b |
| E1000 | 30.9±2.3c |
Values with different superscript letters are significantly different (P<0.05) within the same column; n=10
Fig. 1.
Regression analysis between dietary α-tocopheryl acetate and fillet α-tocopherol content
Tissue TBARs levels in Sparus macrocephalus
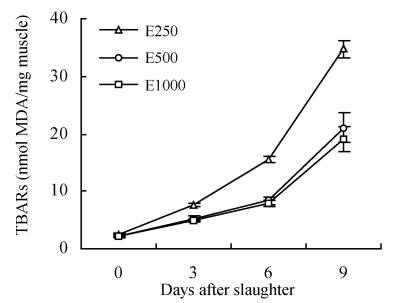
Tissue TBARs levels increased significantly (P<0.05) in Sparus macrocephalus during refrigeration and dietary treatment significantly affected flesh oxidation (Fig.2). The level of TBARs was significantly (P<0.05) lower in fish fed high α-tocopheryl acetate supplemented diets (E500 and E1000) than those receiving low level of α-tocopheryl acetate (E250) on days 0, 3, 6 and 9. Tissue TBARs levels did not change significantly between groups E500 and E1000.
Fig. 2.
Effect of dietary α-tocopheryl acetate supplementation on TBARs (nmol MDA/mg muscle) fillet content of Sparus macrocephalus displayed on ice for 9 d (mean±SD)
DISCUSSION AND CONCLUSION
In the present study, dietary supplementation with α-tocopheryl acetate had no effect on Sparus macrocephalus growth, which is in agreement with studies by Gatta et al.(2000) on sea bass and Ruff et al.(2002) on Atlantic halibut, respectively. Previous studies showed growth differences in Atlantic salmon (Hamre et al., 1997; Tocher et al., 2002), but these results were based on α-tocopherol deficiency. Furthermore, Waagbø et al.(1993) and Gatta et al.(2000) found that α-tocopherol did not affect the proximate composition of Atlantic salmon and sea bass, respectively, which is in agreement with the findings in Sparus macrocephalus fillets of the present study.
Fillet α-tocopherol concentration is most likely affected by various factors, such as fish species and fat content, size and age as well as rearing conditions, while diet α-tocopheryl acetate levels may be the major factor (Ruff et al., 2002). In this study, the average concentrations of α-tocopherol incorporated corresponded very well with the dietary supplementation levels of α-tocopheryl acetate in Sparus macrocephalus. Fig.1 clearly showed an increase in α-tocopherol fillet content, with minimum and maximum values of 14.2 and 30.9 μg/g fillet for groups E250 and E1000, respectively. These findings suggest that Sparus macrocephalus might have the ability to store large amounts of α-tocopherol, when fed high α-tocopheryl acetate supplemented diets. Other aquaculture species such as Atlantic salmon, sea bass, turbot and Atlantic halibut show comparable results (Bjerkeng et al., 1999; Pirini et al., 2000; Ruff et al., 2002; Stéphan et al., 1995).
The level of dietary α-tocopheryl acetate also showed some significant effects on the activities of the serum enzymes of the antioxidant defense system. These effects have to be interpreted within the knowledge of the commonly perceived biochemical mechanisms of these enzyme systems. For instance, SOD is scavenger of active oxygen species, acting on hydrogen peroxide (H2O2) and superoxide (O2 −), respectively (Halliwell and Gutteridge, 1996; Winston and Di Giulio, 1991). Thus, the lower activity of SOD in Sparus macrocephalus fed with the E250 diet and the higher levels of SOD fed with the E500 and E1000 diets are all consistent with the expected pattern. Similar results were also found in other aquatic species, such as turbot (Scophthalmus maximus L.), halibut (Hippoglossus hippoglossus L.), sea bream (Sparus aurata L.) and Litopenaeus vannamei (Tocher et al., 2002; Liu et al., 2007). However, the activity of serum GPX was not related to dietary or tissue α-tocopherol levels in Sparus macrocephalus, which may indicate that elevated dietary α-tocopheryl acetate levels do not have the same effect on different serum antioxidant enzyme activities.
Since α-tocopherol protects against the development of rancidity, α-tocopherol level and TBARs values can be used as indices in fish quality evaluation (Frigg et al., 1990), and high levels of α-tocopherol may have the potential to improve sea food products produced in aquaculture. In the present study, the level of TBARs was significantly (P<0.05) lower in fish fed the high α-tocopheryl acetate supplemented diets (E500 and E1000) than those of E250 treatment on day 0. This is in agreement with the findings of Frigg et al.(1990), Stéphan et al.(1995), Onibi et al.(1996) and Gatta et al.(2000) who demonstrated that high levels of dietary α-tocopherol significantly reduced tissue lipid peroxidation in rainbow trout, juvenile turbot (after induced oxidation), Atlantic salmon and sea bass, respectively. Dietary α-tocopherol levels also had a significant (P<0.05) influence on levels of TBARs on ice storage days 3~9, which was shown in Fig.2. In contrast, Gatta et al.(2000) found lipid oxidation in sea bass fillets stored under refrigeration for up to 12 d was not induced. This may be caused by different storage condition; in our case, all fillets were stored under the same conditions (i.e. low temperature and without handling until day of preparation) with the aim of avoiding oxygen contact and other factors that may initiate lipid oxidation. Moreover, the results also showed that E1000 diet did not show better effect on reducing lipid preoxidation in Sparus macrocephalus than E500 diet. These may indicate that excessive dietary α-tocopheryl acetate supplementation does not have further contribution to shelf-life of fish. These findings above suggested that high levels of α-tocopherol can significantly reduce tissue lipid peroxidation and enhance shelf-life stability, but excessive dose of dietary α-tocopheryl acetate is not necessary. The TBARs value (expressed as MDA) of 0.5 μg/g in stored cooked pork tissue was suggested as a borderline level for the detection of off-flavours by taste panels (Jensen et al., 1998b). Weather this value applies to fish still remains to be investigated. If so, then fish fed the high α-tocopheryl acetate diets would have a distinct flavour advantage over the fillets of fish fed the low α-tocopheryl acetate levels.
The present study has demonstrated relationships between dietary α-tocopheryl acetate levels, the activities of the serum antioxidant enzymes and the levels of fillet lipid peroxidation products. Our findings allow us to conclude that α-tocopherol incorporation was directly related to dietary levels of α-tocopheryl acetate, and that elevated doses of dietary α-tocopheryl acetate significantly reduced lipid peroxidation. Therefore, it is suggested that preslaughter (8 weeks) feeds with about 500 mg/kg of α-tocopheryl acetate for commercial-size Sparus macrocephalus will significantly improve the quality of the final product.
Footnotes
Project (No. 2006c12098) supported by the Science and Technology Department of Zhejiang Province, China
References
- 1.AOAC (Association of Analytical Chemist) International. Official Methods of Analysis of the Association of Analytical Chemists International. 16 Ed. Gaithersburg, Maryland, USA: AOAC International; 1999. [Google Scholar]
- 2.Bjerkeng B, Hamre K, Hatlen B, Wathne E. Astaxanthin deposition in fillets of Atlantic salmon Salmo salar L. fed two dietary levels of astaxanthin in combination with three levels of α-tocopheryl acetate. Aquacult Res. 1999;30(9):637–646. doi: 10.1046/j.1365-2109.1999.00355.x. [DOI] [Google Scholar]
- 3.Frigg M, Prabucki AL, Ruhdel EU. Effect of dietary vitamin E levels on oxidative stability of trout fillets. Aquaculture. 1990;84(2):145–158. doi: 10.1016/0044-8486(90)90344-M. [DOI] [Google Scholar]
- 4.Gatta PP, Pirini M, Testi S, Vignola G, Monetti PG. The influence of different levels of dietary vitamin E on sea bass Dicentrarchus labrax flesh quality. Aquacult Nutr. 2000;6(1):47–52. doi: 10.1046/j.1365-2095.2000.00127.x. [DOI] [Google Scholar]
- 5.Halliwell B, Gutteridge JMC. Lipid Peroxidation: A Radical Chain Reaction. In: Halliwell B, Gutteridge JMC, editors. Free Radicals in Biology and Medicine. Oxford: Clarendon Press; 1996. pp. 188–266. [Google Scholar]
- 6.Hamre K, Waagbø R, Berge RK, Lie Ø. Vitamins C and E interact in juvenile Atlantic salmon (Salmo salar L.) Free Rad Biol Med. 1997;22(1-2):137–149. doi: 10.1016/S0891-5849(96)00281-X. [DOI] [PubMed] [Google Scholar]
- 7.Huang CH, Huang SL. Effect of dietary vitamin E on growth, tissue lipid peroxidation, and liver glutathione level of juvenile hybrid tilapia, Oreochromis niloticus×O. aureus, fed oxidized oil. Aquaculture. 2004;237(1-4):381–389. doi: 10.1016/j.aquaculture.2004.04.002. [DOI] [Google Scholar]
- 8.Jensen C, Birk E, Jokumsen A, Skibsted LH, Bertelsen G. Effect of dietary levels of fat, α-tocopherol and astaxanthin on colour and lipid oxidation during storage of frozen rainbow trout (Oncorhynchus mykiss) and during chill storage of smoked trout. Z Lebensm Unters Forsch. 1998;207(3):189–196. doi: 10.1007/s002170050317. [DOI] [Google Scholar]
- 9.Jensen C, Lauridsen C, Bertelsen G. Dietary vitamin E: quality and storage stability of pork and poultry. Trends Food Sci Technol. 1998;9(2):62–67. doi: 10.1016/S0924-2244(98)00004-1. [DOI] [Google Scholar]
- 10.Kiron V, Puangkaew J, Ishizaka K, Satoh S, Watanabe T. Antioxidant status and nonspecific immune responses in rainbow trout (Oncorhynchus mykiss) fed two levels of vitamin E along with three lipid sources. Aquaculture. 2004;234(1-4):361–379. doi: 10.1016/j.aquaculture.2003.11.026. [DOI] [Google Scholar]
- 11.Kolkovski S, Czesny S, Yackey C, Moreau R, Cihla F, Mahan D, Dabrowski K. The effect of vitamins C and E in (n-3) highly unsaturated fatty acids-enriched Artemia nauplii on growth, survival, and stress resistance of fresh water walleye Stizostedion vitreum larvae. Aquacult Nutr. 2000;6(3):199–206. doi: 10.1046/j.1365-2095.2000.00112.x. [DOI] [Google Scholar]
- 12.Liu Y, Wang WN, Wang AL, Wang JM, Sun RY. Effects of dietary vitamin E supplementation on antioxidant enzyme activities in Litopenaeus vannamei (Boone, 1931) exposed to acute salinity changes. Aquaculture. 2007;265(1-4):351–358. doi: 10.1016/j.aquaculture.2007.02.010. [DOI] [Google Scholar]
- 13.Lygren B, Hamre K, Waagbø R. Effect of induced hyperoxia on the antioxidant status of Atlantic salmon Salmo salar L. fed three different levels of dietary vitamin E. Aquacult Res. 2000;31(4):401–407. doi: 10.1046/j.1365-2109.2000.00459.x. [DOI] [Google Scholar]
- 14.Mourente G, Díaz-Salvago E, Bell JG, Tocher DR. Increased activities of hepatic antioxidant defence enzymes in juvenile gilthead sea bream (Sparus aurata L.) fed dietary oxidised oil: attenuation by dietary vitamin E. Aquaculture. 2002;214(1-4):343–361. doi: 10.1016/S0044-8486(02)00064-9. [DOI] [Google Scholar]
- 15.Onibi GE, Scaife JR, Fletcher TC, et al. Influence of α-Tocopherol Acetate in High Lipid Diets on Quality of Refrigerated Atlantic Salmon (Salmo salar) Fillets; Proceedings of the Conference of IIR Commission C2, Refrigeration and Aquaculture; Mar. 20-22; 1996. pp. 145–152. [Google Scholar]
- 16.Pirini M, Gatta PP, Testi S, Trigari G, Monetti PG. Effects of refrigerated storage on muscle lipid quality of sea bass (Dicentrarchus labrax) fed on diets containing differents levels of vitamin E. Food Chem. 2000;68(3):289–293. doi: 10.1016/S0308-8146(99)00190-9. [DOI] [Google Scholar]
- 17.Ruff N, FitzGerald RD, Cross TF, Kerry JP. Fillet shelf-life of Atlantic halibut Hippoglossus hippoglossus L. fed elevated levels of α-tocopheryl acetate. Aquacult Res. 2002;33(13):1059–1071. doi: 10.1046/j.1365-2109.2002.00770.x. [DOI] [Google Scholar]
- 18.Ruff N, FitzGerald RD, Cross TF, Hamre K, Kerry JP. The effect of dietary vitamin E and C level on market-size turbot (Scophthalmus maximus) fillet quality. Aquacult Nutr. 2003;9(2):91–103. doi: 10.1046/j.1365-2095.2003.00230.x. [DOI] [Google Scholar]
- 19.Scaife JR, Onibi GE, Murray I, Fletcher TC, Houlihan DF. Influence of α-tocopheryl acetate on the short- and long-term storage properties of fillets from Atlantic salmon Salmo salar fed a high lipid diet. Aquacult Nutr. 2000;6(1):65–71. doi: 10.1046/j.1365-2095.2000.00128.x. [DOI] [Google Scholar]
- 20.Stéphan G, Guillaume J, Lamour F. Lipid peroxidation in turbot (Scophthalmus maximus) tissue: effect of dietary vitamin E and dietary n-6 or n-3 polyunsaturated fatty acid. Aquaculture. 1995;130(2-3):251–268. doi: 10.1016/0044-8486(94)00322-F. [DOI] [Google Scholar]
- 21.Tocher DR, Mourente G, van der Eecken A, Evjemo JO, Diaz E, Bell JG, Geuprden I, Lavens P, Olsen Y. Effects of dietary vitamin E on antioxidant defence mechanisms of juvenile turbot (Scophthalmus maximus L.), halibut (Hippoglossus hippoglossus L.) and sea bream (Sparus aurata L.) Aquacult Nutr. 2002;8(3):195–207. doi: 10.1046/j.1365-2095.2002.00205.x. [DOI] [Google Scholar]
- 22.Waagbø R, Sandnes K, Torrisen OJ, Sandvin A, Lie Ø. Chemical and sensory evaluation of fillets from Atlantic salmon (Salmo salar) fed three levels on n-3 polyunsaturated fatty acids at two levels of vitamin E. Food Chem. 1993;46(4):361–366. doi: 10.1016/0308-8146(93)90005-Z. [DOI] [Google Scholar]
- 23.Watanabe F, Goto M, Abe K, Nakano Y. Glutathione peroxidase activity during storage of fish muscle. J Food Sci. 1996;61(4):734–735. doi: 10.1111/j.1365-2621.1996.tb12192.x. [DOI] [Google Scholar]
- 24.Winston GW, Di Giulio RT. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat Toxicol. 1991;19(2):137–161. doi: 10.1016/0166-445X(91)90033-6. [DOI] [Google Scholar]