Abstract
The objectives of this study were to assess the potential of two photosynthetic bacteria (PSB), Rhodopseudomonas palustris HZ0301 and Rhodobacter sphaeroides HZ0302, as probiotics in aquaculture. The viability of HZ0301 and HZ0302 in simulated gastric transit conditions (pH 2.0, pH 3.0 and pH 4.0 gastric juices) and in simulated small intestinal transit conditions (pH 8.0, with or without 0.3% bile salts) was tested. The effects of HZ0301 and HZ0302 on the viability and permeability of intestinal epithelial cell in primary culture of tilapias, Oreochromis nilotica, were also detected. All the treatments were determined with three replicates. The simulated gastric transit tolerance of HZ0301 and HZ0302 strains was pH-dependent and correspondingly showed lower viability at pH 2.0 after 180 min compared with pH 3.0 and pH 4.0. Both HZ0301 and HZ0302 were tolerant to simulated small intestine transit with or without bile salts in our research. Moreover, there was no significant difference (P>0.05) among three treatments including the control and the groups treated with HZ0301 or HZ0302 both in intestinal epithelial cell viability and membrane permeability, showing no cell damage. In summary, this study demonstrated that HZ0301 and HZ0302 had high capacity of upper gastrointestinal transit tolerance and were relatively safe for intestinal epithelial cells of tilapias.
Keywords: Photosynthetic bacteria, Probiotics, Primary culture, Intestinal epithelial cell, Oreochromis nilotica
INTRODUCTION
The world’s population has doubled to 6 billion in less than 40 years, and may reach 12 billion by the end of the 21st century (Bailey, 1997). To sustain an ever-increasing population, adequate food sources of protein must be found, placing increasing demands on aquatic animals (Fast and Menasveta, 2000). However both factors including the disease and pollution caused massive mortality of aquatic animals in the leading aquacultural countries (Smith, 2006).
Chemicals including antimicrobial drugs, pesticides and disinfectants have been conventionally used to control diseases (Gomez-Gil et al., 2000). Unfortunately, the abuse of such antimicrobials in disease prevention and even growth promotion has led to the evolution of resistant strains of bacteria (Esiobu et al., 2002; Fuller, 1989; Sarter et al., 2007; Weston, 1996), so environment-friendly aquaculture has been brought forward to resolve this problem and develop sustainable aquaculture, and research of probiotics for aquatic animals is increasing with this demand (Gatesoupe, 1999). Probiotics are viable bacteria that beneficially influence the host by improving its intestinal microbial balance (Wang and Xu, 2006). The first application of probiotics in aquaculture is relatively recent (Kozasa, 1986), but the interest in such safe and highly effective biological products is increasing rapidly (Wang et al., 2005).
Most probiotics used in aquaculture belong to the lactic acid bacteria, to the genus Bacillus, or to the genus yeast, although other genera or species have also been mentioned (Bogut et al., 1998; Carnevali et al., 2006; Planas et al., 2006; Vázquez et al., 2005). Few studies have been carried out on the potential of photosynthetic bacteria (PSB), Rhodopseudomonas palustris and Rhodobacter sphaeroides, as probiotics in aquaculture. PSB are a group of microorganisms containing abundant nutrient materials and functional factors and exist widely in the world (Sasikala and Ramana, 1995). Probiotics have to firstly survive during the transit through the upper gastrointestinal tract, and then persist in the gut to provide beneficial effects for the host (Chou and Weimer, 1999). In order to be used as potential probiotics, they need to be screened for their capacity of transit tolerance to the upper gastrointestinal tract conditions and their effects on intestinal epithelial cells of aquatic animals.
The objectives of this study were to test the viability of two PSB (R. palustris, HZ0301 and R. sphaeroides, HZ0302) isolated from ponds sludge in simulated gastric transit conditions (pH 2.0, pH 3.0 and pH 4.0 gastric juices) and in simulated small intestinal transit conditions (pH 8.0, with or without 0.3% bile salts), in an attempt to predict candidates that could be used as probiotics in aquaculture. The effects of HZ0301 and HZ0302 on intestinal epithelial cells in primary culture of tilapias, Oreochromis nilotica, were also studied. For detecting the effects of the two candidate probiotics on epithelial cells of tilapias, the cells’ viability (using MTT assay; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide) and permeability (via LDH release; LDH, lactate dehydrogenase) were assayed. Results showed that both HZ0301 and HZ0302 strains had high capacity of upper gastrointestinal transit tolerance and were relatively safe for intestinal epithelial cells of tilapias, and could be used as probiotics potentially.
MATERIALS AND METHODS
Bacterial strains and growth media
The strains of potential probiotics PSB used in this investigation including R. palustris HZ0301 and R. sphaeroides HZ0302 were previously isolated in our laboratory from shrimp ponds sludge. The Hungate (1968) roll tube technique for anaerobic culture of bacteria was applied in the preparation of media, originally described by van Niel (1971), for HZ0301 and HZ0302. The tubes were completely filled with the malate-basal medium (malic acid 4.0 g, NH4Cl 1.0 g, MgSO4·7H2O 0.2 g, K2HPO4 0.2 g, CaCl2·2H2O 0.05 g, FeCl3·6H2O 0.05 g, ZnSO4·7H2O 0.01 g, H3BO4 0.01 g, CuSO4·5H2O 0.5 mg, MnCl2·4H2O 0.5 mg, Co(NO3)2·6H2O 5.0 mg, p-aminobenzoic acid 0.1 mg, thiamine-HCl 0.001 mg, nicotinic acid 0.1 mg and biotin 0.001 mg per liter; pH 6.8~7.0) and closed tightly, incubated at 30 °C for 96 h. The specimens of HZ0301 and HZ0302 were maintained in malate-basal medium with 20% sterile glycerol and stored at −80 °C for more researches. All chemicals (A.R.) used were purchased from Sangon (Canada) and Merck (Germany).
Preparation of washed bacterial cell suspensions
Prior to simulated upper gastrointestinal transit assays, HZ0301 and HZ0302 were serially transferred twice in malate-basal medium and incubated anaerobically at 30 °C for 96 h. Cells in an aliquot (1 ml) of the 96 h culture of each strain were collected by centrifugation (4000×g, 5 min) and washed three times with PBS buffer (0.02% KCl, 0.144% Na2HPO4, 0.8% NaCl and 0.024% KH2PO4, pH 7.0). The count of the washed bacterial cell suspension was determined according to the double-plate method (Sun et al., 2001) prior to assay of transit tolerance. All chemicals (A.R.) used were purchased from Sigma (USA), Roche (USA), Sangon (Canada) and Merck (Germany).
Preparation of simulated gastric and small intestinal juices
Preparation of simulated gastric and small intestinal juices was according to Huang and Adams (2004). Simulated gastric juices were prepared by suspending pepsin (1:10000, Sigma, USA) in sterile saline (0.5%, w/v) to a final concentration of 3 g/L and adjusting the pH to 2.0, 3.0 and 4.0 with concentrated HCl or sterile 0.1 mol/L NaOH using a pH meter (Mettler-Toledo, Delta320, Shanghai, China). Simulated small intestinal juices were prepared by suspending pancreatin (P-1500, Sigma, USA) in the sterile saline (0.5%, w/v) to a final concentration of 1 g/L, with or without 0.3% bile salts and adjusting the pH to 8.0.
Upper gastrointestinal transit tolerance assay
The upper gastrointestinal tolerance of potential probiotics PSB including HZ0301 and HZ0302 was determined in vitro following the method of Charteris et al.(1998). Briefly, an aliquot (0.2 ml) of each washed cell suspension was transferred to a 2.0 ml capacity screw-cap Eppendorf tube, and then mixed with 0.3 ml of sterile saline (0.5%, w/v), and 1.0 ml of simulated gastric (pH 2.0, pH 3.0 and pH 4.0) or small intestinal (pH 8.0) juices. The mixture was then vortexed at maximum setting for 10 s and incubated at 30 °C. The viability of the two strains was then analyzed via calculating live strains according to the double-plate method (Sun et al., 2001) after different incubation time (0 min, 60 min, 90 min, 180 min in simulated gastric juices and 0 min, 1 min, 240 min in small intestinal juices, respectively). All the treatments were determined with three replicates.
Intestinal epithelia viability and permeability assay
Healthy tilapias, Oreochromis nilotica, weighing (7.38±0.92) g were obtained from a local hatchery (Yueteng Aquaculture Co., China) and anesthetized in diluted MS-222 (ethyl 3-aminobenzoate methanesulfonate, Tricaine; Sigma, USA; 1:2500). Intestinal epithelia from tilapias were isolated and cultured in 96-well plates as described by Guzman-Murillo et al.(2000) with minor modification. The treatment was determined with three replicates. In brief, the tilapias were decapitated and the intestines excised from the bodies and rinsed for 2×10 min in 10 ml PBS (Ca2+ and Mg2+ free) containing 200 µg/ml penicillin/streptomycin, 400 µg/ml gentamicin and 250 µg/ml fungizone. The intestines were thereafter transferred to 5 ml of a trypsin solution (PBS without Ca2+ and Mg2+, 0.05% trypsin, 0.02% EDTA) and incubated on a rotating wheel (test-tube HZ-9201K rotator, Taicang, China) for 20 min. The cell suspension was aspirated from the tubes and filtrated through a 100 µm nylon cell strainer into a stopping solution (PBS containing 10% FBS; FBS, foetal bovine serum). Remaining intestines were trypsinised for an additional 20 min and filtrated into the same stopping solution. The cell suspension was centrifuged for 10 min and the cell pellet was washed twice with 5 ml of PBS containing 2% FBS by re-suspension and centrifugation. Thereafter, the cell pellet was re-suspended in culture medium (L-15 medium supplemented with 2 mmol/L L-glutamine, 5% FBS, 100 µg/ml penicillin/streptomycin and 200 µg/ml gentamicin). The cells were maintained in culture flasks or 96-well plates (Millipore, USA) at 30 °C. After 24 h incubation, the cells were rinsed twice with PBS to remove unattached cells and the medium was changed every second day until the experiments started.
At least 24 h before the tests, L-15 medium without antibiotics was used. All of the cells grown in 96-well plates with HZ0301 and HZ0302 respectively (107 CFU/well) were incubated for a period of 3 h. The cell viability (using MTT assay) and the membrane permeability (via LDH release) were measured according to Zödl et al.(2004) and Konjevic et al.(1997). All the chemicals used were purchased from Sigma (USA) and Sangon (Canada).
Statistical analysis
Statistical analysis using one-way ANOVA (Statistical Analysis System, SAS, version 6.03) was performed to find significant difference on various parameters. A significance level of P<0.05 was used.
RESULTS
Simulated gastric juice transit tolerance
The effects of simulated gastric in vitro on viability of PSB strains HZ0301 and HZ0302 are presented in Table 1. The lowest viability (P<0.05) of HZ0301 determined in simulated gastric juice at pH 2.0 was observed in the present study compared with that at pH 3.0 and pH 4.0 besides the initial time (0 min). There was significant difference (P<0.05) between HZ0301 viability at pH 3.0 and pH 4.0 in simulated gastric juice analyzed at 60 min, 90 min and 180 min. As for HZ0302, assays showed similarly higher viability at pH 4.0 (P<0.05) as compared with the rest. The average counts of strains in simulated gastric juice were also found to be lower (P<0.05) at pH 2.0 and pH 3.0 in vitro after 180 min compared with that of the initial whether HZ0301 or HZ0302. However, in the research of viability in simulated gastric tract condition, there was no significant difference (P>0.05) between HZ0301 or HZ0302 viability at pH 4.0 after 180 min compared with that of the initial (0 min) (Table 1). The viability of HZ0301 in simulated gastric juices at pH 2.0 after 180 min was (3.87±0.04) log CFU/ml and lower than that of HZ0302 [(4.48±0.12) log CFU/ml], while the mean viability at pH 3.0 after 180 min was inverse (7.79±0.04) log CFU/ml and (7.21±0.06) log CFU/ml, respectively in HZ0301 and HZ0302 (Table 1). There was no significant difference (P>0.05) between viability of HZ0301 and HZ0302 in simulated gastric juices at pH 4.0 after 180 min in our study.
Table 1.
Effect of simulated gastric juices (pH 2.0, pH 3.0 and pH 4.0) on the viability of photosynthetic bacteria strains HZ0301 and HZ0302 during 180 min of gastric transit
| Strains | pH of simulated gastric juices | Viable count (log CFU/ml) during simulated gastric tolerance |
|||
| 0 min | 60 min | 90 min | 180 min | ||
| HZ0301 | 2.0 | 8.45 (0.07)aA | 5.35 (0.05)bA | 5.29 (0.06)bA | 3.87 (0.04)cA |
| 3.0 | 8.45 (0.07)aA | 8.02 (0.04)bB | 7.90 (0.05)cB | 7.79 (0.04)dB | |
| 4.0 | 8.45 (0.07)aA | 8.44 (0.09)aC | 8.52 (0.12)aC | 8.47 (0.10)aC | |
| HZ0302 | 2.0 | 8.49 (0.06)aA | 5.58 (0.05)bA* | 5.32 (0.03)cA | 4.48 (0.12)dA* |
| 3.0 | 8.49 (0.06)aA | 7.74 (0.10)bB* | 7.38 (0.06)cB* | 7.21 (0.06)dB* | |
| 4.0 | 8.49 (0.06)aA | 8.24 (0.07)bC* | 8.49 (0.06)aC | 8.51 (0.04)aC | |
HZ0301: R. palustris; HZ0302: R. sphaeroides. CFU: Colony-forming units. Results are shown as mean (SE), n=3. Means in the same row with different lowercase superscript were significantly different (P<0.05) in the same strain and at the same pH value. Means in the same column with different uppercase superscript were significantly different (P<0.05) in the same strain. Means in the same columns with asterisk were significantly different (P<0.05) between HZ0301 and HZ0302 at the same pH value
Simulated small intestinal juice transit tolerance
The effects of simulated small intestinal juices (with or without 0.3% bile salts) on the viability of PSB strains HZ0301 and HZ0302 during 240 min of small intestine transit are given in Table 2. There was significantly higher viability (P<0.05) of HZ0301 in the simulated small intestinal tract without bile salts after 240 min as compared with that of the initial (0 min) [(8.62±0.07) log CFU/ml] and (8.45±0.07) log CFU/ml, respectively]. However, as for HZ0302 with absence of bile salts, there was no difference (P>0.05) between the final (240 min) and the initial (0 min) although the average value of viable count at 240 min presented increasing trend. As shown in Table 2, there was no difference in the viable counts when the HZ0301 strains were exposed to simulated small intestine passage through 0.3% bile salts after 240 min compared with that of the initial (0 min). As for HZ0302 strains, assays revealed a relatively lower viable count at 240 min, but it was not significantly different (P>0.05) from that of the initial (0 min) (Table 2). Furthermore, no significant difference was observed in the strains viability between HZ0301 and HZ0302 (P>0.05) at the same time and condition in our research (Table 2).
Table 2.
Effect of simulated small intestinal juices (with or without 0.3% bile salts) on the viability of photosynthetic bacteria strains HZ0301 and HZ0302 during 240 min of small intestine transit
| Strains | Viable count (log CFU/ml) during simulated small intestinal tolerance |
|||||
| Absence of bile salts |
Presence of 0.3% bile salts |
|||||
| 0 min | 1 min | 240 min | 0 min | 1 min | 240 min | |
| HZ0301 | 8.45 (0.07)aA | 8.34 (0.07)aA | 8.62 (0.07)bA | 8.45 (0.07)aA | 8.34 (0.04)aA | 8.52 (0.17)aA |
| HZ0302 | 8.49 (0.06)aA | 8.37 (0.06)aA | 8.55 (0.07)bA | 8.49 (0.06)aA | 8.25 (0.06)bA | 8.34 (0.16)aA |
HZ0301: R. palustris; HZ0302: R. sphaeroides. CFU: Colony-forming units. Results are shown as mean (SE), n=3. Means in the same row with different lowercase superscript were significantly different (P<0.05) in the same strain and at the same condition. Means in the same column with different uppercase superscript were significantly different (P<0.05) between HZ0301 and HZ0302 at the same time and condition
Cell viability and the membrane stability
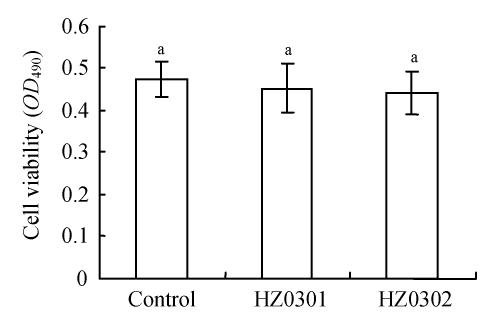
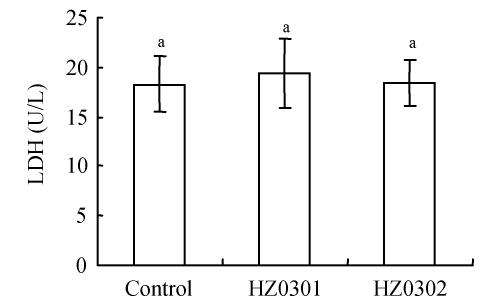
The results of the viability assay (using MTT assay) and the membrane permeability (via LDH release) of tilapia cells are shown in Fig.1 and Fig.2. According to the MTT assay, there was no significant difference (P>0.05) among the three treatments including the control and the groups treated with different potential probiotics (Fig.1). Also no differences (P>0.05) in membrane permeability based on LDH release were found (Fig.2).
Fig. 1.
Viability of Caco-2 cells after incubation with 107 CFU/well of HZ0301 and HZ0302 strains respectively and the controls for 3 h (MTT assay)
HZ0301: R. palustris; HZ0302: R. sphaeroides; MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide; CFU: Colony-forming units. Means with different superscript were significantly different (P<0.05)
Fig. 2.
The membrane stability of Caco-2 cells after incubation with 107 CFU/well of HZ0301 and HZ0302 strains respectively and the controls for 3 h (LDH release)
HZ0301: R. palustris; HZ0302: R. sphaeroides; LDH: Lactate dehydrogenase; CFU: Colony-forming units. Means with different superscript were significantly different (P<0.05)
DISCUSSION
Viability and survival of probiotics were the most important parameters that provide therapeutic functions. The low pH of the stomach and the antimicrobial action of pepsin are known to provide an effective barrier against entry of bacteria into the intestinal tract (Holzapfel et al., 1998). The tests of PSB strains that survive at pH 2.0 and pH 3.0 suggested that the acid tolerance of HZ0301 and HZ0302 is strain-specific, and that pH values of 2.0 and 3.0 could be considered as critical for the selection of potential probiotics PSB (Table 1). However, the presence of feed and feed ingredients had been reported to improve the viability of microorganisms during gastric transit with the suggested mechanism being the pH increase of the gastric content (Charteris et al., 1998; Conway et al., 1987; Huang and Adams, 2004; Zarate et al., 2000). The low acid tolerance of HZ0301 and HZ0302 in gastric transit might be improved with the addition of feed amounts.
The small intestine containing bile salts and pancreatin is another barrier that probiotics must overcome. The pH value of the small intestine was around pH 8.0 (Titus et al., 1991). Unconjugated bile salts, even at low concentrations, can inhibit the in vitro growth of microorganisms. According to Gilliland et al.(1984), 0.3% is considered to be a critical concentration for screening resistant strains. In our research, both strains retained the same viability or even higher viability after 240 min of simulated small intestinal transit in the absence of bile salt and in the presence of 0.3% bile salts (Table 2). Probiotics need to colonize and survive in the small intestine in order to exert a positive effect on the health and wellbeing of a host (Havenaar et al., 1992). The observed high viability of HZ0301 and HZ0302 indicated that these two strains had effective tolerance to small intestine environment with or without bile salts (Table 2).
The cell membrane is one kind of choice permeable membrane and can maintain the relative stability of the cell internal environment (Konjevic et al., 1997). The abnormal increase of cell membrane was one of the cell early damage performances. In the normal condition, the macro-molecule matter LDH could not through the cell membrane except that the cell membrane suffered injury (Konjevic et al., 1997; Ramanan and Rao, 1987). The data (Fig.1 and Fig.2) measured in our research suggested that the potential probiotics including HZ0301 and HZ0302 had relative cell security and that these results were proved by the intestinal epithelial cells morphology using phase-contrast microscope (Olympus CKX41, Japan) in primary culture of tilapias, Oreochromis nilotica.
In summary, this study demonstrated that PSB strains HZ0301 and HZ0302 could provide potential probiotics for future development and was aimed to get further understanding of the action modes of these potential probiotic strains, which obviously have beneficial properties in aquatic animals. It was unlikely that HZ0301 and HZ0302 would cause adverse affects in any other aquatic species, including shrimp and shellfish, although before application, further aquatic animal experiments in vivo need to be undertaken to confirm what we have observed in the laboratory using intestinal epithelial cells model in primary culture of tilapias, Oreochromis nilotica.
Acknowledgments
We acknowledge the valuable assistance provided by Dr. Y. Wang and other colleagues for their many helpful suggestions.
Footnotes
Project (No. 30470021) supported by the National Natural Science Foundation of China
References
- 1.Bailey C. Aquaculture and basic human needs. World Aquac. 1997;28(3):28–31. [Google Scholar]
- 2.Bogut I, Milakovic Z, Bukvic Z, Brkic S, Zimmer R. Influence of probiotic (Streptococcus faecium M74) on growth and content of intestinal microflora in carp (Cyprinus carpio) Czech J Anim Sci. 1998;439(5):231–235. [Google Scholar]
- 3.Carnevali O, Vivo L, Sulpizio R, Gioacchini G, Olivotto L, Silvi S, Cresci A. Growth improvement by probiotic in European sea bass juveniles (Dicentrarchus labrax L.), with particular attention to IGF-1, myostatin and cortisol gene expression. Aquaculture. 2006;258(1-4):430–438. doi: 10.1016/j.aquaculture.2006.04.025. [DOI] [Google Scholar]
- 4.Charteris WP, Kelly PM, Morelli L, Collins JK. Development and application of an in vitro methodology to determine the transit tolerance of potentially probiotic Lactobacillus and Bifidobacterium species in the upper human gastrointestinal tract. J Appl Microbiol. 1998;84(5):759–768. doi: 10.1046/j.1365-2672.1998.00407.x. [DOI] [PubMed] [Google Scholar]
- 5.Chou L, Weimer B. Isolation and characterization of acid and bile tolerant isolates from strains of Lactobacillus acidophilus . J Dairy Sci. 1999;82(1):23–31. doi: 10.3168/jds.S0022-0302(99)75204-5. [DOI] [PubMed] [Google Scholar]
- 6.Conway PL, Gorbach SL, Goldin BR. Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J Dairy Sci. 1987;70(1):1–12. doi: 10.3168/jds.S0022-0302(87)79974-3. [DOI] [PubMed] [Google Scholar]
- 7.Esiobu N, Armenta L, Ike J. Antibiotic resistance in soil and water environments. Int J Environ Health Res. 2002;12(2):133–144. doi: 10.1080/09603120220129292. [DOI] [PubMed] [Google Scholar]
- 8.Fast AW, Menasveta P. Some recent issues and innovations in marine shrimp pond culture. Rev Fish Sci. 2000;8(3):151–233. doi: 10.1080/10641260091129215. [DOI] [Google Scholar]
- 9.Fuller R. Probiotics in man and animals. J Appl Bacteriol. 1989;66(5):365–378. [PubMed] [Google Scholar]
- 10.Gatesoupe FJ. The use of probiotics in aquaculture. Aquaculture. 1999;180(1-2):147–165. doi: 10.1016/S0044-8486(99)00187-8. [DOI] [Google Scholar]
- 11.Gilliland SE, Staley TE, Bush LJ. Importance in bile tolerance of Lactobacillus acidophilus used as a diatery adjunct. J Dairy Sci. 1984;67(12):3045–3051. doi: 10.3168/jds.S0022-0302(84)81670-7. [DOI] [PubMed] [Google Scholar]
- 12.Gomez-Gil B, Roque A, Turnbull JF. The use and selection of probiotic bacteria for use in the culture of larval aquatic organisms. Aquaculture. 2000;191(1-3):259–270. doi: 10.1016/S0044-8486(00)00431-2. [DOI] [Google Scholar]
- 13.Guzman-Murillo MA, Merino-Contreras ML, Ascencio F. Interaction between Aeromonas veronii and epithelial cells of spotted sand bass (Paralabrax maculatofasciatus) in culture. J Appl Microbiol. 2000;88(5):897–906. doi: 10.1046/j.1365-2672.2000.01061.x. [DOI] [PubMed] [Google Scholar]
- 14.Havenaar R, Brink NG, Huis In’t Veld JHJ. Selection of Strains for Probiotics Use. In: Fuller R, editor. Probiotics, the Scientific Basis. London: Chapman & Hall; 1992. pp. 210–224. [Google Scholar]
- 15.Holzapfel WH, Haberer P, Snel J, Schillinger U, Huis In’t Veld JHJ. Overview of gut flora and probiotics. Int J Food Microbiol. 1998;41(2):85–101. doi: 10.1016/S0168-1605(98)00044-0. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, Adams MC. In vitro assessment of the upper gastrointestinal tolerance of potential probiotic dairy propionibacteria. Int J Food Microbiol. 2004;91(3):253–260. doi: 10.1016/j.ijfoodmicro.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Hungate RE. A Roll Tube Method for Cultivation of Strict Anaerobes. In: Morris JR, Robbins DW, editors. Methods in Microbiology. Vol. 3. London: Academic Press; 1968. pp. 117–132. [Google Scholar]
- 18.Konjevic G, Jurišic V, Spuzic I. Corrections to the original lactate dehydrogenase (LDH) release assay for the evaluation of NK cell cytotoxicity. J Immunol Meth. 1997;200(1-2):199–201. doi: 10.1016/S0022-1759(96)00194-9. [DOI] [PubMed] [Google Scholar]
- 19.Kozasa M. Toyocerin (Bacillus toyoi) as growth promotor for animal feeding. Microbiol Aliment Nutr. 1986;4(1):121–135. [Google Scholar]
- 20.Planas M, Pérez-Lorenzo M, Hjelm M, Gram L, Fiksdal IU, Øivind B, Pintado J. Probiotic effect in vivo of Roseobacter strain 27-4 against Vibrio (Listonella) anguillarum infections in turbot (Scophthalmus maximus L.) larvae. Aquaculture. 2006;255(1-4):323–333. doi: 10.1016/j.aquaculture.2005.11.039. [DOI] [Google Scholar]
- 21.Ramanan PN, Rao MN. Antimicrobial activity of cinnamic acid derivatives. Indian J Exp Biol. 1987;25(1):42–43. [PubMed] [Google Scholar]
- 22.Sarter S, Nguyen HNK, Hung LT, Lazard J, Montet D. Antibiotic resistance in Gram-negative bacteria isolated from farmed catfish. Food Control. 2007;18(11):1391–1396. doi: 10.1016/j.foodcont.2006.10.003. [DOI] [Google Scholar]
- 23.Sasikala C, Ramana CV. Biotechnological Potentials of Anoxygenic Phototrophic Bacteria. I. Production of Single-Cell Protein, Vitamins, Ubiquinones, Hormones and Enzymes and Use in Waste Treatment. In: Laskin AI, Bennett JW, Gadd GM, editors. Advances in Applied Microbiology. New York: Academic Press; 1995. pp. 173–226. [DOI] [PubMed] [Google Scholar]
- 24.Smith P. Breakpoints for disc diffusion susceptibility testing of bacteria associated with fish diseases: a review of current practice. Aquaculture. 2006;261(4):1113–1121. doi: 10.1016/j.aquaculture.2006.05.027. [DOI] [Google Scholar]
- 25.Sun JD, Zhao CY, Huang XC, Chen XS, Xiong KZ. Mathematical model of photosynthetic bacteria counting. J Shenyang Agric Univ. 2001;32(5):358–359. (in Chinese) [Google Scholar]
- 26.Titus E, Karasov WH, Ahearn GA. Dietary modulation of intestinal nutrient transport in the teleost fish tilapia. Am J Physiol. 1991;261(6 Pt 2):R1568–R1574. doi: 10.1152/ajpregu.1991.261.6.R1568. [DOI] [PubMed] [Google Scholar]
- 27.van Niel CB. Techniques for the Enrichment, Isolation and Maintenance of the Photosynthetic Bacteria. In: San Pietro A, editor. Methods in Enzymology. London: Academic Press; 1971. pp. 3–28. [Google Scholar]
- 28.Vázquez JA, González MP, Murado MA. Effects of lactic acid bacteria cultures on pathogenic microbiota from fish. Aquaculture. 2005;245(1/4):149–161. [Google Scholar]
- 29.Wang YB, Xu ZR. Effect of probiotics for common carp (Cyprinus carpio) based on growth performance and digestive enzyme activities. Anim Feed Sci Technol. 2006;127(3-4):283–292. doi: 10.1016/j.anifeedsci.2005.09.003. [DOI] [Google Scholar]
- 30.Wang YB, Xu ZR, Xia MS. The effectiveness of commercial probiotics in northern white shrimp Penaeus vannamei ponds. Fisheries Sci. 2005;71(5):1036–1041. doi: 10.1111/j.1444-2906.2005.01061.x. [DOI] [Google Scholar]
- 31.Weston DP. Environmental Considerations in the Use of Antibacterial Drugs in Aquaculture. In: Baird D, Beveridge MVM, Kelly LA, et al., editors. Aquaculture and Water Resource Management. Oxford: Blackwell; 1996. pp. 140–165. [Google Scholar]
- 32.Zarate G, Perez-Chaia A, Gonzalez S, Oliver G. Viability and β-galactosidase activity of dairy propionibacteria subjected to digestion by artificial gastric and intestinal fluids. J Food Prot. 2000;63(9):1214–1221. doi: 10.4315/0362-028x-63.9.1214. [DOI] [PubMed] [Google Scholar]
- 33.Zödl B, Sargazi M, Zeiner M, Roberts NB, Steffan I, Marktl W, Ekmekcioglu C. Toxicological effects of iron on intestinal cells. Cell Biochem Funct. 2004;22(3):143–147. doi: 10.1002/cbf.1065. [DOI] [PubMed] [Google Scholar]