Abstract
The neurosteroid allopregnanolone (ALLO) is a potent positive modulator of γ-aminobutyric acidA (GABAA) receptors that can modulate ethanol (EtOH) withdrawal. The 5α-reductase inhibitor finasteride can block the formation of ALLO and other GABAergic neurosteroids and also reduce certain effects of EtOH. Treatment with finasteride during chronic EtOH exposure decreased EtOH withdrawal severity and blood EtOH concentrations (BECs), suggesting an additional effect of finasteride on EtOH pharmacokinetics. Thus, the purpose of the present study was to determine the effect of finasteride on acute EtOH withdrawal severity, to minimize the effect of finasteride on EtOH metabolism. Male and female C57BL/6J and DBA/2J mice received a pretreatment of finasteride (50 mg/kg i.p.) or vehicle 24 hours prior to an injection of EtOH (4 g/kg i.p.) or saline. Handling-induced convulsions (HICs) were scored at baseline, and then over a 24 hr period after EtOH or saline injection. In another experiment, plasma estradiol and corticosterone levels were assessed at selected time points (0, 2, 8, and 24 hrs). In a final study, retro-orbital blood samples were collected at 30, 60, 120, and 240 minutes post-EtOH administration to access finasteride’s effects on EtOH clearance parameters. Pretreatment with finasteride increased acute EtOH withdrawal severity in female C57BL/6J and DBA/2J mice but decreased withdrawal severity in male mice of both strains. Finasteride did not alter BECs, EtOH clearance, estradiol, or corticosterone concentrations in a manner that appeared to contribute to the sex difference in finasteride’s effect on acute EtOH withdrawal severity. These findings suggest that male and female C57BL/6J and DBA/2J mice differ in their sensitivity to changes in ALLO or other GABAergic neurosteroid levels during acute EtOH withdrawal. Sex differences in the modulation of GABAergic 5α-reduced steroids may be an important consideration in understanding and developing therapeutic interventions in alcoholics.
Keywords: alcohol, allopregnanolone, convulsions, steroid hormones, radioimmunoassay, 5α-reductase
The progesterone metabolite allopregnanolone (ALLO; 3α-hydroxy-5α-pregnan-20-one) is a potent positive modulator of γ-aminobutyric acidA (GABAA) receptors that exerts anxiolytic, sedative, anticonvulsant, and locomotor stimulant effects in animals (Paul and Purdy, 1992; Lambert et al., 1995; Gasior et al., 1999; Rupprecht and Holsboer, 1999; Belelli and Lambert, 2005). ALLO levels peak at approximately 10 minutes following systemic injection, but it is rapidly metabolized as no behavioral effects were seen 3 hours post-injection (Kokate et al., 1994). Ethanol (EtOH) has a pharmacological profile similar to ALLO, with some of its behavioral effects believed to be due to the ability of EtOH to potentiate GABAA receptor function (see reviews by Grobin et al., 1998; Criswell and Breese, 2005). Since EtOH withdrawal can increase seizure susceptibility, ALLO’s anticonvulsant effects may modulate EtOH withdrawal severity (Gasior et al., 1999; Finn et al., 2004a). That is, since ALLO and EtOH both increase GABAergic transmission, endogenous levels of ALLO may be particularly important in determining the net behavioral outcome during EtOH withdrawal. Notably, clinical research documented an inverse relationship between endogenous ALLO levels and symptoms of anxiety and/or depression in alcoholic patients during the early phase of withdrawal (Romeo et al., 1996).
Basal brain and plasma ALLO levels are higher in females than in males, with endogenous levels in females fluctuating from 10 to 30 nM during the estrous cycle and increasing to approximately 100 nM during pregnancy (Paul and Purdy, 1992; Finn and Gee, 1994; Concas et al., 1998). However, ALLO concentrations in males can also increase to 10 to 30 nM following exposure to various stressors (Purdy et al., 1991; Barbaccia et al., 2001). These concentrations observed in vivo are physiologically relevant, since they are in the range of concentrations shown by in vitro studies to potentiate the action of GABA at GABAA receptors (Morrow et al., 1987; Gee et al., 1988; Belelli et al., 1990; Lambert et al., 1995). These findings, in conjunction with the demonstration that manipulation of local hippocampal ALLO concentration unmasked a GABAergic inhibitory tone (Belelli and Herd, 2003), are consistent with the notion that fluctuations in endogenous ALLO levels may modulate GABAA receptors.
Additionally, acute administration of EtOH increased cortical ALLO levels to pharmacologically active concentrations in male rats (Barbaccia et al., 1999; VanDoren et al., 2000), female rats (Morrow et al., 1999), and in male but not in female C57BL/6J mice (Finn et al., 2004b). This sex difference in the effect of EtOH administration on endogenous ALLO levels in mice suggests that the hormonal milieu may impact EtOH’s effects on GABAergic neurosteroids.
Manipulation of ALLO levels can modulate some of EtOH’s pharmacological effects. The 5α-reductase inhibitor finasteride, which can block the formation of ALLO and other GABAergic neurosteroids, has been used as a tool to manipulate the levels of GABAergic neurosteroids and determine the effect on EtOH sensitivity (see Finn et al., 2006 for review). Notably, pretreatment with finasteride (single doses of 50 – 100 mg/kg; multiple doses of 25 – 100 mg/kg) reduced EtOH’s anticonvulsant (VanDoren et al., 2000), antidepressant (Hirani et al., 2002) and anxiolytic (Hirani et al., 2005) effects, altered EtOH’s biphasic effect on dopamine content (Dazzi et al., 2002), but did not alter EtOH-induced ataxia (Khisti et al., 2004) or conditioned place preference (Gabriel et al., 2004; Murphy et al., 2006). It is not known by what mechanism finasteride may decrease certain behavioral and physiological effects of EtOH.
Initial studies from our laboratory examined the effect of finasteride on chronic EtOH withdrawal severity in male and female C57BL/6J and DBA/2J mice. These inbred strains are a well-documented genetic animal model of alcohol withdrawal severity, since DBA/2J mice exhibit more severe handling-induced convulsions (HICs, a sensitive measure of CNS sensitivity) than C57BL/J mice during both chronic (Crabbe et al., 1983; Crabbe, 1998) and acute (Roberts et al., 1992) EtOH withdrawal. Although we hypothesized that decreasing endogenous ALLO levels would decrease GABAergic inhibition and increase chronic EtOH withdrawal severity, treatment with finasteride significantly reduced chronic EtOH withdrawal severity in DBA/2J versus C57BL/6J mice (Finn et al., 2004c). However, the finding that finasteride significantly decreased BEC upon the initiation of withdrawal suggested that it might affect withdrawal severity via an alteration in EtOH pharmacokinetics (Finn et al., 2004c).
In addition to the chronic EtOH administration paradigm, another model of EtOH withdrawal is the use of a single, acute injection of a sedative dose of EtOH. In this procedure for determining acute withdrawal severity, the large dose of EtOH initially produces a depressant effect that is followed by rebound hyperexcitability as the EtOH is metabolized (i.e. at approximately 4–8 hours post-injection). Although the terminal elimination half-life of finasteride is reported to be 2.2 hr in rats (Stuart et al., 2001), there are no comparable data in mice. Based on this result, in conjunction with data in mice and rats indicating that finasteride pre-treatment significantly decreased GABAergic neurosteroid levels at 4–24 hrs post-injection (e.g., VanDoren et al., 2000; Dazzi et al., 2002; Finn, unpublished), we reasoned that pre-treatment with finasteride at 24 hr prior to the acute EtOH withdrawal procedure would decrease GABAergic neurosteroid levels while eliminating the potential interaction with EtOH metabolism.
Thus, the purpose of the present study was to determine the effect of finasteride on acute EtOH withdrawal severity, measured by HICs, in male and female C57BL/6J and DBA/2J mice. We predicted that pretreatment with finasteride would increase acute withdrawal severity, but that there might be strain and sex differences in the effect. Since finasteride blocks the reduction of all 5α-reduced steroids, this inhibitor should affect several hormonal systems (e.g., progesterone, testosterone, and deoxycorticosterone pathways). Due to the fact that estradiol and corticosterone can have proconvulsant effects (e.g., Roberts et al., 1994; Roberts and Keith, 1995; Karst et al., 1999; Reddy, 2004), changes in levels of these steroids could impact acute withdrawal severity. For this reason, a separate study measured plasma levels of estradiol and corticosterone over several time points to see if their change mirrored the time course of HICs. Finally, due to finasteride’s effects on BECs in our chronic study, the third study determined whether finasteride altered EtOH clearance by measuring BECs at several time points following pretreatment with finasteride.
Experimental Procedures
Subjects
Naïve male and female C57BL/6J and DBA/2J mice aged 8–10 weeks were used in all experiments (Jackson Laboratory, Bar Harbor, ME). Animals were separated by strain and sex and maintained in groups of four in individually ventilated cages (Thorens) with ad libitum food and water under a 12:12 hour light/dark cycle at 26 ± 1°C. In our experience, housing males and females in the same room tends to synchronize the estrous cycle in at least 70% of the intact female mice (Finn, unpublished). Since we presumed that the effects of acute EtOH withdrawal and finasteride pretreatment would be greater than the potential variability in estrous cycle state in the different treatment groups of female mice, we did not monitor estrous cyclicity. All procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health and were approved by the local Institutional Animal Care and Use Committee.
Drug Treatments
Finasteride was solubilized in 20% w/v 2-hydroxypropyl-β-cyclodextrin (β-cyclodextrin; Cerestar, USA, Hammond, IN) and prepared as a 5-mg/ml solution to be injected as 0.01 ml/g of body weight. In all experiments, mice received a pretreatment of finasteride (50 mg/kg, i.p.; Steraloids, Newport, RI) or an equivalent volume of saline (i.p.) depending upon group assignment. The following day, mice received an injection of EtOH (4 g/kg, i.p.; Pharmco Products, Brookfield, CT) or an equivalent volume of saline (i.p.) depending upon group assignment. The dose of finasteride was chosen based on preliminary results in our laboratory that a 50-mg/kg dose of finasteride produced an 80% decrease in brain ALLO levels at 24 hr post injection (Finn, unpublished), which was similar to the 75% decrease reported in rats following this dose (VanDoren et al., 2000). Additionally, doses of finasteride in the range of 50 – 100 mg/kg were required to decrease selective behavioral effects of EtOH (e.g., VanDoren et al., 2000; Hirani et al., 2002, 2005).
Experiment 1: Withdrawal Severity
Prior to the injection of finasteride or saline, baseline HICs were measured. Male and female mice received a pretreatment of finasteride (50 mg/kg, i.p.) or saline 24 hrs prior to an injection of EtOH (4 g/kg, i.p.; Pharmco Products, Brookfield, CT) or saline, depending upon group assignment. Following the EtOH or saline injection, mice were scored every hour for 12 hrs and again at 24 and 25 hrs for HIC severity.
Handling-Induced Convulsions
Scoring for HICs was done according to a previously published scale (Finn and Crabbe 1999). This procedure involved lifting the animal by the tail, gently spinning it 180° if necessary, and observing a single, rapid convulsion. HIC scores ranging from 1 to 3 required the gentle spin to elicit a tonic or clonic convulsion, whereas convulsions elicited by merely lifting the mouse by the tail were scored as 4 to 6.
Experiment 2: Hormone Assessment
Mice received a pretreatment of finasteride (50 mg/kg, i.p.) or saline 24 hours prior to an injection of EtOH (4 g/kg, i.p.) or saline. At selected time points (0, 2, 8, or 24 hrs), separate groups of mice were killed and trunk blood collected for subsequent analysis of plasma 17β-estradiol and corticosterone concentrations by radioimmunoassay (RIA) over the time course of withdrawal. Specifically, the 0 hr time point assessed hormone levels prior to an injection of EtOH, but following the pretreatment with finasteride or saline. The 2 hr time point reflected the point prior to initiation of EtOH withdrawal when animals would have high BECs. In our experience, peak withdrawal typically occurred around the 8 hr time point. The 24 hr time point assessed hormone concentrations following the termination of withdrawal. Additionally, data in the saline control groups at each time point gave an indication of the normal fluctuation in basal 17β-estradiol and corticosterone levels across time in the male and female mice.
Radioimmunoassay (RIA)
Due to decreased sensitivity of the antibody used in the ALLO RIA after long-term storage, we were unable to analyze ALLO levels in the present study. RIAs for 17β-estradiol and corticosterone were conducted across several days due to the number of samples to be analyzed. In order to assess the reliability of measures and the consistency of the assay across days, intra- and inter-assay variation was determined.
Estradiol determination
Using 50-μl of plasma, estradiol concentrations were obtained by following the manufacturer’s instructions on the ImmuChem Double Antibody 17β-Estradiol [125I] RIA kit from ICN Pharmaceuticals (Costa Mesa, CA). Counts per minute were normalized and fit to a least-squares regression equation produced by log-logit transformation of the standards (10–3,000 pg/ml) using Prism Version 4 Software (GraphPad Software Inc, San Diego, CA). Mass of samples was calculated by interpolation of the standards. The minimal detectable limit of the assay was 0.34–1.25 pg/ml, based on actual values from individual assays. Intra- and inter-assay coefficients of variation were 1% and less than 11%, respectively, when calculated across 4 assays. The specificity of the assay was fairly high, with 20% cross-reactivity to estrone, 1.5% cross-reactivity to estriol, and less than 1% cross-reactivity to other endogenous steroids (based on the manufacturer’s information).
Corticosterone determination
Plasma (5-μl) was diluted with 100-μl sterile water and stored at 4°C until assayed. Samples were immersed in boiling water for 5 minutes to denature corticosterone-binding globulin. The RIA was adapted from a previously reported procedure (Keith et al., 1978) and employed [125I] corticosterone from ICN Pharmaceuticals and antiserum from Ventrex (Portland, ME). Counts per minute were normalized and fit to a least-squares regression equation produced by log-logit transformation of the standards (10–10,000 pg). Mass of samples was calculated by interpolation of the standards. The minimal detectable limit of the assay was 0.04–0.07 μg/dl, based on actual values from individual assays. Intra- and inter-assay coefficients of variation were 2% and 17%, respectively, when calculated across 4 assays. The specificity of the assay was very high, with only 4% cross-reactivity to deoxycorticosterone, 1% cross-reactivity to 5β-pregnanedione, and <0.6% cross-reactivity to other endogenous steroids (Keith et al., 1978).
Experiment 3: EtOH Clearance
Separate groups of male and female C57BL/6J and DBA/2J received a pretreatment of finasteride (50 mg/kg, i.p.) or saline 24 hours prior to an injection of EtOH (4 g/kg, i.p.). Animals were tested for ethanol clearance rate using previously published methods (Shen et al., 1995). Specifically, retro-orbital blood samples were collected at 30, 60, 120, and 240 minutes post EtOH injection for BEC determination. Mice were briefly restrained while a 20-μl sample of blood was collected rapidly; eyes were alternated for each time point to minimize trauma. Between determinations, the mouse was then released back to its home cage. Recent work suggests that multiple retro-orbital blood sampling did not significantly alter BECs relative to animals that had received a single blood sampling (Kamens et al, 2006).
BEC Determination
A modification of the method originally described by Roach and Creaven (1968) was utilized. Briefly, a 20-μl sample of blood from the tip of the tail was added to 50-μl of chilled 5% ZnSO4 and stored on ice. Distilled water (300-μl) and 0.3N Ba(OH)2 (50-μl) were added to each sample. Each sample was shaken for 5 sec and centrifuged for 5 min at 12,000 rpm. The supernatant was transferred to a crimp top glass vial and analyzed for EtOH concentrations by gas chromatography (Model 6890N, Agilent Technologies, Palo Alto, CA, USA) with flame ionization detection. Seven pairs of EtOH standards (0.25–4.0 mg/ml) were used to establish a standard curve.
Data Analysis
Analysis of variance (ANOVA) was used to assess strain (C57BL/6J versus DBA/2J), sex (male versus female), treatment (EtOH versus saline), and drug (finasteride versus saline) effects on the dependent variables hourly HICs, area under the withdrawal curve (AUC), peak withdrawal, BEC, and hormone concentrations. A repeated measure ANOVA was used to assess the effect of the independent variables on hourly HICs. AUC was calculated over the 25 hr period (AUC25) following the single injection of a 4-g/kg dose of EtOH from the hourly HIC scores. Peak withdrawal was calculated by taking the average of the highest HIC score, the previous hourly score, and the following hourly score.
Linear regression analysis was performed on the retro-orbital BEC time course data for each repeatedly sampled animal. Based on the slope of the regression line, an estimate of clearance rate (mg/ml/hr) was obtained. An estimate of volume of distribution (ml) and volume of distribution accounting for body weight (ml/g) was determined by dividing the amount of EtOH (mg) by the estimated BEC at time = 0 (based on the regression slope and the y-intercept of the regression line). An estimate for total clearance time (min) was determined by the x-intercept of the regression line. A repeated measures ANOVA was used to assess the effects of strain, sex, drug, and time on BECs, while a three-way ANOVA was used to assess the effects of strain, sex, and drug on several of the clearance parameters.
All statistical analyses were conducted using Systat (version 10; Point Richmond, CA). Based on the findings of our chronic study (Finn et al., 2004c), our a priori hypothesis was that finasteride would differentially alter withdrawal in C57BL/6J and DBA/2J male and female mice. Thus, each group was analyzed separately even in the absence of significant interactions. Post-hoc analyses were conducted using Tukey’s post-hoc test. Data are expressed as the mean ± SEM. Significance was set at p ≤ 0.05.
Results
Experiment 1: Withdrawal Severity
HIC Scores and AUC25
Hourly HIC scores, collapsed across time, were significantly higher in DBA/2J versus C57BL/6J mice, in male than in female mice, and in mice treated with EtOH versus saline [Fs(1,184) > 4.13, ps < 0.05] (Figure 1). There were several significant interactions involving strain, sex, treatment, and drug [Fs(1,184) > 6.70, ps < 0.05], indicating that treatment with finasteride and EtOH differentially altered HIC scores in male and female C57BL/6J and DBA/2J mice. Repeated measures ANOVA also indicated that hourly HIC scores changed significantly across time [F(15,2760) = 17.46, p < 0.001]. The significant interactions involving time, strain, sex, treatment, and drug [Fs(15,2760) > 2.027, ps < 0.05] and post-hoc analyses (not shown) provide support for the conclusion that pretreatment with finasteride decreased acute EtOH withdrawal severity in male C57BL/6J and DBA/2J mice and increased withdrawal severity in female mice of both strains. The results of the hourly HIC scores mimicked that of AUC25, another index of EtOH withdrawal severity, which will be discussed in detail.
Figure 1.
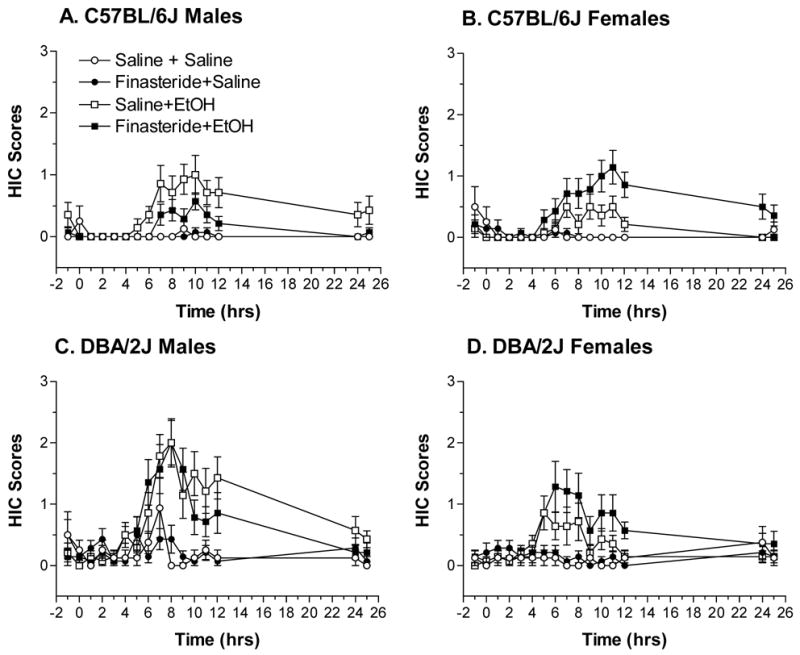
Hourly HIC scores in intact C57BL/6J male (A), C57BL/6J female (B), DBA/2J male (C), and DBA/2J female (D) mice following a 4 g/kg injection of EtOH or saline and pretreatment with finasteride (50 mg/kg) or saline. Values represent the mean ± S.E.M. for n=8–14/group.
AUC25 (Figure 2) was significantly higher in DBA/2J than in C57BL/6J mice and following injection of EtOH [Fs(1,184) > 18.34, ps < 0.05]. There was a trend for AUC25 to be higher in male than in female mice (p = 0.055). The significant three-way interactions between strain, sex, and treatment as well as between sex, treatment, and drug [Fs(1,184) > 4.26, ps < 0.05], suggested that finasteride pretreatment altered acute EtOH withdrawal differently in the male and female mice.
Figure 2.
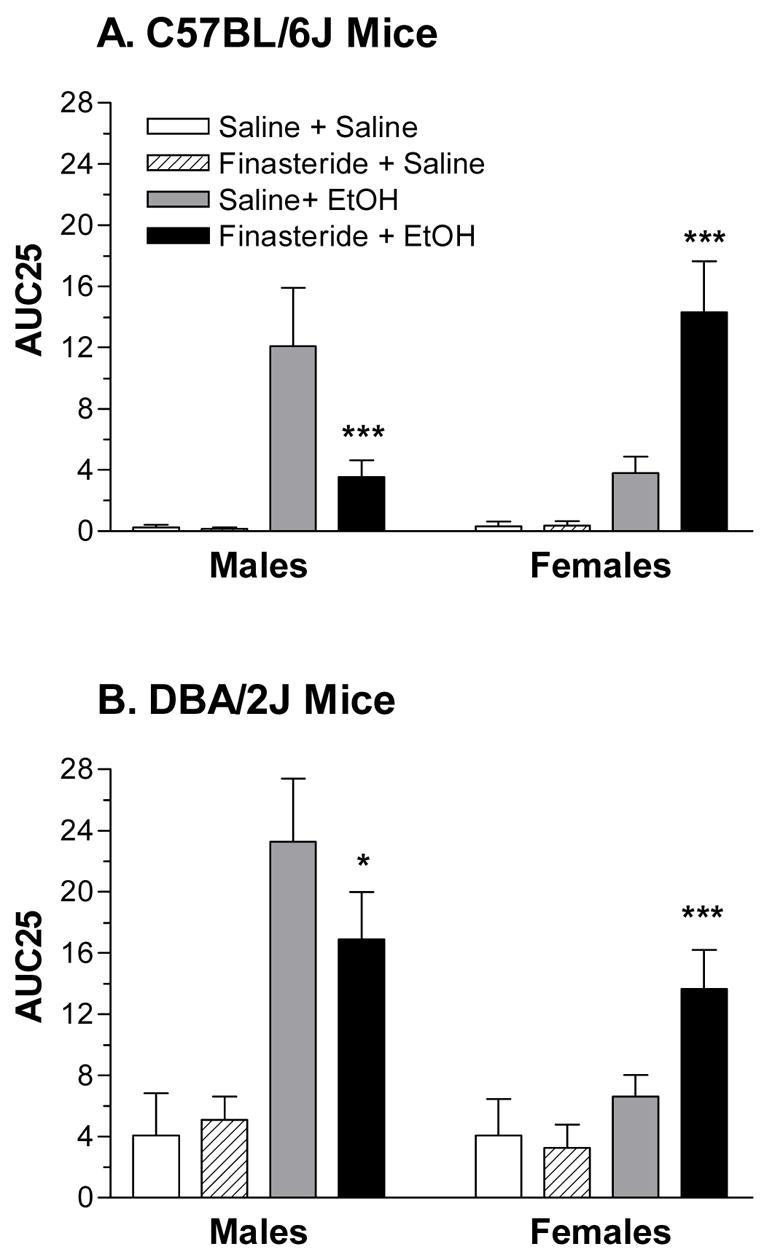
The effect of pretreatment with finasteride on EtOH withdrawal severity, measured by area under the withdrawal curve (AUC25), in C57BL/6J (A) and DBA/2J (B) intact male and female mice. Values represent the mean ± S.E.M. for the animals depicted in Figure 1. *p < 0.05, ***p < 0.001, versus respective saline + EtOH group
For C57BL/6J males (Figure 2A), AUC25 was significantly increased by EtOH injection [F(1,46) = 10.82, p < 0.05] and tended to be decreased by finasteride pretreatment (p = 0.07). Post-hoc tests confirmed that pretreatment with finasteride significantly decreased EtOH withdrawal severity. For C57BL/6J female mice (Figure 2A), there was a significant main effect of treatment (saline < EtOH), a significant main effect of drug (saline < finasteride) and a significant interaction between treatment and drug [Fs(1,46) > 3.32, ps < 0.05]. Post-hoc tests confirmed that pretreatment with finasteride significantly increased EtOH withdrawal severity in female C57BL/6J mice.
In DBA/2J male mice (Figure 2B), injection of EtOH significantly increased AUC25 [F(1,46) = 10.83, p < 0.05], and finasteride pretreatment tended to decrease AUC25 (p = 0.06). Post-hoc tests confirmed that pretreatment with finasteride significantly decreased EtOH withdrawal severity. In DBA/2J females (Figure 2B), AUC25 was significantly increased by EtOH injection [F(1,46) = 9.87, p < 0.05] and tended to be increased by finasteride pretreatment (p = 0.10). The trend for an interaction between treatment and drug (p = 0.07), in conjunction with post-hoc tests, confirmed that pretreatment with finasteride significantly increased EtOH withdrawal severity in DBA/2J female mice.
In EtOH-treated animals, peak withdrawal severity (Table 1) was significantly greater in DBA/2J mice than C57BL/6J mice and was significantly greater in male mice relative to female mice [Fs(1,104) > 4.08, ps < 0.05]. There were multiple significant interactions involving strain, sex, and drug [Fs(1,104) > 6.42, ps < 0.05], suggesting that peak withdrawal was differentially altered by finasteride in the male and female C57BL/6J and DBA/2J mice. In C57BL/6J male mice, pretreatment with finasteride tended to decrease peak withdrawal severity (p = 0.07). In C57BL/6J female mice, pretreatment with finasteride significantly increased peak withdrawal severity [F(1,46) = 4.13, p < 0.05]. There was no significant effect of pretreatment with finasteride on peak withdrawal severity in male or female DBA/2J mice. In EtOH-treated animals, strain, sex, and drug did not significantly affect the hour at which peak withdrawal occurred.
Table 1. Peak Withdrawal Scores and Peak Withdrawal Hour in EtOH-Injected Mice.
Peak withdrawal scores (i.e. the average of the highest HIC score, the previous score, and the following score) and the hour that peak withdrawal occurred were calculated for C57BL/6J and DBA/2J male and female mice. Values represent mean ± S.E.M. for the EtOH-injected animals depicted in Figure 1.
| Strain and Sex | Drug | Peak Withdrawal Score (HIC Score) | Peak Withdrawal Hour |
|---|---|---|---|
| C57BL/6J Males | Saline | 1.12 ± 0.29 (n = 14) | 7.36 ± 1.11 (n = 14) |
| Finasteride | 0.48 ± 0.08 (n = 14)+ | 7.43 ± 0.89 (n = 14) | |
| C57BL/6J Females | Saline | 0.61 ± 0.14 (n = 14) | 7.71 ± 0.78 (n = 14) |
| Finasteride | 1.17 ± 0.21 (n = 14)* | 8.43 ± 0.95 (n = 14) | |
| DBA/2J Males | Saline | 2.02 ± 0.29 (n = 14) | 8.43 ± 0.59 (n = 14) |
| Finasteride | 1.93 ± 0.29 (n = 14) | 6.36 ± 0.59 (n = 14) | |
| DBA/2J Females | Saline | 1.05 ± 0.20 (n = 14) | 6.07 ± 0.68 (n = 14) |
| Finasteride | 1.41 ± 0.58 (n = 14) | 6.93 ± 0.58 (n = 14) |
p < 0.10,
p < 0.05 versus saline pretreatment
Experiment 2: Hormone Assessment
Estradiol Concentrations
Due to the fact that not all groups were equally represented in the 0 hr condition (i.e., since these animals did not receive injections of either EtOH or saline), this time point was analyzed separately from 2, 8, and 24 hrs (Figure 3). At 0 hr, there was a significant main effect of strain (C57BL/6J < DBA/2J) on estradiol concentrations as well as a significant interaction between strain and sex [Fs(1,62) > 4.27, ps < 0.05]. The interaction was due to the fact that estradiol levels tended to be lower in female than in male C57BL/6J mice (p = 0.06) but were significantly higher in female DBA/2J mice than in male DBA/2J mice [F(1,30) = 12.16, p < 0.005].
Figure 3.
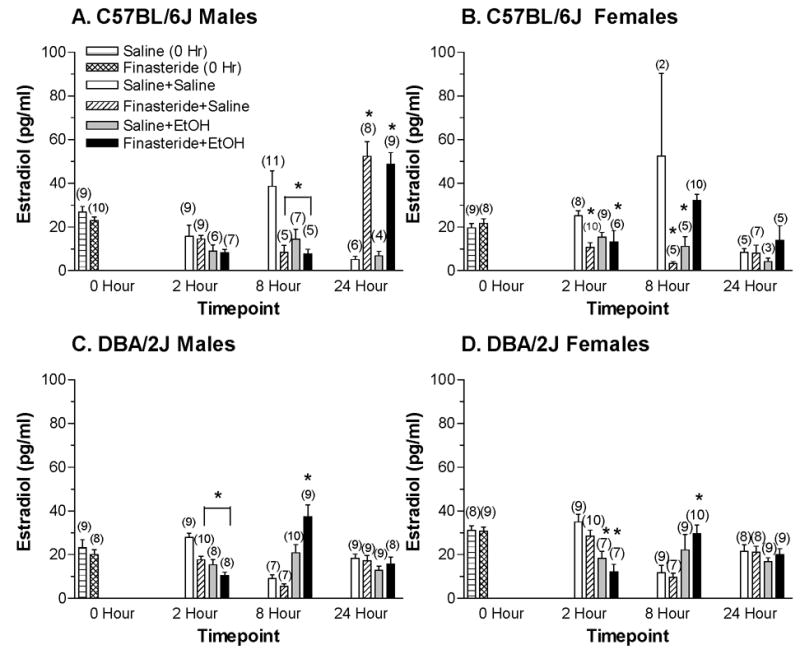
Plasma estradiol concentrations in C57BL/6J male (A), C57BL/6J female (B), DBA/2J male (C), and DBA/2J female (D) mice following a 4 g/kg injection of EtOH or saline that was given 24 hrs after pretreatment with finasteride or saline. Values represent the mean ± S.E.M. for the number of animals in parentheses. * at least p < 0.05, versus respective Saline+Saline group
At 2, 8, and 24 hrs, estradiol concentrations tended to be lower in EtOH-treated mice [F(1,318) = 3.09, p = 0.08], but there were multiple significant interactions involving all of the factors (strain, sex, treatment, drug, and hour) [Fs(1 or 2,318) > 3.98, p < 0.05]. When the analyses were conducted on each strain, there were multiple significant interactions involving sex, treatment, drug, and hour [Fs(1 or 2,137) > 8.36, ps < 0.05] in C57BL/6J mice (Figures 3A and B) and multiple significant interactions involving treatment, drug, and hour [Fs(1 or 2,181) > 6.65, ps < 0.05] in DBA/2J mice (Figures 3C and D). These significant interactions suggested that estradiol levels were changing differently in male and female mice of each inbred strain across time and with EtOH and finasteride treatment.
For C57BL/6J males (Figure 3A), estradiol levels were significantly decreased by EtOH injection, significantly increased by finasteride pretreatment and highest at the 24 hr time point (2 hr < 8 hr < 24 hr) [Fs (1 or 2,74) > 4.74, ps < 0.05]. The significant interaction between drug and hour [F(2,74) = 35.190, p < 0.001] suggested that the effect of finasteride on estradiol levels differed at the various time points. At the 2 hr time point, there was a trend for estradiol levels to be lower in EtOH-treated mice (p = 0.066). At 8 hrs, estradiol levels were significantly lower following finasteride pretreatment [F(1,24) = 7.585, p < 0.05] and tended to be lower following EtOH injection (p = 0.07). The trend for an interaction between treatment and drug (p = 0.09), in conjunction with post-hoc tests, confirmed that finasteride-treated and EtOH-treated animals had lower plasma estradiol concentrations relative to the saline-treated control animals (p < 0.05). At the 24 hr time point, finasteride pretreatment significantly increased estradiol levels [F(1,23) = 59.30, p < 0.001], an effect that was confirmed with post-hoc tests.
For C57BL/6J female mice (Figure 3B), estradiol concentrations were significantly decreased by finasteride pretreatment and were lowest at the 24 hr time point (24 hr < 2 hr < 8 hr) [Fs(1 or 2,63) > 4.60, ps < 0.05]. The multiple interactions between treatment, drug, and hour [Fs (1 or 2,63) > 3.49, ps < 0.05] suggested that the effect of EtOH and finasteride on estradiol levels differed at the various time points. At the 2 hr time point, there was a significant main effect of drug (finasteride < saline) and a significant interaction between treatment and drug [Fs(1,29) > 4.76, ps < 0.05]. Confirmation with post-hoc tests showed that both finasteride-treated groups had lower plasma estradiol levels relative to saline-treated control animals (p < 0.05). Similarly, at 8 hrs, finasteride pretreatment significantly decreased plasma estradiol levels, with a significant interaction between treatment and drug [Fs(1,18) > 2.53, ps < 0.05]. Post-hoc tests revealed that relative to the saline-treated control animals, finasteride + saline-treated and EtOH + saline-treated animals had lower plasma estradiol concentrations (p < 0.005). There were no significant main effects or interactions at 24 hrs.
For DBA/2J males (Figure 3C), there were multiple significant interactions between treatment, drug, and hour [Fs(1 or 2,91) > 6.26, ps < 0.05] on estradiol concentrations, suggesting that they were differentially altered across time by finasteride and EtOH. At the 2 hr time point EtOH injection and finasteride pretreatment significantly decreased estradiol levels [Fs(1,31) > 15.11, ps ≤ 0.001]. Post-hoc tests confirmed that relative to saline-treated control animals, finasteride and EtOH produced a decrease in estradiol concentrations (p < 0.005). At 8 hrs, estradiol levels were significantly increased by EtOH injection, with a significant interaction between treatment and drug [Fs(1,29) > 6.53, ps < 0.05]. Post-hoc tests confirmed that the combination of finasteride and EtOH produced the greatest increase in plasma estradiol concentrations relative to the saline-treated control group (p < 0.001). There were no significant main effects or interactions at the 24 hr time point.
For DBA/2J female mice (Figure 3D), there was a significant interaction between treatment and hour [F(2,90) = 19.12, p < 0.001] on plasma estradiol levels, suggesting that EtOH injection differentially altered estradiol concentration across time. At 2 hrs, estradiol levels were significantly lower in the EtOH-treated mice [F(1,29) = 26.01, p < 0.001], and they tended to be lower in mice pretreated with finasteride (p = 0.06). Post-hoc tests confirmed that treatment with EtOH significantly decreased estradiol concentrations relative to saline-treated counterparts (p < 0.01). However, at the 8 hr time point, EtOH treatment significantly increased plasma estradiol levels [F(1,31) = 10.376, p < 0.05]. This main effect of EtOH was confirmed with post-hoc tests (p < 0.05). There were no significant main effects or interactions at 24 hrs.
Corticosterone Concentrations
Due to the fact that several groups in the DBA/2J male mice fell below the minimum detectable limit (saline+saline at 2 hrs and 24 hrs, as well as the saline+EtOH group at 24 hrs), it was necessary enter the minimal detectable limit of 0.04 μg/dl (see methods) for those animals in order to analyze the data. It should be noted that corticosterone values ≤ 0.04 μg/dl would be consistent with low “non-stressed” basal levels. Since corticosterone levels were measurable in all other DBA/2J male groups as well as in the DBA/2J females and the C57BL/6J males and females, we do not believe that the undetectable levels reflect insensitivity of the assay.
Baseline corticosterone levels were not significantly affected by strain or pretreatment condition (Figure 4). At 2, 8, and 24 hrs, corticosterone levels were significantly higher in EtOH versus saline-injected mice and were highest at the 2 hr time point [Fs(1 or 2,289) > 3.33, ps < 0.001]. There was a trend for corticosterone levels to be higher in DBA/2J mice relative to C57BL/6J mice (p = 0.07). There were multiple significant interactions involving all of the factors (strain, sex, treatment, drug, and time point) [Fs(1 or 2,289) > 4.18, ps < 0.05], suggesting that corticosterone levels were changing across time differently in the male and female mice treated with finasteride and/or EtOH.
Figure 4.
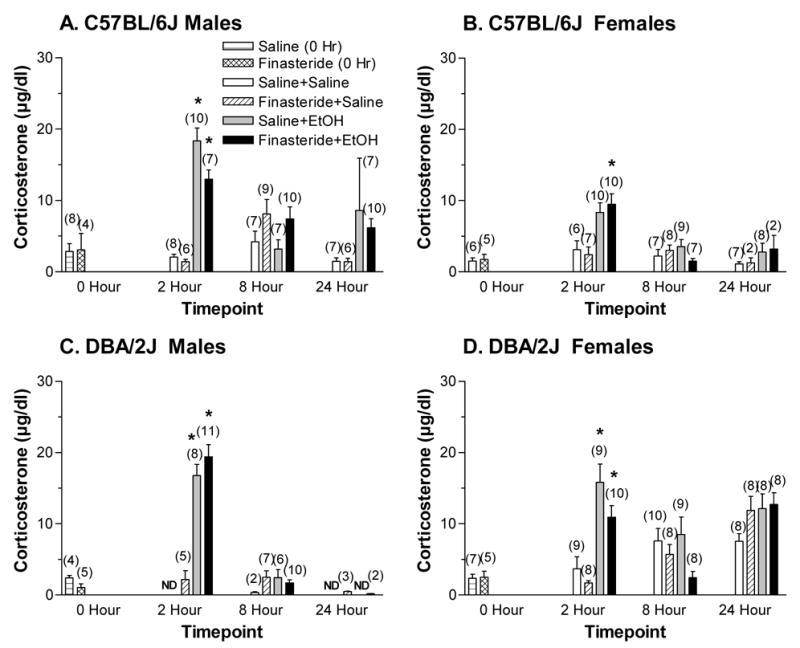
Plasma corticosterone levels in C57BL/6J male (A), C57BL/6J female (B), DBA/2J male (C), and DBA/2J female (D) mice following a 4 g/kg injection of EtOH or saline that was given 24 hrs after pretreatment with finasteride or saline. Values represent mean ± S.E.M. for the number of animals in parentheses. N.D. = not detectable * at least p < 0.05, versus respective Saline+Saline group
For C57BL/6J males (Figure 4A), corticosterone levels were significantly higher in the EtOH-treated group relative to the saline-treated group and were highest at the 2 hr time point (24 hrs < 8 hrs < 2 hrs) [Fs(1 or 2,82) > 3.33, ps < 0.05]. The significant interaction between treatment and hour [F(2,82) = 9.96, p < 0.001] suggested that EtOH treatment differentially altered plasma corticosterone level at various time points. At the 2 hr time point, plasma corticosterone levels were significantly increased by EtOH injection and significantly decreased by finasteride pretreatment [Fs(1,27) > 4.78, ps < 0.05]. The trend for an interaction between the two factors (p = 0.097), in conjunction with post-hoc tests, confirmed that treatment with EtOH increased corticosterone concentrations at 2 hrs (p < 0.001) relative to saline-treated-control groups. At 8 hrs, corticosterone levels were significantly elevated in the mice pretreated with finasteride [F(1,29) = 5.33, p < 0.05]. There were no significant main effects or interactions at the 24 hr time point. However, there was a trend for treatment with EtOH to increase corticosterone levels relative to saline-treated animals (p = 0.10).
For C57BL/6J females (Figure 4B), corticosterone levels were significantly increased by EtOH injection and were highest at the 2 hr time point (24 hrs ≤ 8 hrs < 2 hrs), with a significant interaction between treatment and time [Fs(1 or 2,71) > 7.98, ps < 0.05]. At 2 hrs, corticosterone levels were significantly higher in EtOH-treated animals relative to those treated with saline [F(1,29) = 19.00, p < 0.001]. Post-hoc tests confirmed that the combination of finasteride and EtOH produced a significant increase in corticosterone concentrations relative to saline-treated control animals (p < 0.05). There were no significant main effects or interactions at 8 and 24 hrs.
For DBA/2J males (Figure 4C), plasma corticosterone levels were significantly increased by EtOH injection and were highest at the 2 hr time point (24 hrs < 8 hrs < 2 hrs) [Fs(1 or 2,45) > 18.18, ps < 0.001]. At 2 hrs, corticosterone levels were significantly lower in saline-treated animals than those treated with EtOH [F(1,21) = 34.16, p < 0.001]. Post-hoc tests confirmed this effect (p < 0.001). There were no significant main effects or interactions at the 8 or 24 hr time point. There was a trend for pretreatment with finasteride to increase plasma corticosterone levels at 24 hrs (p = 0.052), but this effect is not physiologically relevant as finasteride merely increased corticosterone levels above the non-detectable range.
For DBA/2J females (Figure 4D), corticosterone levels were higher in EtOH-treated animals relative to those treated with saline and varied significantly across time (8 hrs < 2 hrs < 24 hrs) [Fs(1 or 2,91) > 7.89, ps < 0.05]. The significant interactions between treatment and time point as well as between drug and time point [Fs(2,91) > 3.91, ps < 0.05] suggested that EtOH and/or finasteride altered corticosterone levels differently at the various time points. There was a trend for an interaction between treatment and drug (p < 0.08). At 2 hrs, corticosterone levels were significantly increased by EtOH injection [F(1,32) = 34.77, p < 0.001] and tended to be decreased by finasteride pretreatment (p = 0.07). Post-hoc tests confirmed that relative to the saline-treated control animals, EtOH produced an increase in plasma corticosterone levels (p < 0.05). Finasteride pretreatment also significantly reduced corticosterone levels at 8 hrs [F(1,31) = 4.99, p < 0.05]. Post-hoc tests revealed that there was a trend for finasteride-treatment to decrease corticosterone concentrations (p = 0.10) relative to saline-treated controls. There were no significant main effects or interactions at the 24 hr time point.
Experiment 3: EtOH Clearance
BEC Time course
Retro-orbital BECs were measured at 30, 60, 120, and 240 minutes post EtOH injection (Figure 5). Repeated measures ANOVA indicated that that BECs changed significantly across time [F(3,210) = 229.95, p < 0.001]. There was a significant interaction between time and strain [F(3,210) = 4.98, p < 0.005] and a trend for a significant interaction between time, strain, and drug (p = 0.10).
Figure 5.
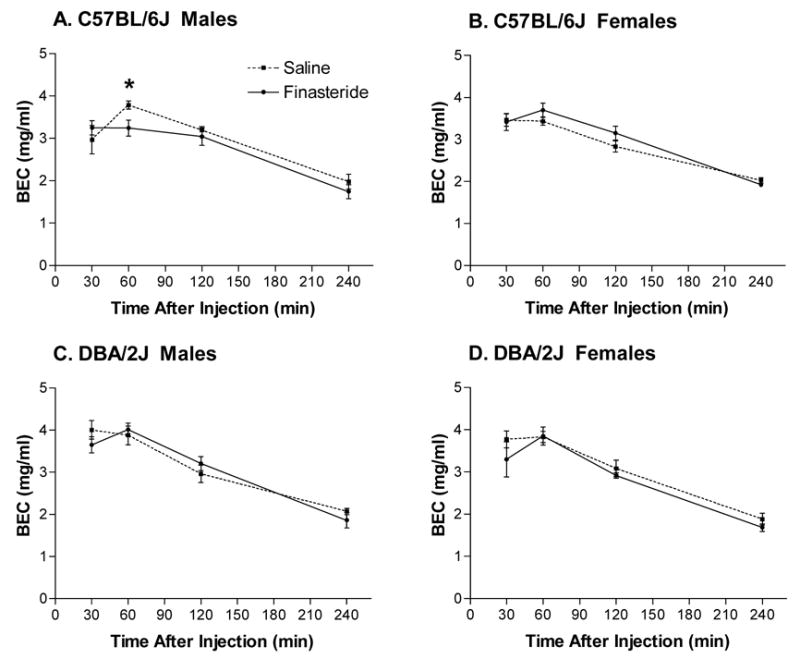
Retro-orbital BECs at 30, 60, 120, and 240 minutes following a 4 g/kg injection of EtOH that was given 24 hrs after pretreatment with finasteride or saline in C57BL/6J male (A), C57BL/6J female (B), DBA/2J male (C), and DBA/2J female (D) mice. Values represent mean ± SEM for n=9–11/group. * p < 0.05 versus saline-treated controls
In C57BL/6J male mice (Figure 5A), pretreatment with finasteride did not significantly alter BECs relative to saline-treated controls at the 30, 120, and 240 minute time points. However, at 60 minutes post EtOH injection, pretreatment with finasteride significantly reduced BECs relative to saline-treated animals [F(1,18) = 6.61, p < 0.02]. In C57BL/6J females, DBA/2J males, and DBA/2J females (Figure 5B–D, respectively), there were no significant differences in BECs at any time point between finasteride-treated and saline-treated animals.
Clearance Parameters
Although BECs were collected at 30, 60, 120, and 240 minutes post-injection, EtOH clearance rates for individual animals were based on the 60 – 240 minute time points (the linear portion of the curve). A comparison of clearance rates (Table 2) revealed that C57BL/6J mice had slower clearance rates than DBA/2J mice [F(1,71) = 14.99, p < 0.001]. For C57BL/6J male and female mice, pretreatment with finasteride did not significantly affect EtOH clearance rates, when compared to saline-treated controls (i.e., pretreatment with finasteride produced only a 12–15% increase versus saline treatment). Similarly, in DBA/2J males, pretreatment with finasteride did not significantly affect EtOH clearance rates (i.e., produced only a 2% increase relative to saline-treated controls). Pretreatment with finasteride produced a non-significant 5% decrease in EtOH clearance rates relative to saline-treated controls in DBA/2J female mice.
Table 2. Ethanol Clearance Parameters.
Retro-orbital blood BECs were collected at 30, 60, 120, and 240 minutes following acute EtOH administration (4 g/kg). EtOH clearance parameters were calculated based on the 60–240 minute time points (i.e. the linear portion of the curve). Values represent the mean ± S.E.M. for n = 9–11/group.
| Clearance Parameters | C57BL/6J Males | C57BL/6J Females | DBA/2J Males | DBA/2J Females | ||||
|---|---|---|---|---|---|---|---|---|
| Saline | Finasteride | Saline | Finasteride | Saline | Finasteride | Saline | Finasteride | |
| BEC at time = 0(mg/ml) | 3.71 ± 0.16 | 3.65 ± 0.17 | 3.75 ± 0.1 | 3.95 ± 0.18 | 4.30 ± 0.21 | 4.27 ± 0.14 | 4.22 ± 0.17 | 3.97 ± 0.29 |
| Clearance Rate(mg/ml/h) | 0.39 ± 0.05 | 0.45 ± 0.03 | 0.43 ± 0.02 | 0.48 ± 0.03 | 0.57 ± 0.06 | 0.58 ± 0.05 | 0.58 ± 0.02 | 0.55 ± 0.09 |
| Vd (ml) | 26.15 ± 1.67 | 26.74 ± 1.62 | 19.76 ± 0.97 | 18.12 ± 0.68 | 19.01 ± 1.07 | 17.52 ± 0.68 | 14.43 ± 0.67 | 15.86 ± 1.3 |
| Vd/body weight(ml/g) | 1.1 ± 0.06 | 1.12 ± 0.05 | 1.08 ± 0.03 | 1.03 ± 0.05 | 0.95 ± 0.05 | 0.94 ± 0.03 | 0.96 ± 0.03 | 1.06 ± 0.09 |
| Total Clearance Time (min) | 394.97 ± 48.26 | 431.14 ± 21.27 | 470.05 ± 22.93 | 432.19 ± 27.55 | 408.22 ± 39.78 | 379.97 ± 25.34 | 412.81 ± 17.6 | 329.85 ± 33.8 * |
p < 0.05 versus respective saline-treated group
For many of the other clearance parameters, there was a significant main effect of strain. Specifically, the estimate of BEC at time 0 (an extrapolation based on the slope) was significantly lower in C57BL/6J mice versus DBA/2J mice [Fs(1,71) < 14.99, ps < 0.002]. Volume of distribution accounting for body weight (ml/g) was significantly lower and total clearance time (minutes) was significantly faster in DBA/2J mice than in C57BL/6J mice [Fs(1,71) < 7.16, ps < 0.03]. For C57BL/6J males and females and DBA/2J males, finasteride pretreatment did not significantly alter any of the clearance parameters. However, for DBA/2J female mice, pretreatment with finasteride significantly decreased total clearance time relative to saline-treated controls [F(1,17) = 4.43, p < 0.05].
Discussion
The present experiments were designed to assess whether male and female C57BL/6J and DBA/2J mice would differ in response to pharmacological inhibition of the neurosteroid biosynthetic enzyme 5α-reductase on acute EtOH withdrawal severity. Pretreatment with finasteride decreased acute EtOH withdrawal severity, measured by hourly HIC and AUC25, in male C57BL/6J and DBA/2J mice. In contrast, pretreatment with finasteride increased acute EtOH withdrawal severity in female mice of both strains. This sex difference in finasteride’s effect on acute EtOH withdrawal was not due to changes in EtOH clearance parameters. Similarly, the finasteride- and EtOH-induced alterations in plasma estradiol and corticosterone levels did not appear to change in a manner that could explain the sex difference in finasteride’s effect on acute EtOH withdrawal severity. Collectively, these findings suggest that male and female C57BL/6J and DBA/2J mice may differ in their sensitivity to manipulation of GABAergic neurosteroids during acute EtOH withdrawal.
HIC Data
Acute EtOH withdrawal severity, measured by hourly HIC scores, AUC, and peak withdrawal severity, was significantly greater in DBA/2J versus C57BL/6J mice, consistent with previously observed strain differences (Crabbe et al., 1983; Crabbe, 1998; Roberts et al., 1992). Another interesting finding was that acute EtOH withdrawal severity (hourly HIC scores, AUC, and peak withdrawal severity) was significantly higher in male mice relative to female mice. This sex difference in acute withdrawal severity is consistent with reports that chronic EtOH withdrawal severity is decreased by approximately 25% in female rodents (e.g. Devaud et al., 2003).
Finasteride pretreatment decreased EtOH withdrawal severity in male mice, a finding that is consistent with a chronic withdrawal study from our laboratory (Finn et al., 2004c). However, finasteride decreased BECs upon the initiation of withdrawal in the chronic study, but did not alter BEC in the male mice in the present study. Thus, the fact that finasteride decreased acute withdrawal severity in male mice does not appear to be due to an effect of finasteride on EtOH metabolism.
In contrast, finasteride increased acute EtOH withdrawal severity in female mice of both strains, which differed from the effect on chronic EtOH withdrawal severity (Finn et al., 2004c). Whereas an indirect effect of finasteride on EtOH pharmacokinetics could have decreased chronic EtOH withdrawal severity in female DBA/2J mice by decreasing the “effective” EtOH dose during the development of physical dependence, it cannot explain the lack of effect on withdrawal in female C57BL/6J mice in the chronic study. These data, in conjunction with the lack of effect on BEC in the present study, suggest that the opposite effect of finasteride on acute versus chronic withdrawal severity in female mice was not solely due to alterations in BECs.
Peak withdrawal was also examined to determine the manner in which finasteride might be altering withdrawal severity. The hour at which peak withdrawal occurred was approximately 1 hr earlier in the DBA/2J mice, consistent with the shorter clearance time in this strain, when compared with C57BL/6J mice. Pretreatment with finasteride did not affect the time at which peak withdrawal occurred, but it tended to decrease peak withdrawal scores in C57BL/6J male mice and to significantly increased peak withdrawal scores in female C57BL/6J mice. Thus, finasteride may be acting to increase or decrease the severity of withdrawal, rather than precipitating withdrawal (i.e., causing withdrawal to occur sooner) or elevating HIC scores for longer duration.
BEC and Clearance Data
There were no systematic changes in BECs in the male or female C57BL/6J or DBA/2J mice following finasteride pretreatment. The finasteride-induced decrease in BEC at 60 minutes in C57BL/6J male mice was not reflected in later time points or in any EtOH clearance parameters. While finasteride significantly decreased total clearance time in female DBA/2J mice, it also increased the time to peak withdrawal. Thus, the current findings indicate that a finasteride-induced alteration in EtOH’s pharmacokinetics was not the cause of the observed changes in acute EtOH withdrawal severity in male and female mice.
Although there were no overall sex and drug effects on any of the EtOH clearance parameters, there were a number of strain differences in the elimination of EtOH. Notably, clearance rates were lower in C57BL/6J mice relative to DBA/2J mice. This finding is compatible with the previous literature, which suggests that C57BL/6J mice have approximately 10–15% slower elimination rates than DBA/2J mice (Faulkner et al., 1990; Grisel et al., 2002). Consistent with the faster elimination rate in DBA/2J mice, estimates for the volume of distribution accounting for body weight and total clearance time were lower in DBA/2J mice relative to C57BL/6J mice.
Hormone Data
One potential mechanism for the differential effect of finasteride on acute EtOH withdrawal severity is via an indirect increase in levels of a steroid hormone (e.g., estradiol or corticosterone) that can influence convulsions (e.g., Roberts et al., 1994; Roberts and Keith, 1995; Karst et al., 1999; Reddy; 2004). By blocking 5α-reductase, an accumulation of testosterone might occur, which could be converted to estradiol via aromatase (since it could not be reduced to dihydrotestosterone). The fact that aromatase expression in rodents appears to be reduced relative to primates (see Syed and Khosla, 2005) suggests that finasteride might not favor an increase in estradiol levels via the aromatization pathway. Consistent with this idea, we did not observe a persistent increase in estradiol levels in the finasteride-treated groups, although estradiol levels were significantly increased at 24 hr in the finasteride-treated C57BL/6J males. While estradiol concentrations were higher in female than in male DBA/2J mice as one would expect, it was surprising that there was no sex difference in estradiol concentrations between male and female C57BL/6J mice. This may be due to the fact that all animals remained intact and that the female mice were not synchronized with regard to hormonal cycling. Based on the estradiol levels in the control female groups, we presume that the majority of the C57BL/6J females were in diestrus (mean values of 19.6, 25.3 and 8.6 pg/ml at the 0, 2 and 24 hr time points), whereas the DBA/2J females were in late diestrus or early proestrus (mean values of 31.1, 35.0, 11.7 and 21.5 pg/ml at the 0, 2, 8 and 24 hr time points). Further studies examining estradiol concentrations in animals that are synchronized with regard to cycling are necessary to further characterize these observed effects.
At the 8 hr time point, when peak withdrawal typically occurs, pretreatment with finasteride produced an increase in plasma estradiol levels relative to saline-treated animals in EtOH-treated male and female DBA/2J mice. However, it is unlikely that this finasteride-induced increase in plasma estradiol at 8 hrs produced an opposite effect on HICs in male and female mice. Although it is possible that male and female mice have different sensitivities to an increase in estradiol levels, other factors (such as 5α-reduced steroids or a differential sensitivity to the neuroprotective effect of estradiol that has been recently reported; e.g., Jung et al., 2002; Rewal et al., 2003) may be mediating the observed sex difference in acute withdrawal severity.
Another potential site of action of finasteride is deoxycorticosterone, which can be converted to 5α-dihydrodeoxycorticosterone via 5α-reductase, or to corticosterone. By blocking 5α-reductase, one might expect to observe an increase in deoxycorticosterone and hence corticosterone levels. Exogenous corticosterone administration can enhance convulsion sensitivity and accelerate kindling epileptogenesis (Roberts et al., 1994; Roberts and Keith, 1995; Karst et al., 1999). In parallel studies, manipulation of plasma corticosterone levels in the range of 5 – 20 μg/dl produced excitatory effects on hippocampal slice electrophysiology and increased convulsion sensitivity (reviewed in Roberts and Keith, 1995). However, corticosterone concentrations did not change in a manner that could explain the HIC and AUC25 data. That is, the EtOH-induced increase in corticosterone levels at 2 hrs in both strains and sexes, which is consistent with previous findings (e.g., Roberts et al., 1992; Rivier, 1993), was not associated with an increase in convulsive behavior (i.e., mice were still fairly sedated from the high dose of EtOH, Figure 1), was not sustained throughout the time course of withdrawal, and was not potentiated by pretreatment with finasteride.
Other Potential Mechanisms
Since finasteride can block the conversion of progesterone, deoxycorticosterone, and testosterone to GABAergic neurosteroids, there may be a complex interaction between finasteride, EtOH, and their subsequent interaction with GABAA receptors (Finn et al., 2006). Specifically, a finasteride-induced decrease in ALLO, 5α-THDOC, or androstanediol levels would thereby decrease GABAergic inhibition, making EtOH withdrawal seizures more pronounced. Recent evidence indicates that fluctuations in endogenous GABAergic neurosteroid levels during the estrous cycle of female C57BL/6J mice can modulate GABAA receptor-mediated tonic inhibition, seizure susceptibility and anxiety (Maguire et al., 2005). Since basal ALLO levels are higher in females than in males, finasteride may produce a larger, more physiologically relevant decrease in GABAergic tonic inhibition in female than in male mice, thereby increasing brain excitability. Although the use of finasteride is an indirect tool to examine how ALLO may contribute to withdrawal severity, we were unable to measure ALLO levels in the present study. Thus, it would be of great interest to directly measure ALLO levels during the course of withdrawal in future studies to determine the correspondence between endogenous ALLO levels and acute EtOH withdrawal severity.
Another possibility is that a finasteride-induced decrease in GABAergic inhibition altered EtOH sensitivity in the male mice, since decreasing endogenous ALLO levels has been shown to decrease sensitivity to some, but not all, effects of EtOH in male rodents (e.g., Dazzi et al., 2002; Van Doren et al., 2000; Hirani et al., 2002, 2005). For example, finasteride altered EtOH’s anticonvulsant, antidepressant, and anxiolytic effects without influencing EtOH-induced place conditioning (Gabriel et al., 2004; Murphy et al., 2006) or motor incoordinating effects (Khisti et al., 2004). These inconsistent findings suggest that finasteride may affect alcohol-related behavioral or physiological responses with a strong GABAergic component. Since EtOH has been reported to have both a direct and indirect effect on GABAA receptor function (e.g., Sanna et al., 2004), with the indirect effect related to steroidogenesis, there may be important sex differences in sensitivity to finasteride’s effects on steroidogenesis and the interaction with EtOH sensitivity. Further studies are necessary to determine whether finasteride can differentially alter the development of physical dependence in male and female mice.
Sex differences in chronic EtOH withdrawal severity, measured by seizure susceptibility, document that the expression of withdrawal severity is lower and the recovery from withdrawal is faster in female than in male rats (reviewed in Devaud et al., 2003). The hippocampus is a key brain region that is activated during chronic EtOH withdrawal (Dave et al., 1990; Morgan et al., 1992), and chronic EtOH exposure and withdrawal produces significant alterations in the expression and peptide levels of several GABAA receptor subunits in the hippocampus of male rats (↓α1, ↑α4, ↑γ2, ↓δ; Matthews et al., 1998; Grobin et al., 2000; Cagetti et al., 2003). Whereas it is possible that chronic EtOH-induced alterations in expression of GABAA receptor subunits modify native GABAA receptor subunit assembly, composition of synaptic vs. extrasynaptic GABAA receptors, or phosphorylation state and trafficking of subunits (see Kumar et al., 2004; Liang et al., 2004), the relevance of these changes to the acute withdrawal model is not known. Since recent data suggests that GABAA receptors containing δ subunits are highly sensitive to low concentrations of EtOH (e.g., Sundstrom-Poromaa et al., 2002; Wallner et al., 2003; Wei et al., 2004) and that expression of the δ subunit fluctuates during the estrous cycle in female C57BL/6J mice (Maguire et al., 2005), it is possible that the plasticity of GABAAreceptors during the estrous cycle contributed to the present sex difference in the effect of finasteride on acute EtOH withdrawal severity.
Conclusions
It is important to note that finasteride’s decrease in acute EtOH withdrawal severity in male mice may have important implications for human men taking finasteride. That is, in men already taking finasteride for the treatment of benign prostatic hyperplasia and androgenetic alopecia, finasteride may not exacerbate withdrawal symptoms following alcohol consumption. The present findings, in conjunction with recent work indicating that finasteride decreased the subject effects of alcohol in humans (Pierucci-Lagha et al., 2005) and EtOH self-administration in C57BL/6J mice (Ford et al., 2005), may be particularly important for a cohort of men who have also been diagnosed with alcoholism. Further studies are necessary to examine the effect of finasteride on withdrawal-related symptoms in humans.
In conclusion, pretreatment with finasteride increased acute EtOH withdrawal severity in female C57BL/6J and DBA/2J mice but decreased withdrawal severity in male mice of both strains. Finasteride did not alter BECs, estradiol or corticosterone concentrations in manner that appeared to contribute to the HIC results. Collectively, these findings suggest that male and female C57BL/6J and DBA/2J mice may differ in their sensitivity to changes in levels of GABAergic neurosteroids during acute EtOH withdrawal. Although additional studies are necessary, sex differences in the modulation of GABAergic neurosteroids may represent a new therapeutic target in the treatment of alcohol dependence.
Acknowledgments
This research was supported by USPHS grants AA012439 and AA010760 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the Department of Veterans Affairs, a training grant stipend from NIAAA (T32 AA07468) and an N.L. Tartar Fellowship from OHSU. We thank Lauren Brown for analyzing the BEC samples, and Drs. John Crabbe and Charles Roselli for their comments and helpful suggestions.
Abbreviations
- ALLO
Allopregnanolone
- BEC
Blood ethanol concentration
- EtOH
Ethanol
- GABAA
γ-aminobutyric acidA
- HIC
Handling-induced convulsion
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barbaccia ML, Affricano D, Trabucchi M, Purdy RH, Colombo G, Agabio R, Gessa GL. Ethanol markedly increases “GABAergic” neurosteroids in alcohol-preferring rats. Eur J Pharmacol. 1999;384:R1–R2. doi: 10.1016/s0014-2999(99)00678-0. [DOI] [PubMed] [Google Scholar]
- Barbaccia ML, Serra M, Purdy RH, Biggio G. Stress and neuroactive steroids. Int Rev Neurobiol. 2001;46:243–272. doi: 10.1016/s0074-7742(01)46065-x. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: Endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–75. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Belelli D, Herd MB. The contraceptive agent Provera enhances GABAA receptor-mediated inhibitory neurotransmission in the rat hippocampus: Evidence for endogenousneurosteroids? J Neurosci. 2003;23:10013–10020. doi: 10.1523/JNEUROSCI.23-31-10013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Lan NC, Gee KW. Anticonvulsant steroids and the GABA/benzodiazepine receptor-chloride ionophore complex. Neurosci Biobehav Rev. 1990;14:315–322. doi: 10.1016/s0149-7634(05)80041-7. [DOI] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci USA. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Jr, Young ER, Kosobud A. Genetic correlations with ethanol withdrawal severity. Pharmacol Biochem Behav. 1983;18(Suppl 1):541–547. doi: 10.1016/0091-3057(83)90233-2. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Provisional mapping of quantitative trait loci for chronic ethanol withdrawal severity in BXD recombinant inbred mice. J Pharmacol Exp Ther. 1998;286:263–271. [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: A commentary. Neuropsychopharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Dave JR, Tabakoff B, Hoffman P. Ethanol withdrawal seizures produce increased c-fos mRNA in mouse brain. Mol Pharmacol. 1990;37:367–371. [PubMed] [Google Scholar]
- Dazzi L, Serra M, Seu E, Cherchi G, Pisu MG, Purdy RH, Biggio G. Progesterone enhances ethanol-induced modulation of mesocortical dopamine neurons: antagonism by finasteride. J Neurochem. 2002;83:1103–1109. doi: 10.1046/j.1471-4159.2002.01218.x. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Alele P, Ritu C. Sex differences in the central nervous system actions of ethanol. Crit Rev Neurobiol. 2003;15:41–59. doi: 10.1615/critrevneurobiol.v15.i1.20. [DOI] [PubMed] [Google Scholar]
- Faulkner TP, Cantleberry SB, Watts VJ, Hussain AS. Comparative pharmacokinetics of ethanol in inbred strains of mice using doses based on total body weight. Alcohol Clin Exp Res. 1990;14:82–86. doi: 10.1111/j.1530-0277.1990.tb00451.x. [DOI] [PubMed] [Google Scholar]
- Finn DA, Gee KW. The estrus cycle, sensitivity to convulsants and the anticonvulsant effect of a neuroactive steroid. J Pharmacol Exp Ther. 1994;271:164–170. [PubMed] [Google Scholar]
- Finn DA, Crabbe JC. Chronic ethanol differentially alters susceptibility to chemically induced convulsions in Withdrawal Seizure-Prone and -Resistant mice. J Pharmacol Exp Ther. 1999;288:782–790. [PubMed] [Google Scholar]
- Finn DA, Beadles-Bohling AS, Beckley EH, Ford MM, Gililland KR, Gorin-Meyer RE, Wiren KM. A new look at the 5α-reductase inhibitor finasteride. CNS Drug Rev. 2006;12:53–76. doi: 10.1111/j.1527-3458.2006.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Ford MM, Wiren KM, Roselli CE, Crabbe JC. The role of pregnane neurosteroids in ethanol withdrawal: behavioral genetic approaches. Pharmacol Ther. 2004a;101:91–112. doi: 10.1016/j.pharmthera.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Finn DA, Sinnott RS, Ford MM, Long SL, Tanchuck MA, Phillips TJ. Sex differences in the effect of ethanol injection and consumption on brain allopregnanolone levels in C57BL/6J mice. Neurosci. 2004b;123(4):813–9. doi: 10.1016/j.neuroscience.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Finn DA, Long SL, Tanchuck MA, Crabbe JC. Interaction of chronic ethanol exposure and finasteride: sex and strain differences. Pharmacol Biochem Behav. 2004c;78:435–443. doi: 10.1016/j.pbb.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Ford MM, Nickel JD, Finn DA. Treatment with and withdrawal from finasteride alters ethanol intake patterns in male C57BL/6J mice: potential role of endogenous neurosteroids? Alcohol. 2005;37:25–33. doi: 10.1016/j.alcohol.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel KI, Cunningham CL, Finn DA. Allopregnanolone does not influence ethanol-induced conditioned place preference in DBA/2J mice. Psychopharmacology. 2004;176:50–56. doi: 10.1007/s00213-004-1862-2. [DOI] [PubMed] [Google Scholar]
- Gasior M, Carter RB, Witkin JM. Neuroactive steroids: potential therapeutic use in neurological and psychiatric disorders. Trends Pharmacol Sci. 1999;20:107–112. doi: 10.1016/s0165-6147(99)01318-8. [DOI] [PubMed] [Google Scholar]
- Gee KW, Bolger MB, Brinton RE, Coirini H, McEwen BS. Steroid modulation of the chloride ionophore in rat brain: structure-activity requirements, regional dependence and mechanism of action. J Pharmacol Exp Ther. 1988;246:803–812. [PubMed] [Google Scholar]
- Grisel JE, Metten P, Wenger CD, Merrill CM, Crabbe JC. Mapping of quantitative trait loci underlying ethanol metabolism in BXD recombinant inbred mouse strains. Alcohol Clin Exp Res. 2002;26:610–616. [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol. Psychopharmacology. 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Papadeas ST, Morrow AL. Regional variations in the effects of chronic ethanol administration on GABAA receptor expression: potential mechanisms. Neurochem Int. 2000;37:453–461. doi: 10.1016/s0197-0186(00)00058-9. [DOI] [PubMed] [Google Scholar]
- Hirani K, Khisti RT, Chopde CT. Behavioral action of ethanol in Porsolt’s forced swim test: modulation by 3alpha-hydroxy-5alpha-pregnan-20-one. Neuropharmacology. 2002;43:1339–1350. doi: 10.1016/s0028-3908(02)00330-1. [DOI] [PubMed] [Google Scholar]
- Hirani K, Sharma AN, Jain NS, Ugale RR, Chopde CT. Evaluation of GABAergic neuroactive steroid 3α-hydroxy-5α-pregnan-20-one as a neurobiological substrate for the anti-anxiety effect of ethanol in rats. Psychopharmacology. 2005;180:267–278. doi: 10.1007/s00213-005-2169-7. [DOI] [PubMed] [Google Scholar]
- Jung ME, Yang SH, Brun-Zinkernagel AM, Simpkins JW. Estradiol protects against cerebellar damage and motor deficit in ethanol-withdrawn rats. Alcohol. 2002;26:83–93. doi: 10.1016/s0741-8329(01)00199-9. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ. Ethanol-related traints in mice selectively bred for differential sensitivity to methamphetamine-induced activation. Behav Neurosci. 2006;120:1356–1366. doi: 10.1037/0735-7044.120.6.1356. [DOI] [PubMed] [Google Scholar]
- Karst H, de Kloet ER, Joëls M. Episodic corticosterone treatment accelerates kindling epileptogenesis and triggers long-term changes in hippocampal CA1 cells, in the fully kindled state. Eur J Neurosci. 1999;11:889–898. doi: 10.1046/j.1460-9568.1999.00495.x. [DOI] [PubMed] [Google Scholar]
- Keith LD, Winslow JR, Reynolds RW. A general procedure for estimation of corticosteroid response in individual rats. Steroids. 1978;31:523–531. doi: 10.1016/0039-128x(78)90034-x. [DOI] [PubMed] [Google Scholar]
- Khisti RT, VanDoren MJ, Matthews DB, Morrow AL. Ethanol-induced elevation of 3α-hydroxy-5α-pregnan-20-one does not modulate motor incoordination in rats. Alcohol Clin Exp Res. 2004;28:1249–1256. doi: 10.1097/01.alc.0000134232.44210.06. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BJ, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- Kumar S, Fleming RL, Morrow AL. Ethanol regulation of γ-aminobutyric acidA receptors: genomic and nongenomic mechanisms. Pharmacol Ther. 2004;101:211–226. doi: 10.1016/j.pharmthera.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- Liang J, Cagetti E, Olsen RW, Spigelman I. Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. J Pharmacol Exp Ther. 2004;310:1234–1245. doi: 10.1124/jpet.104.067983. [DOI] [PubMed] [Google Scholar]
- Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nature Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Devaud LL, Fritschy JM, Sieghart W, Morrow AL. Differential regulation of GABAA receptor gene expression by ethanol in the rat hippocampus versus cerebral cortex. J Neurochem. 1998;70:1160–1166. doi: 10.1046/j.1471-4159.1998.70031160.x. [DOI] [PubMed] [Google Scholar]
- Morgan PF, Nadi NS, Karanian J, Linnoila M. Mapping rat brain structures activated during ethanol withdrawal: role of glutamate and NMDA receptors. Eur J Pharmacol. 1992;225:217–223. doi: 10.1016/0922-4106(92)90023-o. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, Grant KA. Neurosteroids mediate pharmacological effects of ethanol: A new mechanism of ethanol action? Alcohol Clin Exp Res. 1999;23:1933–1940. doi: 10.1111/j.1530-0277.1999.tb04094.x. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987;142:483–485. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Sakoori K, Okabe C. Lack of evidence of a role for the neurosteroid allopregnanolone in ethanol-induced reward and c-fos expression in DBA/2 mice. Brain Res. 2006;1094:107–118. doi: 10.1016/j.brainres.2006.03.109. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Pierucci-Lagha A, Covault J, Feinn R, Nellissery M, Hernandez-Avila C, Oncken Morrow AL, Kranzler HR. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30:1193–203. doi: 10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS. Testosterone modulation of seizure susceptibility is mediated by neurosteroids 3α-androstanediol and 17β-estradiol. Neurosci. 2004;129:195–207. doi: 10.1016/j.neuroscience.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Rewal M, Jung ME, Wen Y, Brun-Zinkernagel AM, Simpkins JW. Role of the GABAA system in behavioral, motoric, and cerebellar protection by estrogen during ethanol withdrawal. Alcohol. 2003;31:49–61. doi: 10.1016/j.alcohol.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Rivier C. Female rats release more corticosterone than males in response to alcohol: influence of circulating sex steroids and possible consequences for blood alcohol levels. Alcohol Clin Exp Res. 1993;17:854–859. doi: 10.1111/j.1530-0277.1993.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Roach MK, Creaven PJ. A micro-method for the determination of acetaldehyde and ethanol in blood. Clin Chim Acta. 1968;21:275–278. doi: 10.1016/0009-8981(68)90138-1. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Crabbe JC, Keith LD. Genetic differences in hypothalamic-pituitary-adrenal axis responsiveness to acute ethanol and acute ethanol withdrawal. Brain Res. 1992;596:296–302. doi: 10.1016/0006-8993(92)90064-g. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Crabbe JC, Keith LD. Corticosterone increases severity of acute withdrawal from ethanol, pentobarbital, and diazepam in mice. Psychopharmacology. 1994;115:278–284. doi: 10.1007/BF02244784. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Keith LD. Corticosteroids enhance convulsion susceptibility via central mineralocorticoid receptors. Psychoneuroendocrinology. 1995;20:891–902. doi: 10.1016/0306-4530(95)00016-x. [DOI] [PubMed] [Google Scholar]
- Romeo E, Brancati A, De Lorenzo A, Fucci P, Furnari C, Pompili E, Sasso GF, Spalletta G, Troisi A, Pasini A. Marked decrease of plasma neuroactive steroids during alcohol withdrawal. Clin Neuropharmacology. 1996;19:366–369. doi: 10.1097/00002826-199619040-00011. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci. 2004;24:6521–6530. doi: 10.1523/JNEUROSCI.0075-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen EH, Harland RD, Crabbe JC, Phillips TJ. Bidirectional selective breeding for ethanol effects on locomotor activity: Characterization of FAST and SLOW mice through selection generation 35. Alcohol Clin Exp Res. 1995;19:1234–1245. doi: 10.1111/j.1530-0277.1995.tb01606.x. [DOI] [PubMed] [Google Scholar]
- Stuart JD, Lee FW, Noel DS, Kadwell SH, Overton LK, Hoffman CR, Kost TA, Tippin TK, Yeager RL, Batchelor KW, Bramson HN. Pharmacokinetic parameters and mechanisms of inhibition of rat type 1 and 2 steroid 5α-reductases: determinants for different in vivo activities of GI198745 and finasteride in the rat. Biochem Pharmacol. 2001;62:933–942. doi: 10.1016/s0006-2952(01)00728-6. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nature Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed F, Khosla S. Mechanisms of sex steroid effects in bone. Biochem Biophy Res Comm. 2005;328:688–696. doi: 10.1016/j.bbrc.2004.11.097. [DOI] [PubMed] [Google Scholar]
- VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment tonic inhibition mediated by δ subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


