Abstract
In the present work, the potential of acaricidal activity of chamomile flowers’ extract was studied against engorged Rhipicephalus annulatus tick under laboratory condition. For this purpose, the engorged females of Rhipicephalus annulatus were exposed to two-fold serial dilutions of chamomile flowers’ extract (0.5%, 1.0%, 2.0%, 4.0% and 8.0%) using “dipping method” in vitro. The engorged ticks were immersed in different plant dilutions (five ticks for each dilution) for 1 min and they were immediately incubated in separate Petri dishes for each replicate at 26 °C and 80% relative humidity. Mortality rate for each treatment was recorded 5 d after incubation.
The mortality rate caused by different dilutions of chamomile flowers’ extract ranged from 6.67% to 26.7%, whereas no mortality was recorded for non-treated control group. The mass of produced eggs varied from 0.23 g (in 8.0% solution) to 0.58 g (in control), with no statistical differences between the treatments and control (P>0.05). Also the chamomile flowers’ extract in highest concentration used (8.0%) caused 46.67% failure in egg laying in engorged females while no failure was observed for non-treated control group. Macroscopic observations indicated that in effective concentrations of plant (4.0% and 8.0%), patchy hemorrhagic swelling appeared on the skin of treated ticks. The results presented for the first time in this study imply that chamomile may be considered as a promising plant for biocontrol of cattle fever tick disease in the field condition.
Keywords: Matricaria chamomile, Chamomile, Rhipicephalus annulatus, Botanical acaricidal agent
INTRODUCTION
Despite the major role in control of Acari, synthetic acaricides suffer from a large limitation. Because of the hard delayed degradation, their residues usually remain in agricultural environment where they adversely affect the life of living organisms in natural ecosystem. Likewise, they are able to induce the production of resistant strains of ticks (Drummond, 1977).
The interest in alternative methods for the control of ticks has been dramatically increased in recent years, in accordance with increasing demands for safer animal products and environmental protection (Georghiou and Lagunes-Tejada, 1991; Georghiou, 1986). Ticks are blood-sucking ectoparasites of mammals, birds and reptiles throughout the world, so they are potentially involved in transmission of the widest variety of pathogens including bacteria, protozoa and viruses between different animals in nature (Rak, 1976).
Besides of their adverse effects of synthetic acaricides on ecosystem, some recent studies have shown that chemical substances widely used for pest control have a considerable genotoxic and cytotoxic effect on human target cells (Ündeğer and Başaran, 2005), so it is necessary to look for alternative methods which are adaptable, safer and cheaper than chemical substance. The bacteria that are pathogenic for arthropods (Hassanain et al., 1997; Zhioua et al., 1999), as well as entomopathogenic fungi (Onofre et al., 2001; Samish and Rehacek, 1999) have been widely used in biocontrol of parasites. Application of medicinal plants as antagonistic agents for harmful microorganisms has a long history of a thousand years. Some investigations have been designed to use herbal medicine such as Melaleuca alternifolia (tea tree oil) (Iori et al., 2005) and extract of neem seed oil (Azadirachta indica A. juss) (Abdel-Shafy and Zayed, 2002) against nymph of Ixodes ricinus and adult stage of Hyalomma anatolicum excavatum (Ixodoidea: Ixodidae), respectively. Recently, Macchioni et al. (2004) showed the antagonistic activity of chamomile (Matricaria chamomile) flowers’ extract against mite Psoroptes caniculi. In the present study, we examined the acaricidal potential of the extract obtained from the flowers of chamomile (M. chamomile) against the females of Rhipicephalus annulatus to elucidate its ability as a cattle fever tick biocontrol agent.
MATERIALS AND METHODS
Plant extract
Extract form chamomile flowers was prepared by Barij Essence Co. Kashan, Iran.
Tick rearing
Rhipicephalus annulatus adults were collected from naturally infested cattle in Mazandaran Province in the north of Iran. Female ticks were selected morphologically and maintained in the laboratory at 26 °C and 80% relative humidity (RH) in test tubes for laying eggs. After hatching of the eggs, tick larvae were fed on healthy 1~3 month-old Holstein calves. Female ticks were collected from the infested calves and transferred to laboratory during 3~4 h to perform the subsequent experiments.
Bioassay on ticks
Two-fold serial dilutions of extract (0.5%, 1.0%, 2.0%, 4.0% and 8.0%) were prepared in 60% EtOH and then the acaricidal effect of each dilution was tested. For this purpose, engorged females were washed with 70% EtOH. After drying, five females per replicate were immersed in each plant dilution and in control solution (ethanol 60%) for 1 min. The experiment was carried out in triplicate. Ticks were then transferred to Petri dishes containing moist filter paper and incubated for 5 d at 26 °C and 80% RH in the dark. For all dilutions, a control group of five ticks were also treated in the same manner except that they were immersed in 60% ethanol.
Ticks were studied with dissecting microscope and the mortality rate and weight of produced eggs in groups were recorded. The mortality rate was recorded daily by counting dead ticks and leaving cadavers in other dishes to observe the effects of extract on the ticks. The dead ticks were diagnosed based on the signs of cuticle darkness, stopped Malpighian tube movement and hemorrhagic skin lesions.
Failure in egg laying was then calculated. The failure in egg laying was qualitatively noticed via direct observation. For quantitating the egg laying ability, the initial female weight (IFW) for each treatment was obtained by initial weighing of engorged females using an analytical scale, before putting them in Petri dishes. The related egg laying weight (EW) was then calculated by subtraction of the weight of dead females from their IFW based on Onofre et al.(2001).
Statistical analysis
The data were expressed as the mean±SEM. Groups were compared using one-way ANOVA for repeated measurements. Tukey test was used for post hoc analysis. A value of P<0.05 was considered significant.
RESULTS
Initial qualitative assay
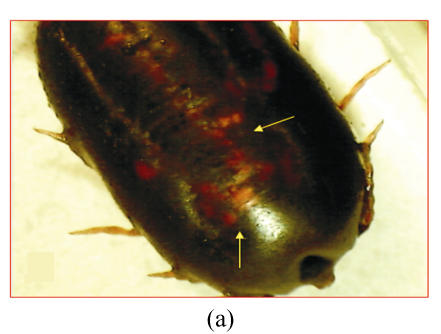
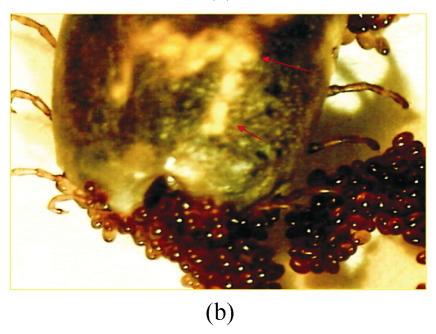
Based on the morphological observation of ticks treated and/or non-treated with chamomile flower’s extract, mortality rate and also egg laying inhibition were noted (Fig.1). The cuticle of dead ticks was dark with these ticks becoming immotile. Malpighian tubes movement was also stopped and haemorrhagic swelling lesions appeared on the skin surface (Fig.1a). An engorged non-treated tick was able to lay approximately 2 000 eggs in our study (Fig.1b), while a treated tick could not lay egg especially in higher concentrations of the plant extract (Fig.1a).
Fig. 1.
Comparing a chamomile-treated dead tick (a) with a non-treated live tick (b). Tick inoculated with chamomile flowers’ extract shows the major signs of death including a dark cuticle, immobilized Malpighian tubes, and haemorrhagic swelling skin lesions (arrows). In non-treated live tick, the colour of cuticle is normal and motile Malpighian tubes are easily visible (arrows). Egg laying is stopped for dead tick (a), while it is normal for live control tick (b)
Quantification of tick inhibition
1. Mortality rate
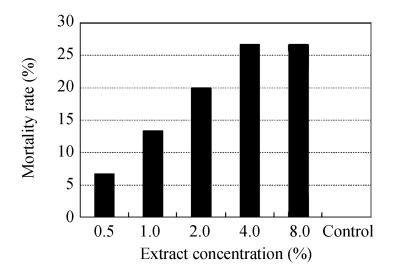
Fig.2 shows low to moderate acaricidal effect caused by different concentrations of chamomile flowers’ extract. The most mortality was reported 26.67% at the highest plant concentration (8.0%). According statistical analysis chamomile flowers’ extract caused low to moderate acaricidal effect which occurred in first 24 h post inoculation and was dose-dependent.
Fig. 2.
Mortality rate of Rhipicephalus annulatus females caused by different concentrations of chamomile flowers’ extract
2. Egg laying and mass of produced eggs
The acaricidal efficacies of different concentrations of chamomile flowers’ extract are reported in Table 1 that shows average failure in egg laying (%) and mass of produced eggs (g) compared with non-treated control. The analysis of variance showed no significant differences between the different dosages used in all of the parameters. The most failure in egg laying was reported for the highest concentration of extract (8.0%), which caused the eggs failure of 46.67% compared with control (Table 1). The mass of produced eggs was in the range from 0.23 g for 8.0% concentration to 0.36 g in 0.5% concentration compared with the control group which was 0.58 g at the end of experiment period.
Table 1.
Failure in egg laying and mass of produced eggs of Rhipicephalus annulatus engorged females treated with different concentrations of chamomile flowers’ extract after 5 d post inoculation
| Concentration of extract (%) | Failure in egg laying (%)* | Mass of produced eggs (g)* |
| 0.5 | 13.33±6.67 | 0.36±0.11 |
| 1.0 | 26.67±13.3 | 0.32±0.13 |
| 2.0 | 26.67±6.7 | 0.28±0.03 |
| 4.0 | 40.00±11.54 | 0.34±0.04 |
| 8.0 | 46.67±17.64 | 0.23±0.10 |
| Control | 0.0 | 0.58±0.03 |
The data are expressed as mean±SEM; n=3
DISCUSSION
During the last decades, plant extracts were widely used against phytophagous pests and mosquitoes (Arnason et al., 1987; Balandrin et al., 1985; Chavan and Nikam, 1988).
It has been shown that the extract of flower heads of chamomile (Matricaria chamomile) contains angelic acid (2-meyhyl-2-butenoic acid), azolen, chamazulene (1,4-dimethyl-7-etazulene), α-bisablol, sineol, maricarin and matricin as major constituents (Franz, 1980). Chamomile has been used in traditional medicine for thousands of years, and it has been widely used in Europe. It is a popular treatment for numerous ailments, including sleep disorders, anxiety, digestion/intestinal conditions, skin infections/inflammation (including eczema), wound healing, infantile colic, teething pains, and diaper rash. In the US, chamomile is best known as an ingredient in herbal tea preparations advertised for mild sedating effects.
The acaricidal activity of the chamomile flowers’ extract against cattle fever tick, Rhipicephalus annulatus, had not been previously reported. There are only reports of the acaricidal properties of decoctions, infusions and macerates of dried flower heads of chamomile in vitro against the mite Psoroptes caniculi in which all the extracts tested showed highly significant acaricidal activity when compared with controls. Among them, a decoction of 10% was the only formulation which gave 100% activity (Macchioni et al., 2004). In another study, the acaricidal activity of Chamomile roman essential oil obtained from Anthemis nobilis was reported against Dermanyssus gallinae (red mite) (Kim et al., 2004). The results by these researchers showed that Chamomile roman essential oil might be good candidates for naturally occurring D. gallinae control. Our results justify further efforts toward practical application of plant extracts as anti-tick acaricidal agents. Chamomile flowers’ extract showed a low to moderate effect against Rhipicephalus annulatus. The highest effect appeared after the first 24 h in 8.0% concentration of the extract, and the mortality rates were reported to be highly dose-dependent. Our protocol was not designed to address the mode of action of chamomile flowers’ extract, but its anti-mycotic and anti-bacterial properties have been reported already. The results indicating that a significant acaricidal effect may be apparent only after exposure of at least 90 min and that this activity seems to be dose-independent, needs to be further established for Rhipicephalus annulatus in future studies. The absence or smallness of the effected lower exposure time could be linked to the peculiar respiratory habits of hard ticks that, when resting, open their spiracles 1~2 times per hour (Needham and Teel, 1986). The present works imply that chamomile may be a promising candidate for considering as a preventing agent in control of cattle fever tick, Rhipicephalus annulatus, in field condition. In order to substantiate this hypothesis, tests are currently being performed using larger recipient and larger doses than tested in the present study in our laboratory.
Footnotes
Project partly supported by the Research Fund of Shahrekord University, Iran
References
- 1.Abdel-Shafy S, Zayed AA. In vitro acaricidal effect of plant extract of neem seed oil (Azadirachta indica) on egg, immature, and adult stages of Hyalomma anatolicum excavatum (Ixodoidea: Ixodidae) Vet Parasitol. 2002;106(1):89–96. doi: 10.1016/S0304-4017(02)00023-7. [DOI] [PubMed] [Google Scholar]
- 2.Arnason JT, Philogene BJR, Donsko N, Kubo I. Limonoides from the Meliaceae and Rutacea reduce feeding growth and development of Ostrinia nubilalis . Entomol Exp Appl. 1987;43(3):221–226. doi: 10.1007/BF00343552. [DOI] [Google Scholar]
- 3.Balandrin MF, Klocke JA, Wurtele ES, Bollinger WH. Natural plant chemicals: sources of industrial and medicinals. Science. 1985;228(4704):1154–1160. doi: 10.1126/science.3890182. [DOI] [PubMed] [Google Scholar]
- 4.Chavan SR, Nikam S. Investigation of alkones from neem leaves and neem larvicidal activity. Pesticides. 1988;22(7):32–33. [Google Scholar]
- 5.Drummond RO. Resistance in Ticks and Insects of Veterinary Importance Reprinted from: Pesticide Management and Insecticide Resistance. San Francisco: Academic Press, Inc; 1977. pp. 303–319. [Google Scholar]
- 6.Franz C. Content and composition of the essential oil in flower heads of Matricaria chamomilla L. during ontogenetical development. Acta Hort. 1980;96:317–321. [Google Scholar]
- 7.Georghiou GP. Pesticide Resistance: Strategies and Tactics for Management. Washington, DC: National Academy Press; 1986. The Magnitude of the Resistance Problem; pp. 14–43. [Google Scholar]
- 8.Georghiou GP, Lagunes-Tejada A. The Occurrence of Resistance to Pesticide in Arthropods: An Index of Cases Reported through 1989. Rome: FAO; 1991. [Google Scholar]
- 9.Hassanain MA, el Garhy MF, Abdel-Ghaffar FA, el-Sharaby A, Abdel Megged KN. Biological control studies of soft and hard ticks in Egypt: I. The effect of Bacillus thuringiensis varieties on soft and hard ticks (Ixodidae) Parasitol Res. 1997;83(3):209–213. doi: 10.1007/s004360050235. [DOI] [PubMed] [Google Scholar]
- 10.Iori A, Grazioli D, Gentile E, Marano G, Salvatore G. Acaricidal properties of the essential oil of Melaleuca alternifolia Cheel (tea tree oil) against nymphs of Ixodes ricinus . Vet Parasitol. 2005;129(1-2):173–176. doi: 10.1016/j.vetpar.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 11.Kim SI, Yi JH, Tak JH, Ahn YJ. Acaricidal activity of plant essential oils against Dermanyssus gallinae (Acari Dermanyssidae) Vet Parasitol. 2004;120(4):297–304. doi: 10.1016/j.vetpar.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Macchioni F, Perrucci S, Cecchi F, Cioni PL, Morelli I, Pampiglione S. Acaricidal activity of aqueous extracts of chamomile flowers, Marticaria chamomilla, against the mite Psoroptes caniculi . Med Vet Entomol. 2004;18(2):205–207. doi: 10.1111/j.0269-283X.2004.00488.x. [DOI] [PubMed] [Google Scholar]
- 13.Needham GR, Teel PD. Water Balance by Ticks between Blood Meals. In: Sauer JR, Hair JA, editors. Morphology, Physiology and Behavioral Biology of Ticks. Chichester, England: Ellis Horwood Limited; 1986. pp. 100–151. [Google Scholar]
- 14.Onofre SB, Miniuk CM, Debarros NM, Azevedo JL. Pathogenicity of four strains of entomopathogenic fungi against the bovine tick Boophilus microplus . AJVR. 2001;62(9):1478–1480. doi: 10.2460/ajvr.2001.62.1478. [DOI] [PubMed] [Google Scholar]
- 15.Rak H. Tick-Borne Diseases and Their Vectors in Iran. In: Wilde JKH, editor. Proceeding of International Congress on Tick-Borne Diseases and Their Vectors. Edinburgh, UK: 1976. pp. 163–166. [Google Scholar]
- 16.Samish M, Rehacek JA. Pathogens and predators of tick and their potential in biological control. Annu Rev Entomol. 1999;44(1):159–182. doi: 10.1146/annurev.ento.44.1.159. [DOI] [PubMed] [Google Scholar]
- 17.Ündeğer Ü, Başaran N. Effects of pesticides on human peripheral lymphocytes in vitro: induction of DNA damage. Arch Toxicol. 2005;79(3):169–176. doi: 10.1007/s00204-004-0616-6. [DOI] [PubMed] [Google Scholar]
- 18.Zhioua E, Heyer K, Browning M, Ginsberg HS, LeBrun RA. Pathogenicity of Bacillus thuringiensis variety kurstaki to Ixodes scapularis (Acari Ixodidae) J Med Ent. 1999;36(6):900–902. doi: 10.1093/jmedent/36.6.900. [DOI] [PubMed] [Google Scholar]