Abstract
Consumption of arsenic contaminated drinking water has been linked to higher rates of coronary disease, stroke, and peripheral arterial disease. Recent evidence suggests that early life exposures may play a significant role in the onset of chronic adult diseases. To investigate the potential for in utero exposure to accelerate the onset of cardiovascular disease we exposed pregnant ApoE-knockout (ApoE−/−) mice to arsenic in their drinking water and examined the aortic trees of their male offspring for evidence of early disease 10 and 16 weeks after birth. Mice were maintained on normal chow after weaning. ApoE−/− mice are a commonly used model for atherogenesis and spontaneously develop atherosclerotic disease. Mice exposed to arsenic in utero showed a >2-fold increase in lesion formation in the aortic roots as well as the aortic arch compared to control mice at both 10 and 16 weeks of age. The mice exposed to arsenic also had a 20 – 40% decrease in total triglycerides, but no change in total cholesterol, phospholipids and total abundance of VLDL or HDL particles. Subfractionation of VLDL particles showed a decrease in large VLDL particles. In addition, the arsenic exposed mice showed a vasorelaxation defect in response to acetylcholine suggesting disturbance of endothelial cell signalling. These results indicate that in utero arsenic exposure induces an early onset of atherosclerosis in ApoE−/− mice without a hyperlipidemic diet and support the hypothesis that in utero arsenic exposure may be atherogenic in humans.
Keywords: Arsenic, atherosclerosis, ApoE mice, vasorelaxation defect, aortic lesions, aortic valve lesions, in utero exposure
Introduction
Inorganic arsenic is a worldwide natural drinking water contaminant and is a high priority hazardous substance in the United States. The role that exposure to arsenic in drinking water plays in disease is a major concern in the U.S. because large areas of the country have elevated arsenic in the ground water. Inorganic arsenic is metabolized in the liver by humans and rodents to mono- and dimethylated forms (1–4). Both inorganic and organic arsenic species cross the placenta in humans (5) and rodents (6). Thus, in utero arsenic exposure may play an important role in environmental arsenic induced disease.
Cardiovascular disease accounts for more than half the deaths in the United States. Chronic ingestion of arsenic contaminated drinking water has been implicated in development of cardiovascular disease (3). The contribution of arsenic ingestion to cardiovascular disesase in the U.S. is unknown but is likely significant. Nearly 4,000 wells supplying public water in the U.S. have arsenic levels greater than 10 μg/L (7). In addition, many people living in rural areas consume arsenic laden water from their own wells as exemplified by the documentation of chronic arsenicism in rural Michigan as reported in the Detroit Free Press (8). Consumption of arsenic contaminated drinking water is associated with mortality from arterial disease in the U.S. (9,10). Data from epidemiological studies performed in the arsenic endemic area of Taiwan suggest dose dependence of the incidence of both peripheral vascular disease and cardiovascular disease related mortality (11–15). A role for transplacental arsenic exposure in development of arterial disease is suggested by reports of myocardial infarction in infants whose mothers consumed water with high levels of arsenic (16,17).
There is a need to investigate the role of in utero arsenic exposure on the etiology of vascular disease leading to myocardial infarction and stroke. Apolipoprotein E knockout (ApoE−/−) mice (18,19) represent the most relevant and useful model currently available, and are used extensively in atherosclerosis research (20,21). In ApoE−/− mice, the lack of apolipoprotein E, which is a ligand for lipoprotein recognition and clearance by the lipoprotein receptor, results in delayed clearance of lipoproteins. The mice develop a phenocopy of human type III hyerplipidemia with severe hypercholesterolemia on a normal chow diet due to the accumulation of chylomicrons and VLDL remnant lipoproteins, and spontaneously develop atherosclerosis (18,19,22,23). We used these mice to determine whether early life exposure to arsenic can accelerate the atherosclerotic process. We show here that transplacental arsenic exposure of ApoE−/− mice maintained on normal chow accelerates development of aortic lesions and vasorelaxation defects.
Methods
Chemicals
Sodium arsenite (As3+), phenylephrine, acetylcholine and sodium nitroprusside (SNP) were purchased from Sigma Chemical Company (St. Louis, MO).
Animal housing and husbandry
ApoE−/− mice (B6.129P2-Apoetm1Unc/J, Jax Labs, Bar Harbor, ME) were housed and bred in a temperature- and humidity-controlled room with a 12 h/12 h light/dark cycle following the guidelines of the Association for the Accreditation of Laboratory Animal Care-approved animal care facility. Matings were performed with 1 male to breed 2 females separated by 3 days. Females were monitored daily for the presence of a seminal plug. Pregnant dams were housed individually and with their respective litters until weaning. Post-weaning, the mice were housed in cages with 3 mice per cage, and screened regularly for the presence of common adventitious mouse pathogens. The mice were weaned at 3 weeks of age and were maintained on a standard chow diet (PicoLab Rodent Chow 20 containing 4.5 % fat by weight and 0.02 % cholesterol). Arsenic exposed pregnant mice were provided drinking water containing 85 mg/L NaAsO2 starting on gestational day 8 and ending on day of birthing (day 20). Controls were provided tap water. Studies were performed under protocols approved by the University of Louisville Institutional Animal Care and Use Committee.
Plasma Lipoprotein Analysis
At 10 and 16 weeks of age, mice were anesthetized with pentobarbital (150 mg/kg), and the blood was withdrawn by cardiac puncture. Disodium EDTA (3 mM) was used as an anti-coagulant. The blood was centrifuged at 300 x g for 10 min at room temperature to remove the cells. The plasma was centrifuged at 13,000 x g for 10 min at 4 ºC to remove the chylomicrons. Plasma cholesterol, phospholipids and triglycerides were measured using commercial kits from Wako Chemicals USA (Richmond VA). Lipoprotein subclass profiles were measured by Liposcience Inc. (Raleigh, NC) using nuclear magnetic resonance spectroscopy as described (24). Differences between groups of arsenic exposed and unexposed mice were assessed using t-test analyses.
Atherosclerotic Lesion Analysis
For the morphometric analysis, the entire aorta from the heart, extending to 5 mm after bifurcation of the iliac arteries and including the subclavian right and left common carotid arteries, was removed and dissected for lesion analysis en face, using Sudan IV staining. The aortic arch was defined as the region from ascending arch to 3 mm distal to subclavian artery (25). Percent lesion area was calculated using Metamorph imaging software.
For the analysis of aortic roots, the tissue was frozen in OCT reagent and serial cryosections of 8 μm-thickness were taken from the origin of the aortic valve leaflets, throughout the aortic sinus and stained with oil red O and counter-stained with hematoxylin. Mean lesion area was calculated from the analysis of digital images obtained from 9–12 sections/mouse, using Metamorph imaging software. Differences between groups of arsenic exposed and unexposed mice were assessed using t-test analyses.
Measurement of vascular tone
For the vascular tone measurements, the distal aorta was separated from the surrounding tissue and placed in ice-cold PSS (118 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 12.5 mM NaHCO3, and 11.1 mM glucose; pH 7.4). The PSS solution was continuously aerated (20% O2, 5% CO2, and 75% N2). Adherent fat and connective tissue were removed and descending aorta was cut into rings 2–2.5 mm in length for vascular tone measurements.
Aortic rings were mounted separately on two tungsten wires, attached to a myograph (Kent Scientific Corp., Litchfield, CT) and placed in a 5 mL organ bath filled with PSS (37°C) and constantly aerated with O2:CO2 95%:5%. Rings were stretched gradually to obtain an optimal resting tension of 1g and equilibrated for at least 30 min. The change of tension was measured and recorded in computer program (26,27). To check the vessel integrity, rings were allowed to contract in the presence of high K+ (80 mM KCl) for 20 min. The baths were washed three times with PSS at 10 min intervals following high K+ and monitored to keep the initial tension of 1 g prior to investigating the cumulative response to phenylephrine (10−9 M – 10−5 M) to precontract submaximally, followed by endothelial-dependent relaxation induced by acetylcholine (10−9 M – 10−5 M) which induces NO production by endothelial nitric oxide synthase (eNOS) signaling smooth muscle relaxation. The rings then were washed three times with PSS at 10 min intervals and equilibrated for 30 min to allow tension to return to baseline. Then, the rings were precontracted submaximally with 10−5 M phenylephrine and endothelium independent relaxation was assessed using sodium nitroprusside, a direct NO-generating agent. Differences between arsenic exposed and unexposed mice were assessed using paired t-test analyses.
Results
Plasma Lipoprotein Profile
To asses lipid profiles, we measured plasma total cholesterol, phospholipids and triglyceride levels by conventional enzymatic methods. We saw no difference in plasma cholesterol or phospholipid concentrations between in utero arsenic-exposed and control mice (Fig. 1, panels A and B), at 10 weeks of age. However, we observed a significant decrease (40%, p<0.01) in plasma triglyceride levels in 10 week old arsenic-exposed mice. Next we performed a detailed analysis of lipoprotein particle subpopulations and mean particle size by proton NMR in the 10 week old mice (Fig. 1, panel C). Consistent with the conventional methods, NMR analysis of plasma showed a significant decrease in the total triglyceride levels (31%, p<0.05) and VLDL triglycerides (28%, p<0.05) in the arsenic-exposed mice. NMR analysis of the subclasses of plasma lipoproteins showed the concentrations of VLDL and HDL particles (Fig. 1, panels D and E) of arsenic-exposed mice were comparable to that of controls. The mean particle size of VLDL and HDL particles (Fig. 1, panels F and G)of the arsenic-exposed were also comparable to the controls. Sub-classification of the VLDL particle, however showed a significant decrease in the abundance of the large VLDL particle (>60 nm diameter; Fig. 1, panel J) in arsenic-exposed mice, whereas small (27–35 nm; Fig. 1, panel H) and medium (35–60 nm; Fig. 1, panel I) VLDL concentrations were comparable in arsenic-exposed and control mice. Thus, the only observable differences in plasma lipids was a decrease in total triglycerides and large VLDL particles.
Figure 1.
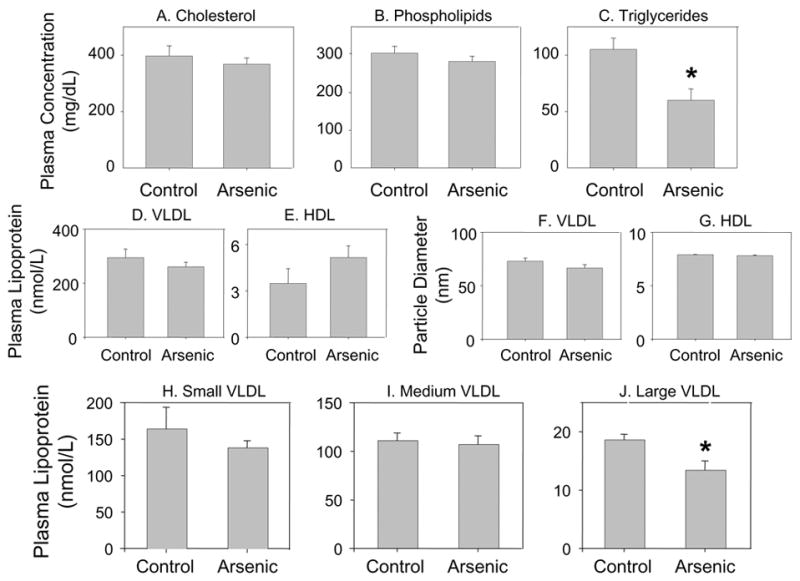
Plasma lipid and lipoprotein analyses of 10 week old ApoE−/− mice exposed and unexposed to arsenic in utero. Pregnant ApoE−/− mice were maintained either on tap water or tap water supplemented with 85 mg/L NaAsO2 from gestational day 8 – 20. Male offspring were maintained on normal chow and sacrificed at 10 weeks of age. Plasma cholesterol (panel A), phospholipids (panel B) and triglycerides (panel C) were measured as described in methods. Values are expressed as Mean ± SE; * indicates p<0.05. Plasma lipoprotein subclass analysis of ApoE−/− mice exposed and unexposed to arsenic in utero performed by NMR. Particle concentrations of total VLDL (panel D) and HDL (panel E). Particle size of VLDL (panel F) and HDL (panel G). Particle concentrations of VLDL subclasses: small VLDL (panel H), medium VLDL (panel I), large VLDL (panel J) . Values are expressed as Mean ± SE; * indicates p<0.05.
Atherosclerotic Lesion Formation Following Arsenic Exposure in Utero
We examined the atherosclerotic lesion formation throughout the aortic tree at 10 and 16 weeks of age. In 10 week old mice, at most, a few small lesions were observed in the aortic arch or the distal aorta of the control mice. Similar to the controls, 10 week old arsenic-exposed mice showed no lesions in the distal aorta. However, five of the twelve arsenic-exposed mice showed appreciable lesion formation in the aortic arch. The lesions were primarily localized in the area of low shear stress. The most pronounced lesions were observed in the innominate and right common carotid arteries, followed by left common carotid artery, left subclavian artery and lesser curvature of the aortic arch (Fig. 2, panel A). Quantitation of the lesion area, indicated that lesion formation in the aortic arch was > 2-fold greater in the arsenic-exposed mice (p<0.05; Fig. 2, panel B). Similar to the aortic arch, five of the ten arsenic-exposed mice showed increased lipid accumulation in the aortic valves (Fig. 2, panels C and D). As a group, arsenic-exposed mice showed a 2- fold increase in the lesion size in the aortic sinus (lesion area 0.5 –1.2 in controls vs 0.6 – 3.8% in arsenic-exposed, p<0.05, Fig. 4).
Figure 2.
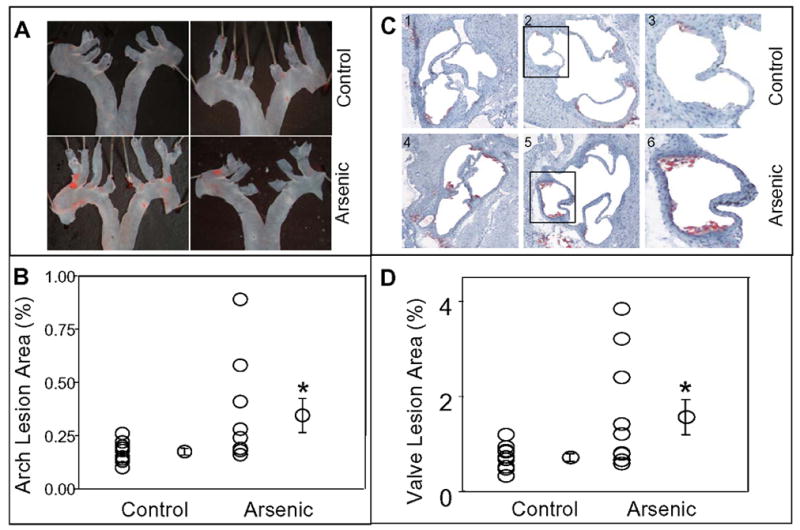
In utero arsenic exposure accelerated atherogenesis in 10 week old ApoE−/− mice. Panel A shows representative photographs of Sudan IV staining in the aortic arch en face of mice exposed (Arsenic) and unexposed (Control) to arsenic in utero. Quantitation of lesions as per cent of aortic arch surface area is illustrated in panel B. Panel C (frames 1 – 6) shows the representative photomicrographs (10x) of aortic valves of mice exposed to arsenic in utero (frames 1 – 3) and unexposed (frames 4 – 6). Lipids were visualized with oil red O staining. Frames 3 and 6 show higher magnification image of the marked sections of images 2 and 4 respectively. Panel D, shows quantitation of lesions as per cent of aortic valve area. Values are expressed as Mean ± SE; * indicates p<0.05.
Figure 4.

In utero arsenic exposure accelerated atherogenesis in 16 week old ApoE−/− mice. Panel A shows representative photographs of Sudan IV staining in the aortic arch en face of mice exposed (Arsenic) and unexposed (Control) to arsenic in utero. Quantitation of lesions as per cent of aortic arch surface area is illustrated in panel B. Panel C (frames 1 – 4) shows the representative photomicrographs (10x) of aortic valves of mice exposed to arsenic in utero (frames 3 and 4) and unexposed (frames 1 and 2). Lipids were visualized with oil red O staining. Panel D, shows quantitation of lesions as per cent of aortic valve area. Values are expressed as Mean ± SE; * indicates p<0.05.
Next we examined the lipoprotein profile and atherosclerotic lesion formation in 16 week old arsenic-exposed mice. Plasma cholesterol and phospholipid levels of the 16 week old arsenic-exposed mice were comparable to the age matched controls (Fig. 3, panels A and B). However, similar to the 10 week old mice, the 16 week old mice had significantly lower concentrations of plasma triglycerides (> 20 % decrease; p< 0.05; Fig. 3, panel C). Despite a significant decrease in the plasma triglycerides, four of the eight, 16 week old arsenic-exposed mice displayed marked increase in the lesion area both in the aortic arch (Fig. 4, panels A and B) as well as aortic roots (Fig. 4, panels C and D), as compared to the age matched controls. As a group 16 week old arsenic-exposed mice had a 2- fold increase in lesion area in both aortic arch (p<0.05) and aortic sinus (p<0.05). None of the 16 week old arsenic-exposed or control mice displayed any visible lesions in the distal aorta.
Figure 3.

Plasma lipid analyses of 16 week old ApoE−/− mice exposed and unexposed to arsenic in utero. Pregnant ApoE−/− mice were maintained either on tap water or tap water supplemented with 85 mg/L NaAsO2 from gestational day 8 – 20. Male offspring were maintained on normal chow and sacrificed at 10 weeks of age. Plasma cholesterol (panel A), phospholipids (panel B) and triglycerides (panel C) were measured as described in methods. Values are expressed as Mean ± SE; * indicates p<0.05.
Vasoreactivity studies were performed on aortic vessels from arsenic treated and control mice. Initially, aortic rings were allowed to contract in a PSS buffer containing 80 mM potassium chloride (KCl). High concentration of KCl opens the voltage-gated ion channels and allows extracellular calcium entry into the cells thus causing maximal contraction. Under this condition no significant differences were observed between the in utero exposed and unexposed groups indicating lack of gross abnormality from arsenic treatment on the voltage–gated ion channels. Experiments performed using a cumulative dose of phenylephrine (10−9–10−5 M) also showed no significant differences between the in utero exposed and unexposed groups in the contractile responses of the vessels (Fig. 5). Phenylephrine binds to α-adrenergic receptor, a G-protein coupled receptor, that mobilizes the intracellular calcium via phospholipase C /inositol-3-phosphate pathway. Contraction occurs via the phosphorylation of myosin light chain kinase. Following maximal contraction at 10−5 M phenylephrine, relaxation of the rings was determined using cumulative doses of acetylcholine (10−9–10−5 M). Relaxation was reduced significantly in vessels from in utero exposed mice (Fig 5). Acetylcholine mediates the release of nitric oxide (NO) through the stimulation of nitric oxide synthase (NOS). Therefore, it appears that in utero arsenic exposure may block NO availability which could be due to either inhibition of NOS or oxidation of NO. Furthermore, when vessels were contracted with 10−5 M phenylephrine, and sodium nitroprusside (SNP, a NO donor), was added, the relaxation responses were comparable between the groups. The SNP experiment verifies the likely NO bioavailability defect in vessels from in utero arsenic-exposed animals and eliminates the defect being in the guanylate cyclase system in the smooth muscle cells of the vessels.
Figure 5.
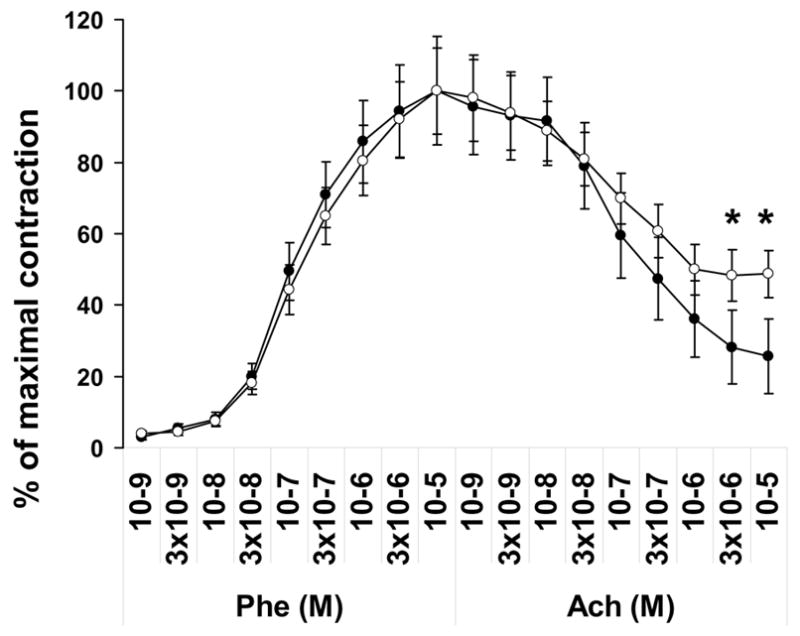
Arsenic exposure in utero affects vasorelaxation of mouse aortic rings. Contraction in response to cumulative doses of phenylephrine (Phe) and relaxation response to cumulative doses of acetylcholine (Ach) were assessed for mice exposed (○) and unexposed (●) to arsenic in utero. Data are normalized to contraction induced by 80 mM KCl and expressed as Mean ± SE; * indicates p<0.05.
Discussion
The evidence for chronic arsenic exposure causing cardiovascular disease is based on a limited number of small epidemiological studies (reviewed in (28)). One clinical manifestation of chronic arsenicosis associated atherosclerosis is gangrene of the extremities. In Taiwan, the gangrene most commonly manifests in the feet and the syndrome is known as Blackfoot Disease. More recently, a high frequency of gangrene of the toes has been noted in reports from Bangladesh and West Bengal in India (29–31), another area in which chronic consumption of arsenic contaminated drinking water has occurred on a large scale. Earlier reports from this area discussed skin lesions and cancers but did not mention gangrene (31–35), suggesting that the manifestations of arterial disease may have appeared later. The reports do not contain enough detail to determine whether the exposures in people with and without gangrene differ in terms of fetal exposures. Our results suggest that early life arsenic exposure accelerates atherosclerotic changes. Early life exposure to high levels of arsenic in drinking water have been associated with arteriosclerosis and myocardial infarction in infants as young as 1 year old in the Antofagosta region of Chile (17). Significant transfer of arsenic from mother to infant via breast milk is unlikely because arsenic levels in human breast milk of mothers consuming arsenic contaminated water are very low (36). Thus, it is likely that transplacental arsenic plays a significant role in development of arterial lesions.
Transplacental arsenic exposure also is likely to play a role in other adult onset diseases. Arsenic is a transplacental carcinogen in mice. In utero arsenic exposure was first shown to induces tumor in the liver, adrenal, lung, and ovary in C3H mice (37). More recent studies using CD1 mice showed that transplacental arsenic induced urogenital system tumors, mostly benign tumors of the ovary and uterus, and adrenal adenoma, and that combining in utero arsenic with post-natal exposure to diethylstilbestrol induced malignant urogenital tumors (38). Early life exposures to arsenic are also likely to play a role in human carcinogenesis and pulmonary disease. An epidemiological study of the population in Antofagasta, Chile that experienced early life exposure to high levels of arsenic in drinking water showed an increase in mortality from lung cancer and bronchiectasis in the exposed population (39). These data indicate that early life arsenic exposure may be a serious risk factor for a variety of adult onset diseases and that there is a need for more research on the mechanisms of action.
The cholesterol levels in ApoE−/− mice are typically 400 to 600 mg/dL, as compared to < 100 mg/dL in wild-type mice (19). As they age, the ApoE−/− mice develop atherosclerotic lesions spontaneously; the rate of atherogenesis is enhanced further by a high fat / high cholesterol (HF/HC) diet, which increases their cholesterol to 2,000 – 4,000 mg/dL. We maintained the mice on normal chow and their total cholesterol levels at the time of sacrifice averaged ~380 mg/dL and were unchanged by the in utero arsenic exposure.
The only effect of arsenic exposure on plasma lipids we observed was a significant decrease in the concentration of plasma triglycerides and the abundance of large VLDL particles. Despite this decrease, the in utero arsenic-exposed mice showed appreciable increase in lesion formation in the aortic roots and the aortic arch at 10 or 16 weeks of age. In humans, increased risk of coronary artery disease is associated with elevated concentrations of triglycerides and VLDL (40,41). Thus, because transplacental arsenic exposure caused arterial lesions in these ApoE−/− mice in spite of decreases in triglycerides and large VLDL particles, the mechanism was not related to an induction of hyperlipidemia. Significantly, Hsueh et al (42) have observed that arsenic-related ischemic heart disease in humans is independent of the changes in the serum lipids. The observed cholesterol independent increase in atherosclerotic lesion formation in the present study is consistent with observations by Simeonova et al (43), who showed that prolonged arsenic exposure (20 or 100 mg/L sodium arsenite for 24 weeks) results in a 1.6–2.3 fold increase in the atherosclerotic lesion formation in the aorta of female ApoE−/− mice without any significant difference in the serum cholesterol levels. Similarly, Bunderson et al (44) reported that adult ApoE−/−/LDLR−/− mice exposed to sodium arsenite (133 mg/L) for 18 weeks had a significant increase in atherosclerotic lesions in the innominate arteries. Our observation of increased atherosclerotic lesion formation in the aortic roots as well as aortic sinus of 10 and 16 week old offspring of mice drinking water containing sodium arsenite (85 mg/L) for only two weeks while pregnant is therefore quite remarkable.
The underlying mechanisms responsible for the transplacental arsenic induced atherosclerotic lesion formation remain to be investigated. However, the mechanism may be related to arsenic’s well established potential to cause oxidative stress. In vitro studies have shown that arsenic causes oxidative stress in vascular cells (44–48) and enhances the generation of pro-inflammatory cytokines and chemokines (43,48). In humans, increased blood arsenic level correlates with increased oxidative stress, compromised oxidative defense capacity and induction of proinflammatory cytokines and chemokines including IL-1β, IL-6 and monocyte chemotactic protein-1 which could cause endothelial dysfunction (49). Our results suggest that in utero arsenic exposure has lasting effects on susceptibility to atherosclerosis in adulthood. Because the arsenic exposure occurred in utero long before the onset of arterial disease, the arsenic is unlikely to still be resident and to be directly causing oxidative stress in the arterial wall. In utero arsenic exposure induced epigenetic changes consistent with adult onset tumorigenesis in the transplacental carcinogenesis model (50). Thus, it is likely that the in utero arsenic exposure in our studies is inducing epigenetic effects that are long lasting and in the ApoE−/− mouse model result in early onset of vascular disease. One of these effects appears to be disruption of the nitric oxide signaling system in the endothelium. Our results indicate that arsenic exposure in utero affects the contractile apparatus of aortic vessels of young adult mice. This damaging effect of arsenic appears to affect the ability of the blood vessel to dilate in response to acetylcholine. This could be an effect on endothelial nitric oxide synthase (eNOS). Alternatively, arsenic can generate free radicals that in turn may react with nitric oxide to form peroxynitrite (51), thus decreasing the bioavailability of nitric oxide.
Conflicting observations make it unclear how the endothelial NO system is affected by arsenic. eNOS is inhibited in endothelial cells exposed to high concentrations of arsenic in vitro, (52,53), but exposure to low concentrations activates eNOS and stimulates angiogenesis (52). Others have observed increased reactive oxygen species but not reactive nitrogen species (46). Epidemiologic evidence associates chronic arsenic exposure with decreased blood levels of nitric oxide suggesting inhibition of eNOS (54). Remediation of arsenic exposure appears to reverse the vascular dysfunction (55). In vitro arsenic exposure inhibits eNOS mediated relaxation in aortic rings of rats (55–57). Further studies are required to determine if eNOS expression or function is altered in mice exposed to arsenic in utero.
In summary, we have demonstrated that the atherosclerotic process is accelerated by transplacental inorganic arsenic exposure administered via drinking water to pregnant mice. These results support the hypothesis that early life arsenic exposure plays a role in development of cardiovascular disease and highlight the need for more research to gain an understanding of the potential for early life arsenic exposure to mediate disease progression in adults.
Acknowledgments
The authors thank Ms. Heather L. Miller for expert technical assistance. This work was supported in part by a pilot project grant from the University of Louisville Center for Genetics and Molecular Medicine and U. S. Public Health Service grants R01HL65618 and P01ES011860.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aposhian HV. Enzymatic methylation of arsenic species and other new approaches to arsenic toxicity. Annu Rev Pharmacol Toxicol. 1997;37:397–419. doi: 10.1146/annurev.pharmtox.37.1.397. [DOI] [PubMed] [Google Scholar]
- 2.Kitchin KT. Recent advances in arsenic carcinogenesis: modes of action, animal model systems, and methylated arsenic metabolites. Toxicol Appl Pharmacol. 2001;172:249–261. doi: 10.1006/taap.2001.9157. [DOI] [PubMed] [Google Scholar]
- 3.National Research Council. Arsenic in Drinking Water. National Academy Press; Washington DC: 1999. [Google Scholar]
- 4.Thomas DJ, Styblo M, Lin S. The cellular metabolism and systemic toxicity of arsenic. Toxicol Appl Pharmacol. 2001;176:127–144. doi: 10.1006/taap.2001.9258. [DOI] [PubMed] [Google Scholar]
- 5.Concha G, Vogler G, Lezcano D, Nermell B, Vahter M. Exposure to inorganic arsenic metabolites during early human development. Toxicol Sci. 1998;44:185–190. doi: 10.1006/toxs.1998.2486. [DOI] [PubMed] [Google Scholar]
- 6.Devesa V, Adair BM, Liu J, Waalkes MP, Diwan BA, Styblo M, Thomas DJ. Arsenicals in maternal and fetal mouse tissues after gestational exposure to arsenite. Toxicology. 2006;224:147–155. doi: 10.1016/j.tox.2006.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frost FJ, Muller T, Petersen HV, Thomson B, Tollestrup K. Identifying US populations for the study of health effects related to drinking water arsenic. J Expo Anal Environ Epidemiol. 2003;13:231–239. doi: 10.1038/sj.jea.7500275. [DOI] [PubMed] [Google Scholar]
- 8.Detroit Free Press. 167. 1997. Nov 19, Water Making Many Ill; pp. 1–9. [Google Scholar]
- 9.Engel RR, Smith AH. Arsenic in drinking water and mortality from vascular disease: an ecologic analysis in 30 counties in the United States. Arch Environ Health. 1994;49:418–427. doi: 10.1080/00039896.1994.9954996. [DOI] [PubMed] [Google Scholar]
- 10.Engel RR, Hopenhayn-Rich C, Receveur O, Smith AH. Vascular effects of chronic arsenic exposure: a review. Epidemiol Rev. 1994;16:184–209. doi: 10.1093/oxfordjournals.epirev.a036150. [DOI] [PubMed] [Google Scholar]
- 11.Chen CJ, Chiou HY, Chiang MH, Lin LJ, Tai TY. Dose-response relationship between ischemic heart disease mortality and long-term arsenic exposure. Arterioscler Thromb Vasc Biol. 1996;16:504–510. doi: 10.1161/01.atv.16.4.504. [DOI] [PubMed] [Google Scholar]
- 12.Chiou HY, Huang WI, Su CL, Chang SF, Hsu YH, Chen CJ. Dose-response relationship between prevalence of cerebrovascular disease and ingested inorganic arsenic. Stroke. 1997;28:1717–1723. doi: 10.1161/01.str.28.9.1717. [DOI] [PubMed] [Google Scholar]
- 13.Tseng CH, Chong CK, Chen CJ, Tai TY. Dose-response relationship between peripheral vascular disease and ingested inorganic arsenic among residents in blackfoot disease endemic villages in Taiwan. Atherosclerosis. 1996;120:125–133. doi: 10.1016/0021-9150(95)05693-9. [DOI] [PubMed] [Google Scholar]
- 14.Wang CH, Jeng JS, Yip PK, Chen CL, Hsu LI, Hsueh YM, Chiou HY, Wu MM, Chen CJ. Biological gradient between long-term arsenic exposure and carotid atherosclerosis. Circulation. 2002;105:1804–1809. doi: 10.1161/01.cir.0000015862.64816.b2. [DOI] [PubMed] [Google Scholar]
- 15.Wu MM, Kuo TL, Hwang YH, Chen CJ. Dose-response relation between arsenic concentration in well water and mortality from cancers and vascular diseases. Am J Epidemiol. 1989;130:1123–1132. doi: 10.1093/oxfordjournals.aje.a115439. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg HG. Systemic arterial disease with myocardial infarction. Report on two infants Circulation. 1973;47:270–275. doi: 10.1161/01.cir.47.2.270. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg HG. Systemic arterial disease and chronic arsenicism in infants. Arch Pathol. 1974;97:360–365. [PubMed] [Google Scholar]
- 18.Breslow JL. Transgenic mouse models of lipoprotein metabolism and atherosclerosis. Proc Natl Acad Sci U S A. 1993;90:8314–8318. doi: 10.1073/pnas.90.18.8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Breslow JL. Mouse models of atherosclerosis. Science. 1996;272:685–688. doi: 10.1126/science.272.5262.685. [DOI] [PubMed] [Google Scholar]
- 20.Fazio S, Linton MF. Mouse models of hyperlipidemia and atherosclerosis. Front Biosci. 2001;6:D515–D525. doi: 10.2741/fazio. [DOI] [PubMed] [Google Scholar]
- 21.Jawien J, Nastalek P, Korbut R. Mouse models of experimental atherosclerosis. J Physiol Pharmacol. 2004;55:503–517. [PubMed] [Google Scholar]
- 22.Okoji RS, Yu RC, Maronpot RR, Froines JR. Sodium arsenite administration via drinking water increases genome-wide and Ha-ras DNA hypomethylation in methyl-deficient C57BL/6J mice. Carcinogenesis. 2002;23:777–785. doi: 10.1093/carcin/23.5.777. [DOI] [PubMed] [Google Scholar]
- 23.Zhao CQ, Young MR, Diwan BA, Coogan TP, Waalkes MP. Association of arsenic-induced malignant transformation with DNA hypomethylation and aberrant gene expression. Proc Natl Acad Sci U S A. 1997;94:10907–10912. doi: 10.1073/pnas.94.20.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammad SM, Powell-Braxton L, Otvos JD, Eldridge L, Won W, Lyons TJ. Lipoprotein subclass profiles of hyperlipidemic diabetic mice measured by nuclear magnetic resonance spectroscopy. Metabolism. 2003;52:916–921. doi: 10.1016/s0026-0495(03)00058-1. [DOI] [PubMed] [Google Scholar]
- 25.Webb NR, Bostrom MA, Szilvassy SJ, van der Westhuyzen DR, Daugherty A, de Beer FC. Macrophage-expressed group IIA secretory phospholipase A2 increases atherosclerotic lesion formation in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:263–268. doi: 10.1161/01.atv.0000051701.90972.e5. [DOI] [PubMed] [Google Scholar]
- 26.Tsakadze NL, Srivastava S, Awe SO, Adeagbo AS, Bhatnagar A, D'Souza SE. Acrolein-induced vasomotor responses of rat aorta. Am J Physiol Heart Circ Physiol. 2003;285:H727–H734. doi: 10.1152/ajpheart.00269.2003. [DOI] [PubMed] [Google Scholar]
- 27.Lominadze D, Tsakadze N, Sen U, Falcone JC, D'Souza SE. Fibrinogen and fragment D-induced vascular constriction. Am J Physiol Heart Circ Physiol. 2005;288:H1257–H1264. doi: 10.1152/ajpheart.00856.2004. [DOI] [PubMed] [Google Scholar]
- 28.Navas-Acien A, Sharrett AR, Silbergeld EK, Schwartz BS, Nachman KE, Burke TA, Guallar E. Arsenic exposure and cardiovascular disease: a systematic review of the epidemiologic evidence. Am J Epidemiol. 2005;162:1037–1049. doi: 10.1093/aje/kwi330. [DOI] [PubMed] [Google Scholar]
- 29.Alam MG, Allinson G, Stagnitti F, Tanaka A, Westbrooke M. Arsenic contamination in Bangladesh groundwater: a major environmental and social disaster. Int J Environ Health Res. 2002;12:235–253. doi: 10.1080/0960312021000000998. [DOI] [PubMed] [Google Scholar]
- 30.Anawar HM, Akai J, Mostofa KM, Safiullah S, Tareq SM. Arsenic poisoning in groundwater: health risk and geochemical sources in Bangladesh. Environ Int. 2002;27:597–604. doi: 10.1016/s0160-4120(01)00116-7. [DOI] [PubMed] [Google Scholar]
- 31.Guha Mazumder DN. Chronic arsenic toxicity: clinical features, epidemiology, and treatment: experience in West Bengal. J Environ Sci Health Part A Tox Hazard Subst Environ Eng. 2003;38:141–163. doi: 10.1081/ese-120016886. [DOI] [PubMed] [Google Scholar]
- 32.Guha Mazumder DN, Haque R, Ghosh N, De BK, Santra A, Chakraborty D, Smith AH. Arsenic levels in drinking water and the prevalence of skin lesions in West Bengal, India. Int J Epidemiol. 1998;27:871–877. doi: 10.1093/ije/27.5.871. [DOI] [PubMed] [Google Scholar]
- 33.Mazumder DN, Das GJ, Santra A, Pal A, Ghose A, Sarkar S. Chronic arsenic toxicity in west Bengal--the worst calamity in the world. J Indian Med Assoc. 1998;96:4–7. 18. [PubMed] [Google Scholar]
- 34.Subramanian KS. Arsenic poisoning in West Bengal. Science. 1996;274:1287–1288. [PubMed] [Google Scholar]
- 35.Subramanian KS, Kosnett MJ. Human exposures to arsenic from consumption of well water in West Bengal, India. Int J Occup Environ Health. 1998;4:217–230. doi: 10.1179/oeh.1998.4.4.217. [DOI] [PubMed] [Google Scholar]
- 36.Concha G, Vogler G, Nermell B, Vahter M. Low-level arsenic excretion in breast milk of native Andean women exposed to high levels of arsenic in the drinking water. Int Arch Occup Environ Health. 1998;71:42–46. doi: 10.1007/s004200050248. [DOI] [PubMed] [Google Scholar]
- 37.Waalkes MP, Ward JM, Liu J, Diwan BA. Transplacental carcinogenicity of inorganic arsenic in the drinking water: induction of hepatic, ovarian, pulmonary, and adrenal tumors in mice. Toxicol Appl Pharmacol. 2003;186:7–17. doi: 10.1016/s0041-008x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 38.Waalkes MP, Liu J, Ward JM, Powell DA, Diwan BA. Urogenital carcinogenesis in female CD1 mice induced by in utero arsenic exposure is exacerbated by postnatal diethylstilbestrol treatment. Cancer Res. 2006;66:1337–1345. doi: 10.1158/0008-5472.CAN-05-3530. [DOI] [PubMed] [Google Scholar]
- 39.Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von EO, Steinmaus C, Bates MN, Selvin S. Increased mortality from lung cancer and bronchiectasis in young adults after exposure to arsenic in utero and in early childhood. Environ Health Perspect. 2006;114:1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carmena R, Duriez P, Fruchart JC. Atherogenic lipoprotein particles in atherosclerosis. Circulation. 2004;109:III2–III7. doi: 10.1161/01.CIR.0000131511.50734.44. [DOI] [PubMed] [Google Scholar]
- 41.Hodis HN, Mack WJ, Azen SP, Alaupovic P, Pogoda JM, LaBree L, Hemphill LC, Kramsch DM, Blankenhorn DH. Triglyceride- and cholesterol-rich lipoproteins have a differential effect on mild/moderate and severe lesion progression as assessed by quantitative coronary angiography in a controlled trial of lovastatin. Circulation. 1994;90:42–49. doi: 10.1161/01.cir.90.1.42. [DOI] [PubMed] [Google Scholar]
- 42.Hsueh YM, Wu WL, Huang YL, Chiou HY, Tseng CH, Chen CJ. Low serum carotene level and increased risk of ischemic heart disease related to long-term arsenic exposure. Atherosclerosis. 1998;141:249–257. doi: 10.1016/s0021-9150(98)00178-6. [DOI] [PubMed] [Google Scholar]
- 43.Simeonova PP, Hulderman T, Harki D, Luster MI. Arsenic exposure accelerates atherogenesis in apolipoprotein E(−/−) mice. Environ Health Perspect. 2003;111:1744–1748. doi: 10.1289/ehp.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bunderson M, Brooks DM, Walker DL, Rosenfeld ME, Coffin JD, Beall HD. Arsenic exposure exacerbates atherosclerotic plaque formation and increases nitrotyrosine and leukotriene biosynthesis. Toxicol Appl Pharmacol. 2004;201:32–39. doi: 10.1016/j.taap.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 45.Barchowsky A, Dudek EJ, Treadwell MD, Wetterhahn KE. Arsenic induces oxidant stress and NF-kappa B activation in cultured aortic endothelial cells. Free Radic Biol Med. 1996;21:783–790. doi: 10.1016/0891-5849(96)00174-8. [DOI] [PubMed] [Google Scholar]
- 46.Barchowsky A, Roussel RR, Klei LR, James PE, Ganju N, Smith KR, Dudek EJ. Low levels of arsenic trioxide stimulate proliferative signals in primary vascular cells without activating stress effector pathways. Toxicol Appl Pharmacol. 1999;159:65–75. doi: 10.1006/taap.1999.8723. [DOI] [PubMed] [Google Scholar]
- 47.Lynn S, Gurr JR, Lai HT, Jan KY. NADH oxidase activation is involved in arsenite-induced oxidative DNA damage in human vascular smooth muscle cells. Circ Res. 2000;86:514–519. doi: 10.1161/01.res.86.5.514. [DOI] [PubMed] [Google Scholar]
- 48.Lee PC, I, Ho C, Lee TC. Oxidative stress mediates sodium arsenite-induced expression of heme oxygenase-1, monocyte chemoattractant protein-1, and interleukin-6 in vascular smooth muscle cells. Toxicol Sci. 2005;85:541–550. doi: 10.1093/toxsci/kfi101. [DOI] [PubMed] [Google Scholar]
- 49.Wu MM, Chiou HY, Wang TW, Hsueh YM, Wang IH, Chen CJ, Lee TC. Association of blood arsenic levels with increased reactive oxidants and decreased antioxidant capacity in a human population of northeastern Taiwan. Environ Health Perspect. 2001;109:1011–1017. doi: 10.1289/ehp.011091011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen H, Li S, Liu J, Diwan BA, Barrett JC, Waalkes MP. Chronic inorganic arsenic exposure induces hepatic global and individual gene hypomethylation: implications for arsenic hepatocarcinogenesis. Carcinogenesis. 2004 doi: 10.1093/carcin/bgh161. epub Apr 8. [DOI] [PubMed] [Google Scholar]
- 51.Bunderson M, Coffin JD, Beall HD. Arsenic induces peroxynitrite generation and cyclooxygenase-2 protein expression in aortic endothelial cells: possible role in atherosclerosis. Toxicol Appl Pharmacol. 2002;184:11–18. [PubMed] [Google Scholar]
- 52.Kao YH, Yu CL, Chang LW, Yu HS. Low concentrations of arsenic induce vascular endothelial growth factor and nitric oxide release and stimulate angiogenesis in vitro. Chem Res Toxicol. 2003;16:460–468. doi: 10.1021/tx025652a. [DOI] [PubMed] [Google Scholar]
- 53.Lee MY, Bae ON, Chung SM, Kang KT, Lee JY, Chung JH. Enhancement of platelet aggregation and thrombus formation by arsenic in drinking water: a contributing factor to cardiovascular disease. Toxicol Appl Pharmacol. 2002;179:83–88. doi: 10.1006/taap.2001.9356. [DOI] [PubMed] [Google Scholar]
- 54.Pi J, Kumagai Y, Sun G, Yamauchi H, Yoshida T, Iso H, Endo A, Yu L, Yuki K, Miyauchi T, Shimojo N. Decreased serum concentrations of nitric oxide metabolites among Chinese in an endemic area of chronic arsenic poisoning in inner Mongolia. Free Radic Biol Med. 2000;28:1137–1142. doi: 10.1016/s0891-5849(00)00209-4. [DOI] [PubMed] [Google Scholar]
- 55.Pi J, Yamauchi H, Sun G, Yoshida T, Aikawa H, Fujimoto W, Iso H, Cui R, Waalkes MP, Kumagai Y. Vascular dysfunction in patients with chronic arsenosis can be reversed by reduction of arsenic exposure. Environ Health Perspect. 2005;113:339–341. doi: 10.1289/ehp.7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bilszta JL, Dusting GJ, Jiang F. Arsenite increases vasoconstrictor reactivity in rat blood vessels: role of endothelial nitric oxide function. Int J Toxicol. 2006;25:303–310. doi: 10.1080/10915810600746130. [DOI] [PubMed] [Google Scholar]
- 57.Lee MY, Jung BI, Chung SM, Bae ON, Lee JY, Park JD, Yang JS, Lee H, Chung JH. Arsenic-induced dysfunction in relaxation of blood vessels. Environ Health Perspect. 2003;111:513–517. doi: 10.1289/ehp.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]


