Abstract
Neural stimuli, such as suckling or cold exposure, increase TRH mRNA in the paraventricular nucleus (PVN) of the rat hypothalamus, yet only suckling induces prolactin secretion. As TRH co-localizes with cocaine-and amphetamine-regulated transcript (CART) in hypophysiotropic neurons of the PVN, and CART inhibits TRH-induced prolactin release but not TRH-induced TSH release in adenohypophyseal cell cultures, we raised the possibility that differential regulation of CART gene expression in the PVN may explain the differences in prolactin secretion following each of the two stimuli. Primiparous female rats were mated and handled daily during the pre- and postpartum periods. After delivery, the litter was adjusted to 8 pups and at mid-lactation, dams were separated from their pups for 8 hours and exposed to either 1h of cold or 30 min of suckling. Long term effects of suckling were studied by separating pups from their mothers for 24h, followed by a 12h period of continuous suckling. Serum TSH levels increased in response to cold exposure, while prolactin levels were increased by suckling and diminished by cold exposure. CART mRNA levels increased in rostral and mid parts of the medial parvocellular PVN following cold exposure but not after suckling stimulation. These data demonstrate a differential regulation of CART gene expression in hypophysiotropic neurons in response to stimuli that increase TRH mRNA levels, and suggest that CART activation in the PVN may contribute to the decrease in PRL release when the thyroid axis is activated by cold exposure.
Section: Regulatory systems
Keywords: Cocaine- and Amphetamine-Regulated Transcript; TRH; suckling; cold; prolactin, hypothalamic paraventricular nucleus; in situ hybridization
INTRODUCTION
Thyrotropin-releasing hormone (TRH) neurons involved in the regulation of anterior pituitary TSH and prolactin secretion (Grosven or and Mena, 1980; Haisenleder et al., 1992) are confined to the medial and periventricular parvocellular subdivisions of the hypothalamic paraventricular nucleus (PVN), primarily organized in mid- to caudal portions of the PVN (Lechan and Toni, 1992; Fekete et al., 2000). Most of these “hypophysiotropic” TRH neurons contain a second peptide, cocaine and amphetamine-regulated transcript (CART) (Broberger, 1999; Elias et al., 2001; Fekete et al., 2000), originally identified in the striatum as a transcript up-regulated by psychostimulants (Douglass et al., 1995). While CART neurons from the arcuate nucleus have been shown to exert important effects on energy homeostasis including those on the hypothalamic-pituitary-thyroid axis through their action on hypophysiotropic TRH neurons (Fekete et al., 2000; Stanley et al., 2001), the importance of the coexistence of CART with TRH in the PVN is not yet understood. Hypothyroidism regulates both TRH and CART gene expression in the PVN; reduction in circulating thyroid hormone levels leads to an increase in both TRH and CART mRNA only in the medial and periventricular parvocellular subdivisions of the PVN (Kakucska et al., 1992; Raptis et al., 2004). Nevertheless, while there is an increase in TRH in the portal blood (Rondeel et al., 1988) and TRH is a potent prolactin secretogogue (Jacobs et al., 1971; Tashjian et al., 1971; Yuan and Pen, 2002), prolactin does not rise with hypothyroidism in the rat (Jahnke et al., 1980; Tohei et al., 1997). The possibility that CART could be directly modulating pituitary secretion was corroborated in primary cultures of anterior pituitary cells from male rats; CART inhibits prolactin release after 15 min incubation (Kuriyama et al., 2004) while at longer times, prevents TRH-induced prolactin release without affecting TSH release (Raptis et al., 2004).
Included among the physiologic stimuli associated with activation of hypophysiotropic TRH neurons are suckling and cold exposure that induce TRH release (Arancibia et al., 1983; de Greef and Visser, 1981; Fink and Ben-Aroya, 1983; Hefco et al., 1975) and a rapid (30-60 min) and transient increase in proTRH mRNA levels in the PVN (Rage et al., 1994; Sanchez et al., 2001; Uribe et al., 1993; van Haasteren et al., 1996). However, the response of pituitary target cells is specific to the stimulus. In lactating dams TSH is released after cold exposure, while it is modestly or not affected by suckling, and the opposite is observed for prolactin, released only after suckling but not cold stimulus (Adels et al., 1986; Haisenleder et al., 1992; Sánchez et al., 2001; van Haasteren et al., 1996). The main goal of this study was to determine whether activation of CART neurons in the PVN is stimulus-dependent, in concert with the selective pituitary response. We therefore evaluated the effects of cold exposure and acute or chronic suckling on CART mRNA expression in the PVN of lactating rats.
RESULTS
Effect of suckling or cold exposure on serum levels of PRL, TSH and corticosterone in lactating females
No significant differences were observed in serum corticosterone levels between control animals that were housed at normal room temperature in an adjacent room in the vivarium (RT suckling controls), and those that were transported into a room adjacent to the cold room (RT, cold controls) (Fig. 1A). Suckling produced a small but insignificant increase in corticosterone levels after 30’ of suckling stimulation, while a 6-fold increase in corticosterone was observed after 1hr of cold exposure (Fig. 1A). PRL secretion increased 2.5-fold after suckling, but decreased after cold stimulation (Fig. 1B) TSH levels increased 4.3-fold only after cold exposure (Fig. 1C).
Figure 1.
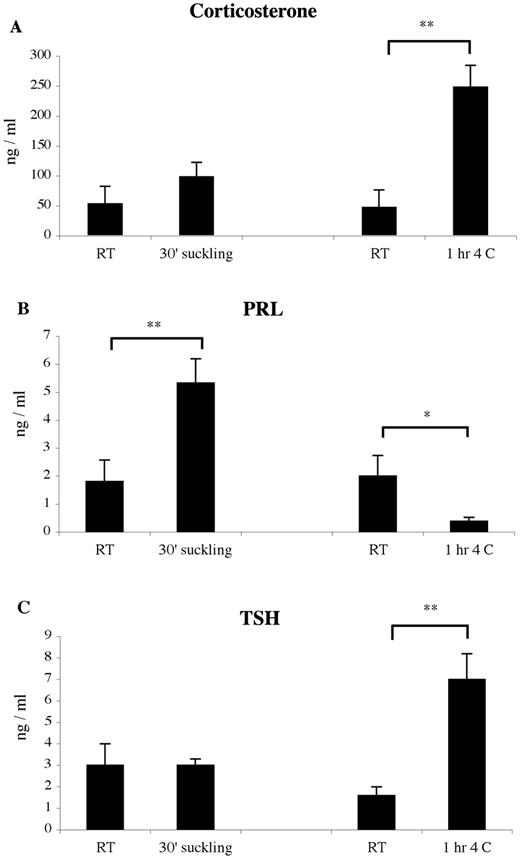
Serum hormone concentrations of control, suckling and cold stimulated lactating females quantified by radioimmunoassay (5 animals per group). Values represent mean ± sem. (* P < 0.05 or ** P < 0.001 statistically different by Newman-Keuls post hoc testing)
Effect of suckling or cold exposure on CART mRNA levels in the PVN as determined by RT-PCR
PCR amplification for CART mRNA generated two bands similar to that reported by Douglass et al (Douglass et al., 1995) corresponding to different polyadenylation start sites on the CART transcript (Fig. 2). The ratio of doublet CART/Cyclophilin signal showed a significant increase in the PVN after 1hr of cold exposure (Fig. 2A). No differences were observed in response to 30’ of suckling (Fig. 2B), despite an increase in TRH expression in the same hypothalamic RNA preparations (Fig. 2C and 2D).
Figure 2.
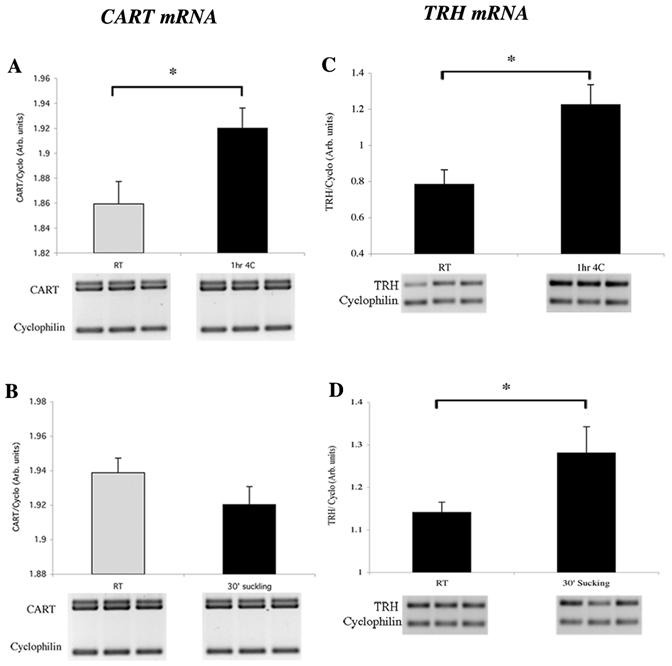
Semiquantitative RT-PCR for CART mRNA and TRH mRNA in the PVN of lactating rats exposed to cold (A, C) or suckling (B, D) stimulation. Densitometric analysis showed a significant increase in the PVN for CART mRNA after 1hr of cold stimulation and a tendency to decrease after 30’ of suckling (optical density = Arbitrary units). TRH mRNA was significantly increased after cold or suckling stimulation. A total of 5 animals per group were analyzed in each condition. t-Test * P < 0.05 statistically different from control RT animals
Effect of suckling or cold exposure on CART mRNA levels in the PVN as determined by ISH
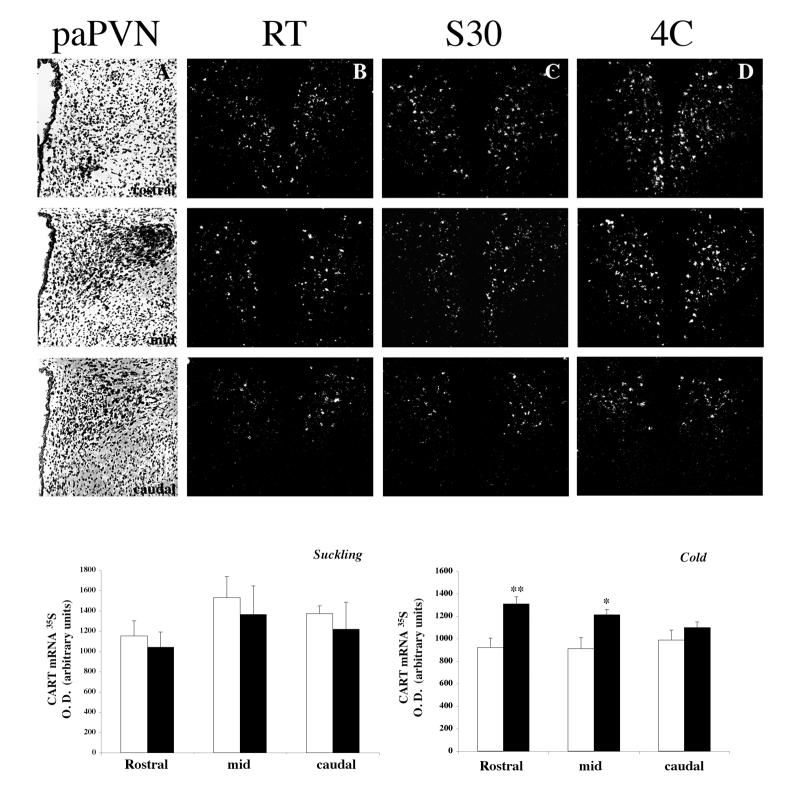
The distribution of CART mRNA in PVN neurons of control lactating rats was similar to that reported for male rats (Douglass et al., 1995; Elias et al., 2001); CART mRNA was observed in parvocellular and magnocellular PVN neurons (Fig. 3 B-D). No changes were observed in CART mRNA levels quantified in the rostral, mid or caudal portions of the parvocellular part of the PVN after 30 min of suckling (Fig. 3 C). In contrast, 1 h of cold exposure increased CART mRNA expression in rostral and mid portions of the parvocellular PVN (Fig. 3 D). No significant changes in CART mRNA expression were present in caudal PVN sections.
Figure 3.
Distribution of CART mRNA in the parvocellular PVN of lactating rats exposed to 30 min of suckling (column C) or 1h of cold exposure at 4C (column D). CART mRNA was detected by in situ hybridization using a 35S -labeled probe. Note the increase in the density of silver grains in cold exposed animals in the rostral (1st row) and mid (2nd row) sections of the parvocellular PVN (paPVN). First column (A): bright-field illumination showing representative hematoxilin-eosin stained sections trough the rostro-caudal paPVN. Second column (B): CART mRNA pattern in control RT lactating rats; third (C), in lactating rats suckled for 30 min (S30) and last (D) on lactating rats exposed 1h to cold, without pups. Graphs show semiquantitative analysis of CART mRNA after 30’ of suckling or 1hr of cold exposure. Values represent mean ± sem from 4, 8 or 4 slices/animal/group in the rostral, mid and caudal paPVN respectively. (* P < 0.05 or ** P < 0.01 statistically different from control RT animals; Newman-Keuls post hoc testing)
Effect of prolonged suckling on CART mRNA in the PVN
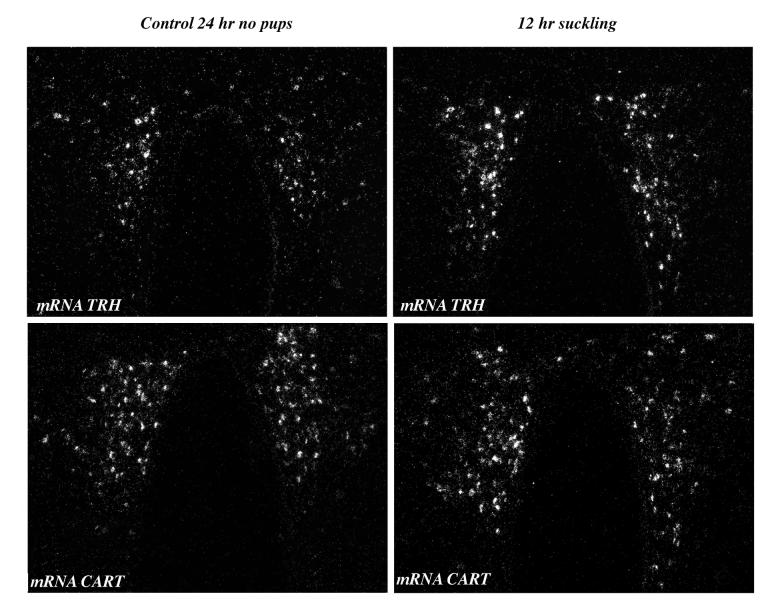
To determine whether CART expression could be modified in the PVN by a more prolonged suckling stimulus, pups were separated from their mothers on day 12 for 24 h, and then allowed to suckle for a 12 h period. CART mRNA levels were not modified at any level of the parvocellular PVN [control 905 ± 111, 12h suckling 1, 493 ± 293 arbitrary units; p = 0.43]. TRH mRNA increased 2-fold [controls 193 ± 32, 12h suckling 387 ± 41 arbitrary units; P = 0.006] (Fig. 4).
Figure 4.
TRH and CART mRNA expression in the PVN of lactating females separated from their pups 24 h on day 12, and then reunited with them for a 12 h period. TRH and CART mRNA were detected by in situ hybridization using 35S-labeled probes. Note the significant increase in silver grains for TRH mRNA after 12 h of continuous suckling in the mid PVN while no changes can be detected for CART mRNA.
DISCUSSION
CART is one of most abundant of the many peptides synthesized in the hypothalamus. High concentrations are found in the arcuate nucleus where it plays an important role in energy homeostasis, but neurons are also found in the paraventricular nucleus where most are neuroendocrine and some, pre-autonomic neurons (Vrang, 2006; Fekete et al., 2000). Forty percent of CART neurons concentrated in the anterior periventricular zone coexpress somatostatin (Larsen et al., 2003; Vrang, 2006; Vrang et al., 1999) while the medial parvocellular PVN CART neurons extensively colocalize with TRH (Broberger, 1999; Fekete et al., 2000). As CART is the only known peptide that coexists with TRH in the PVN (Fekete et al., 2000) it is feasible that under the appropriate circumstances, the genes for both peptides may become simultaneously activated. The paradigm of the lactating rat subject to either suckling or cold exposure, conditions when TRH neurons are rapidly activated in the PVN (Sánchez et al., 2001), exemplify in vivo situations where TRH is released but the secretion of TSH and prolactin diverge (Adels et al., 1986; Arancibia et al., 1983; de Greef and Visser, 1981; Sanchez et al., 2001; van Haasteren et al., 1996). Using this paradigm, we demonstrate herein a differential activation of CART neurons in the PVN, stimulated by cold exposure but not by suckling.
Neither acute, nor continuous suckling modified CART expression in any area of the PVN. These observations are in agreement with previous reports that failed to observe changes in CART mRNA expression in the PVN of lactating rats continuously suckled compared to female rats in diestrus, even when a significant increase was observed in the supraoptic nucleus (Smith et al., 2006). In contrast to suckling, or chronic cold exposure (Kong et al., 2003), CART mRNA levels were enhanced in the rostral and mid portions of the PVN by acute cold stimulation. The distribution of cold-responsive CART neurons resembles the distribution of cold-responsive TRH neurons in the PVN, namely up to 2.4 times greater in the rostral and the mid PVN compared to the caudal portion, and different from those responding to suckling which are present only in the mid-PVN (Sánchez et al., 20001). As CART is also co-expressed with somatostatin in neurons localized in the periventricular zone of rostro-mid PVN (Larsen et al; 2003, Vrang 2006), it is possible that these neurons could also participate in the cold-induced CART response, particularly since somatostatin expression is known to rapidly increase 15 min after cold stimulation (Rage et al., 1994). Quantification of CART-cold responsive neurons expressing TRH or other peptides in lactating vs. male rats will require further study.
The potential importance of the differential response of suckling and cold exposure on CART expression in the PVN may be seen in light of the inhibitory effect of CART on prolactin secretion shown in vitro and in vivo. Dispersed cells from male pituitaries incubated for 15 min with 100 nM of CART55-102 show decreased prolactin secretion without affecting the release of other pituitary hormones (Kuriyama et al., 2004), and an inhibitory effect on prolactin secretion in response to TRH (Raptis et al., 2004). In vivo, central administration of CART inhibits basal prolactin release and activates dopamine turnover in the median eminence of ovariectomized-estrogen primed female, but not in normal male rats (Yang and Shieh 2004). Thus, the central actions of CART could have a dual regulatory action on prolactin secretion as a result of direct release into the portal system from hypophysiotropic PVN neurons and through effects on TIDA neurons. Dopamine released from the tuberoinfundibular dopaminergic neurons (TIDA) exerts a tonic inhibitory tone on prolactin secretion and factors that inhibit or stimulate their activity consequently stimulate or inhibit prolactin release (Freeman et al., 2000). CART terminals impinge on TIDA terminals and increase DA turnover leading to a decrease in serum prolactin (Yang and Shieh 2004). Cold exposure, therefore, would appear to not only have an effect on the HPT axis by increasing TSH secretion, but also inhibiting prolactin release as a result of the simultaneous release of TRH and CART. The upregulation of CART expression in the hypophysiotropic neurons of lactating rats, exclusively by cold stimulation, gives further support for a relevant role in the control of prolactin secretion, adding this peptide to the host of hypothalamic prolactin-inhibiting factors (Freeman et al., 2000).
The lack of response of CART expression to suckling contrast the upregulation observed in TRH neurons of the mid-PVN; it remains to be shown whether all TRH neurons responding to suckling express CART in order to differentiate if this apparent functional heterogeneity of TRH hypophysiotropic neurons is due or not to a different population of those responding to cold stimulation (Sánchez et al., 2001). We cannot exclude the possibility that the neuronal signals that mediate the effects of cold and suckling engage different transduction pathways. Cold stimulation on TRH gene expression is proposed to be mediated by noradrenergic pathways (Arancibia et al., 1996) and in vitro, noradrenaline increases pro-TRH transcription, and TRH release, through the activation of PKA (Cote et al., 2005). Since elevated cAMP is known to stimulate both TRH and CART gene expression (Cote et al., 2005; Perez-Martinez et al., 1998; Dominguez, 2006), phosphorylated CREB is likely responsible for the increased synthesis of TRH and CART. The pathways that activate the TRH neurons during suckling are less understood; the exclusive effect on TRH but not CART gene expression suggests the involvement of second messenger pathways within the TRH neurons that do not activate CART promoter.
Activation of CART neurons in the rostral PVN, where the colocalization of TRH and CART is negligible under basal conditions (Fekete et al., 2000), suggest the involvement of other circuits. The rostral PVN has been implicated in the autonomic thermogenesis response (Yoshida et al., 2005) and, although the nature of CART responsive neurons in the rostral PVN awaits characterization (Vrang, 2006), non-neuroendocrine TRH neurons project their axons to other hypothalamic nuclei and/or other brain areas as the brain stem (Markakis and Swanson, 1997). The simultaneous activation of CART and TRH neurons in this area, together with the hypophysiotropic ones, supports a coordinate expression of a somatomotor, autonomic and neuroendocrine response to cold exposure.
In conclusion, CART neurons are activated in the PVN by cold exposure but not by suckling. Given the inhibitory effect of CART on TRH-induced prolactin release (Raptis et al., 2004), these results strengthen a physiological role for CART neurons of the parvicellular PVN which, upon stimulation, could release CART and serve to restrain TRH-induced prolactin secretion under specific physiologic circumstances.
EXPERIMENTAL PROCEDURE
Animals
All animal care and procedures followed the “Guidelines for Use of Animals in Neuroscience Research” of the Society for Neuroscience (USA), and were approved by the Institutional Animal Care and Ethics Committee of the Instituto de Biotecnología UNAM, and Tufts-New England Medical Center.
Animal Preparation
Lactating rats were maintained on a 12-hour light/dark cycle (lights on at 7 a.m.) and fed Purina chow (PMI 5001) and water ad libitum.
Paradigm A:
Primiparous Wistar female rats were mated, handled daily and kept in individual cages as previously described (Uribe et al., 1993). The litter was adjusted to 8 pups following delivery, and after 12-14 days of lactation, dams were separated from their pups at 7 a.m. for 8 hours. Half the group of dams were transported at 7 a.m. from the vivarium to a room adjacent to the cold room (RT, 25 °C), some were exposed to 4 °C for 1 hour in the cold room at 3 p.m., and the others, maintained at room temperature (cold controls without pups). The remaining half were divided into two groups: in one, dams were reunited at 3 p.m. with their pups and allowed to suckle for 30 min; the other, remained without pups in an adjacent room of the vivarium (suckling controls). Two independent experiments were performed.
All animals were killed at 3:30-4:30 p.m. by decapitation with a guillotine and trunk blood collected. The blood was allowed to clot at room temperature and serum aliquots were kept at -20 °C. The brains were rapidly removed from the skull, placed on dry ice and stored at -70 °C.
Paradigm B:
To determine the effect of prolonged suckling on CART mRNA in the PVN, Sprague-Dawley lactating dams were separated from their pups on day 12. The pups were kept on a warm plate for 24 h and then reunited with their mothers. Pups were allowed to suckle for 12 h, the dams were then anesthetized with sodium pentobarbital (50 mg/kg BW ip) and immediately perfused transcardially with 20 ml 0.01 M PBS, pH 7.4, containing 15,000 U/liter heparin sulfate followed by 150 ml 4% paraformaldehyde in PBS. Brains were removed and post-fixed by immersion in the same fixative for 2 h at room temperature. Tissue blocks containing the hypothalamus were cryoprotected in 20% sucrose in PBS at 4°C overnight then snap frozen on dry ice and stored at -70 °C (Raptis et al., 2004).
Total RNA extraction and RT-PCR
To measure CART, TRH and cyclophilin mRNA expression in the PVN by RT-PCR, a group of rats were kept under the conditions of paradigm A. After guillotine decapitation, brains were rapidly removed from the skull, placed on dry ice and stored at -70 °C. Brains were sectioned coronally in a cryostat until an area corresponding to approximately -1.3 mm cadual to the Bregma (based on Paxinos and Watson, 2005) was reached. The brain was then removed, kept frozen on pulverized dry ice, and a 1 mm coronal tissue slab containing the PVN was obtained using a razor blade. The PVN was dissected from the tissue slab using the micropunch technique (Palkovits and Brownstein, 1988) and kept frozen in a tube on dry ice. Total RNA was extracted (Chomczynski and Sacchi, 1987) and RNA concentration was determined by absorbance at 260 nm. Only those samples showing 260/280 nm ratio of >1.8 and adequate 28S/18S ratio, verified by gel electrophoresis in agarose 1 %-TBE 1x, were used.
One microgram of RNA was transcribed with M-MLV reverse transcriptase and oligo-dT and PCR reactions were performed as reported (Pérez Martínez et al., 1998), except that antisense oligonucleotide primers (15 pmol for CART, 25 pmol for TRH, 50 pmol for cyclophilin probes) were used, and 0.5 μl Taq DNA polymerase (5 U/μl) (Biotecnologías Universitarias, UNAM, DF, Mexico). Sequences for PCR oligonucleotides were verified as described (de Gortari et al., 2006) and synthesized at the Institute of Biotecnology. Cyclophilin (nucleotides (nt) 166-422 of cDNA (Danielson et al., 1988); fin al product (fp): 256 bp; sense (s): 5’-GGGGAGAAAGGATTTGGATA-3’, antisense (as): 5’-ACATGATTGCCATCCAGCA-3’. Pro-TRH [nt 586-943 of cDNA (Lechan et al., 1986); fp: 358 bp; s: 5’-CCCTGGATGGAGTCTGATGT-3’, as: 5’-GACAGCTAGTGAAGGGAACAGG-3’. CART (Douglass et al., 1995) fp: 592 bp; s: 5′-GGC-GCC-GCC-CTA-CTG-CTG-CTG-CTA-CCT-TTG-3′, as: 5′-AGA-ATG-TGT-CAG-GTG-GAT-AAA-ACG-AGA-GAA-3′.
To determine the adequacy of the conditions for mRNA semi-quantification of each probe, cDNAs prepared from 1.0 μg of RNA from PVN were amplified for different PCR cycles (Mastercycler, Ependorff, Hamburg, Germany) and the number of cycles chosen for each cDNA was taken in the ascending part of the curve relating to optical density. Cyclophilin, 1 μl of cDNA [94 °C 1’15”, 57 °C 30”, 72 °C 1’] × 25 cycles + 5 min at 72 °C for a final extension; pro-CART, 6 μl of cDNA [94 °C 1’, 64 °C 1’, 72 °C 1’] × 30 cycles + 7 min at 72 °C for a final extension; pro-TRH, 6 μl of cDNA [95 °C 1’, 64 °C 1’, 72 °C 1’] × 30 cycles + 10 min at 72 °C for a final extension. Products were separated by gel electrophoresis in agarose 2 %-TBE 1x, running buffer 0.5x, stained with ethidium bromide and the density quantified with a Fluor-S MultiImager (BioRad). Samples of control and experimental animals from the same group were included in the same gel. The relative values of mRNAs were calculated as the signal ratio against cyclophilin cDNA.
In situ hybridization histochemistry (ISH)
Frozen brains from a different set of animals prepared as described in paradigm A were sectioned coronally with a cryostat at 12 μm and stored at -70 °C until use. Sections from the rostral-caudal extent of the PVN were taken for in situ hybridization histochemistry. The rostral zone was considered around -1.3 mm caudal to the Bregma, the mid zone starting at -1.56 mm and the caudal, from -1.8 mm to -2.04 [Paxinos and Watson 2005; Swanson, 1998]. The rostral, mid, and caudal zones of the parvocellular PVN were identified by brightfield microscopic analysis of hematoxylin/eosin stained sections. Serial sections were hybridized with a 866-base pair single stranded [35S] UTP labeled cRNA probe for CART (Couceyro et al., 1997) (kindly provided by Dr MJ Kuhar) and processed as previously described (Sanchez et al., 2001) except that final rinses were done in descending concentrations of SSC 2x, 1x, 0.5× and 0.1× at RT, two washes in 0.1× SSC for 30 min each at 60 °C and treatment with ascending concentrations of ethanol 75%, 80% and 95% for 2 min each. Specific labeling was detected with Kodak Bioma× film exposed for 7 days at room temperature. Slides were then dipped into Kodak NTB2 autoradiography emulsion (Eastman Kodak, Rochester, NY) and the autoradiograms developed after 21 days of exposure at 4 °C.
Serial coronal sections through the PVN from brains prepared as described for paradigm B were similarly subjected to in situ hybridization histochemistry using either the single stranded [35S] UTP labeled cRNA probe for CART or, a 1,241-base pair single stranded [35S] UTP labeled cRNA probe for pro-TRH as previously described (Dyess et al., 1988; Kakucska et al., 1992).
Image Analysis
CART ISH signal was visualized under dark field illumination using a CCD Sony video camera and analyzed using Explora Nova imaging software (France). Hematoxylin-eosin counterstained sections were used to define the confines of the parvocellular PVN subdivision as described by Swanson and Kuypers (Swanson and Kuypers, 1980). Sections were visualized by bright-field microscopy and the parvocellular PVN was delineated. Proper filters were then exchanged to visualize the preparation by dark-field illumination in the delineated zone on both sides of the PVN and optical density (density × area) was measured in each animal. Four slices from the rostral, 8 from the mid and 4 from the caudal portions of the PVN were analyzed in each animal. The mean of the optical density values of slices/zone/animal were calculated and considered as one determination.
Hormone Quantification
Corticosterone was measured by radioimmunoassay (ICN diagnostic kit). TSH and prolactin were iodinated using reagents and protocols of the NIDDK. Intra- and inter-assay variation coefficients were <10%. In each experimental paradigm those rats that did not show an increase in TSH following cold exposure, or prolactin after suckling, were excluded from further analysis.
Statistical Analysis
Comparison of groups was performed with one-way ANOVA followed by multiple comparisons (Newman-Keuls post hoc testing), or t-Test where stated. Significance was considered for * P < 0.05 or ** P < 0.01. Results are presented as the mean ± sem. At least two different experiments were performed and each analyzed twice by independent ISH trials on different sections, giving similar results.
Acknowledgments
The authors thank Sergio González Trujillo for his assistance in animal handling and Alfonso Carreón-Rodriguez, Miguel Cisneros, Fidelia Arteaga and Manuel Villa for their technical assistance. This work was partially funded by CONACYT 43503-Q, DGAPA IN 222603 and Grant NIH DK-37021
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adels LE, Leon M, Wiener SG, Smith MS. Endocrine response to acute cold exposure by lactating and non-lactating Norway rats. Physiol. Behav. 1986;36:179–181. doi: 10.1016/0031-9384(86)90093-4. [DOI] [PubMed] [Google Scholar]
- Arancibia S, Tapia-Arancibia L, Assenmacher I, Astier H. Direct evidence of short-term cold-induced TRH release in the median eminence of unanesthetized rats. Neuroendocrinology. 1983;37:225–228. doi: 10.1159/000123547. [DOI] [PubMed] [Google Scholar]
- Arancibia S, Rage F, Astier H, Tapia-Arancibia L. Neuroendocrine and autonomous mechanisms underlying thermoregulation in cold environment. Neuroendocrinology. 1996;64:257–267. doi: 10.1159/000127126. [DOI] [PubMed] [Google Scholar]
- Broberger C. Hypothalamic cocaine- and amphetamine-regulated transcript (CART) neurons: histochemical relationship to thyrotropin-releasing hormone, melanin-concentrating hormone, orexin/hypocretin and neuropeptide Y. Brain Res. 1999;848:101–113. doi: 10.1016/s0006-8993(99)01977-0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cote-Velez A, Perez-Martinez L, Diaz-Gallardo MY, Perez-Monter C, Carreon-Rodriguez A, Charli JL, Joseph-Bravo P. Dexamethasone represses cAMP rapid upregulation of TRH gene transcription: identification of a composite glucocorticoid response element and a cAMP response element in TRH promoter. J Mol Endocrinol. 2005;34:177–97. [Google Scholar]
- Couceyro PR, Koylu EO, Kuhar MJ. Further studies on the anatomical distribution of CART by in situ hybridization. J. Chem. Neuroanat. 1997;12:229–241. doi: 10.1016/s0891-0618(97)00212-3. [DOI] [PubMed] [Google Scholar]
- Danielson PE, Forss-Petter S, Brow MA, Calavetta L, Douglass J, Milner RJ, Sutcliffe JG. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988;7:261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- de Gortari P, Uribe RM, Garcia-Vazquez A, Aguilar-Valles A, Martinez A, Valdes A, Charli JL, Fernandez-Guardiola A, Joseph-Bravo P. Amygdala kindling differentially regulates the expression of the elements involved in TRH transmission. Neurochem. Int. 2006;48:31–42. doi: 10.1016/j.neuint.2005.08.003. [DOI] [PubMed] [Google Scholar]
- de Greef WJ, Visser TJ. Evidence for the involvement of hypothalamic dopamine and thyrotrophin-releasing hormone in suckling-induced release of prolactin. J. Endocrinol. 1981;91:213–223. doi: 10.1677/joe.0.0910213. [DOI] [PubMed] [Google Scholar]
- Dominguez G. The CART gene: structure and regulation. Peptides. 2006;27:1913–1918. doi: 10.1016/j.peptides.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Douglass J, McKinzie AA, Couceyro P. PCR differential display identifies a rat brain mRNA that is transcriptionally regulated by cocaine and amphetamine. J. Neurosci. 1995;15:2471–2781. doi: 10.1523/JNEUROSCI.15-03-02471.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyess EM, Segerson TP, Liposits Z, Paull WK, Kaplan MM, Wu P, Jackson IM, Lechan RM. Triiodothyronine exerts direct cell-specific regulation of thyrotropin-releasing hormone gene expression in the hypothalamic paraventricular nucleus. Endocrinology. 1988;123:2291–2297. doi: 10.1210/endo-123-5-2291. [DOI] [PubMed] [Google Scholar]
- Elias CF, Lee CE, Kelly JF, Ahima RS, Kuhar M, Saper CB, Elmquist JK. Ch aracterization of CART neurons in the rat and human hypothalamus. J. Comp. Neurol. 2001;432:1–19. doi: 10.1002/cne.1085. [DOI] [PubMed] [Google Scholar]
- Fekete C, Mihaly E, Luo LG, Kelly J, Clausen JT, Mao Q, Rand WM, Moss LG, Kuhar M, Emerson CH, Jackson IM, Lechan RM. Association of cocaine- and amphetamine-regulated transcript-immunoreactive elements with thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and its role in the regulation of the hypothalamic-pituitary-thyroid axis during fasting. J. Neurosci. 2000;20:9224–9234. doi: 10.1523/JNEUROSCI.20-24-09224.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GKY, Ben-Aroya N. In: TRH in hypophysial portal blood: Characteristics of release and relationship to thyrotropin and prolactin secretion; In Thyrotropin-releasing Hormone. Griffiths EC, Bennett GW, editors. Raven Press; New York: 1983. pp. 127–144. [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. [Google Scholar]
- Grosvenor CE, Mena F. Evidence that thyrotropin-releasing hormone and a hypothalamic prolactin-releasing factor may function in the release of prolactin in the lactating rat. Endocrinology. 1980;107:863–868. doi: 10.1210/endo-107-4-863. [DOI] [PubMed] [Google Scholar]
- Haisenleder DJ, Ortolano GA, Dalkin AC, Yasin M, Marshall JC. Differential actions of thyrotropin (TSH)-releasing hormone pulses in the expression of prolactin and TSH subunit messenger ribonucleic acid in rat pituitary cells in vitro. Endocrinology. 1992;130:2917–2923. doi: 10.1210/endo.130.5.1572303. [DOI] [PubMed] [Google Scholar]
- Hefco E, Krulich L, Illner P, Larsen PR. Effect of acute exposure to cold on the activity of the hypothalamic-pituitary-thyroid system. Endocrinology. 1975;97:1185–1195. doi: 10.1210/endo-97-5-1185. [DOI] [PubMed] [Google Scholar]
- Jacobs LS, Snyder PJ, Wilber JF, Utiger RD, Daughaday WH. Increased serum prolactin after administration of synthetic thyrotropin releasing hormone (TRH) in man. J. Clin. Endocrinol. Metab. 1971;33:996–998. doi: 10.1210/jcem-33-6-996. [DOI] [PubMed] [Google Scholar]
- Kakucska I, Rand W, Lechan RM. Thyrotropin-releasing hormone gene expression in the hypothalamic paraventricular nucleus is dependent upon feedback regulation by both triiodothyronine and thyroxine. Endocrinology. 1992;130:2845–2850. doi: 10.1210/endo.130.5.1572297. [DOI] [PubMed] [Google Scholar]
- Kong WM, Stanley S, Gardiner J, Abbott C, Murphy K, Seth A, Connoley I, Ghatei M, Stephens D, Bloom S. A role for arcuate cocaine and amphetamine-regulated transcript in hyperphagia, thermogenesis, and cold adaptation. Faseb J. 2003;17:1688–1690. doi: 10.1096/fj.02-0805fje. [DOI] [PubMed] [Google Scholar]
- Kuriyama G, Takekoshi S, Tojo K, Nakai Y, Kuhar MJ, Osamura RY. Cocaine- and amphetamine-regulated transcript peptide in the rat anterior pituitary gland is localized in gonadotrophs and suppresses prolactin secretion. Endocrinology. 2004;145:2542–2550. doi: 10.1210/en.2003-0845. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Seier V, Fink-Jensen A, Holst JJ, Warberg J, Vrang N. Cocaine- and amphetamine-regulated transcript is present in hypothalamic neuroendocrine neurones and is released to the hypothalamic-pituitary portal circuit. J. Neuroendocrinol. 2003;15:219–226. doi: 10.1046/j.1365-2826.2003.00960.x. [DOI] [PubMed] [Google Scholar]
- Lechan RM, Toni R. Thyrotropin-releasing hormone neuronal systems in the central nervous system. In: Nemeroff CB, editor. Neuroendocrinology. CRC Press; Boca Raton, Florida: 1992. pp. 279–330. [Google Scholar]
- Lechan RM, Wu P, Jackson IM, Wolf H, Cooperman S, Mandel G, Goodman RH. Thyrotropin-releasing hormone precursor: characterization in rat brain. Science. 1986;231:159–561. doi: 10.1126/science.3079917. [DOI] [PubMed] [Google Scholar]
- Markakis EA, Swanson LW. Spatiotemporal patterns of secretomotor neuron generation in the parvicellular neuroendocrine system. Brain Res. Rev. 1997;24:255–291. doi: 10.1016/s0165-0173(97)00006-4. [DOI] [PubMed] [Google Scholar]
- Palkovits MBM. Maps and Guide to Microdissection of the Rat Brain. Elsevier Science; N.Y.: 1988. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereoaxic coordinates. 5th Ed. Elsevier; San Diego, CA.: [Google Scholar]
- Perez-Martinez L, Carreon-Rodriguez A, Gonzalez-Alzati ME, Morales C, Charli JL, Joseph-Bravo P. Dexamethasone rapidly regulates TRH mRNA levels in hypothalamic cell cultures: interaction with the cAMP pathway. Neuroendocrinology. 1998;68:345–354. doi: 10.1159/000054383. [DOI] [PubMed] [Google Scholar]
- Rage F, Lazaro JB, Benyassi A, Arancibia S, Tapia-Arancibia L. Rapid changes in somatostatin and TRH mRNA in whole rat hypothalamus in response to acute cold exposure. J. Neuroendocrinol. 1994;6:19–23. doi: 10.1111/j.1365-2826.1994.tb00550.x. [DOI] [PubMed] [Google Scholar]
- Raptis S, Fekete C, Sarkar S, Rand WM, Emerson CH, Nagy GM, Lechan RM. Cocaine- and amphetamine-regulated transcript co-contained in thyrotropin-releasing hormone (TRH) neurons of the hypothalamic paraventricular nucleus modulates TRH-induced prolactin secretion. Endocrinology. 2004;145:1695–1699. doi: 10.1210/en.2003-1576. [DOI] [PubMed] [Google Scholar]
- Rondeel JM, de Greef WJ, van der Schoot P, Karels B, Klootwijk W, Visser TJ. Effect of thyroid status and paraventricular area lesions on the release of thyrotropin-releasing hormone and catecholamines into hypophysial portal blood. Endocrinology. 1988;123:523–527. doi: 10.1210/endo-123-1-523. [DOI] [PubMed] [Google Scholar]
- Sanchez E, Uribe RM, Corkidi G, Zoeller RT, Cisneros M, Zacarias M, Morales-Chapa C, Charli JL, Joseph-Bravo P. Differential responses of thyrotropin-releasing hormone (TRH) neurons to cold exposure or suckling indicate functional heterogeneity of the TRH system in the paraventricular nucleus of the rat hypothalamus. Neuroendocrinology. 2001;74:407–422. doi: 10.1159/000054707. [DOI] [PubMed] [Google Scholar]
- Smith SM, Vaughan JM, Donaldson CJ, Fernandez RE, Li C, Chen A, Vale WW. Cocaine- and amphetamine-regulated transcript is localized in pituitary lactotropes and is regulated during lactation. Endocrinology. 2006;147:1213–1223. doi: 10.1210/en.2005-1392. [DOI] [PubMed] [Google Scholar]
- Stanley SA, Small CJ, Murphy KG, Rayes E, Abbott CR, Seal LJ, Morgan DG, Sunter D, Dakin CL, Kim MS, Hunter R, Kuhar M, Ghatei MA, Bloom SR. Actions of cocaine- and amphetamine-regulated transcript (CART) peptide on regulation of appetite and hypothalamo-pituitary axes in vitro and in vivo in male rats. Brain Res. 2001;893:186–194. doi: 10.1016/s0006-8993(00)03312-6. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. 2nd rev. Ed. Elsevier; Amsterdam: 1998. [Google Scholar]
- Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J. Comp. Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- Tashjian AH, Jr., Barowsky NJ, Jensen DK. Thyrotropin releasing hormone: direct evidence for stimulation of prolactin production by pituitary cells in culture. Biochem. Biophys. Res. Commun. 1971;43:516–523. doi: 10.1016/0006-291x(71)90644-9. [DOI] [PubMed] [Google Scholar]
- Tohei A, Akai M, Tomabechi T, Mamada M, Taya K. Adrenal and gonadal function in hypothyroid adult male rats. J. Endocrinol. 1997;152:147–154. doi: 10.1677/joe.0.1520147. [DOI] [PubMed] [Google Scholar]
- Uribe RM, Redondo JL, Charli JL, Joseph-Bravo P. Suckling and cold stress rapidly and transiently increase TRH mRNA in the paraventricular nucleus. Neuroendocrinology. 1993;58:140–145. doi: 10.1159/000126523. [DOI] [PubMed] [Google Scholar]
- van Haasteren GA, van Toor H, Klootwijk W, Handler B, Linkels E, van der Schoot P, van Ophemert J, de Jong FH, Visser TJ, de Greef WJ. Studies on the role of TRH and corticosterone in the regulation of prolactin and thyrotrophin secretion during lactation. J. Endocrinol. 1996;148:325–336. doi: 10.1677/joe.0.1480325. [DOI] [PubMed] [Google Scholar]
- Vrang N. Anatomy of hypothalamic CART neurons. Peptides. 2006;27:1970–1980. doi: 10.1016/j.peptides.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Vrang N, Tang-Christensen M, Larsen PJ, Kristensen P. Recombinant CART peptide induces c-Fos expression in central areas involved in control of feeding behaviour. Brain. Res. 1999;818:499–509. doi: 10.1016/s0006-8993(98)01349-3. [DOI] [PubMed] [Google Scholar]
- Yang SC, Shieh KR. Effects of the cocaine- and amphetamine-regulated transcript peptide on the turnover of dopamine in tuberoinfundibular neurons and serum prolactin levels: studies using estrogen, melanin concentrating hormone, and melanocortin. Neuropharmacology. 2004;47:1070–1080. doi: 10.1016/j.neuropharm.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Konishi M, Nagashima K, Saper CB, Kanosue K. Fos activation in hypothalamic neurons during cold or warm exposure: projections to periaqueductal gray matter. Neuroscience. 2005;133:1039–1046. doi: 10.1016/j.neuroscience.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Yuan ZF, Pan JT. Involvement of angiotensin II, TRH and prolactin-releasing peptide in the estrogen-induced afternoon prolactin surge in female rats: studies using antisense technology. Life Sci. 2002;71:899–910. doi: 10.1016/s0024-3205(02)01773-3. [DOI] [PubMed] [Google Scholar]