Abstract
A common pathology in heart failure is a detrimental change in the mechanics of both contraction and filling. In familial hypertrophic cardiomyopathy, a genetic disease characterized by left ventricular hypertrophy and myofiber disarray, left ventricular diastolic dysfunction is common and contributes to congestive heart failure. In dilated cardiomyopathy, a common correlate to reduced wall thickening and increased chamber volume is an asynchronous activation of the left ventricle due to left bundle branch block. Local measures of the timing and magnitude of myocardial shortening and relaxation can be obtained with magnetic resonance (MR) tissue tagging, MR cine phase contrast, or MR cine displacement encoding. In familial hypertrophic cardiomyopathy, these methods have been shown to quantify the restrictive filling of the ventricle. Characterizing the regions of the failing heart which are activated late has allowed investigators to measure the change in protein expression in those regions compared to normal myocardium. Also, these MR imaging methods have led to a better quantification of the asynchronous activation in dilated cardiomyopathy, which can be used to predict response to resynchronization therapy with pacing.
Keywords: Heart failure, Tagging, Myocardium, Function, MRI
Introduction
Heart failure is the leading cause of hospitalizations among the elderly. It is estimated that just less than 5 million people in the US have heart failure. Heart failure comprises a broad spectrum of pathologies in which the heart undergoes changes in its geometrical dimensions and its pump function is reduced. This can involve myocardium, which is too thick and stiff in the case of hypertrophic cardiomyopathy (HCM) or is too thin in the case of dilated cardiomyopathy (DCM). In both cases, the heart experiences a reduction in pump function due to a poor geometry and/or asynchronous activation of the myocardium. The myocardium itself may be compromised by the infiltration of fibrous tissue secondary to previous infarct, or due to idiopathic etiology.
When evaluating the patient with heart failure, it is necessary to measure the geometry of the heart, the mechanics of the contracting ventricles, hemodynamics, and the classification of the myocardium as viable or nonviable. Magnetic resonance imaging (MRI) can fill all of these needs. Methods currently exist for the precise measurement of local 3-dimensional myocardial motion noninvasively with MRI tagging.1 From these motion estimates, strain images representing the local deformation of the myocardium can be formed to show local myocardial contraction.2,3 These images clearly show the sequence of mechanical events during the activation and relaxation of the heart, making them ideal to visualize abnormalities caused by asynchronous electrical activation or ischemia. Flow imaging can be used for measuring hemodynamics5 and late enhancement Gd-diethylenetriamine pentaacetic acid imaging can be used to highlight nonviable myocardium.6-8
In this article, we will focus our attention on the ability of MRI to measure the mechanical properties of the failing heart.
Measuring mechanics with MRI
Magnetic resonance imaging has made significant contributions to the understanding of myocardial mechanics through the use of numerous “tagging” methods for tracking myocardial motion noninvasively.1 In MRI tagging, a set of saturation pulses placed in the tissue provide a spatially varying signal intensity pattern that is an intrinsic part of the tissue; the change in shape of the intensity pattern in the image reflects the change in shape of the underlying body containing the intensity pattern.9-11 Although these methods were initially difficult to perform in large patient populations, they are now available on almost all commercial MRI scanners, and the advent of new processing algorithms has made it possible to analyze the data in a reasonable time.12 This has led to the use of tagging in large clinical trials.13,14 Recent work on alternative motion-tracking methods has also opened up opportunities of high-resolution imaging of myocardial motion,15,16 but these have yet to be used in large clinical trials. Reviews of these techniques exist,17,18 so we will not cover the details here. Fig. 1 shows a short axis slice of the heart at 2 time points in the heart cycle: just after the electrocardiogram trigger and at end-systole. The ability to measure local mechanics from the deformation of the tagging pattern is very clear.
Fig. 1.
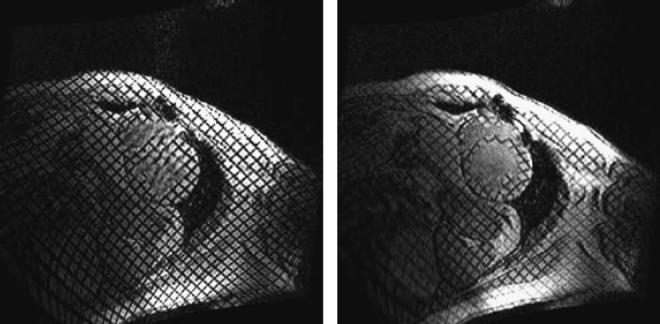
Two images of a short axis slice in a heart that has been tagged with a grid pattern. The left image shows the heart just after electrocardiogram detection and tag pattern application. The right picture shows the mechanical deformation of the myocardium close to end-systole. Note the severe deformation of the thinned septum away from the center of the left ventricle indicating “paradoxical” systolic stretching in this region of myocardium. These lines can be tracked and the regional circumferential shortening calculated.
Measuring mechanics in HCM
Hypertrophic cardiomyopathy is a condition in which the myocardium becomes too thick, thereby impairing function and sometimes obstructing the outflow tract in the left ventricle. Hypertrophic cardiomyopathy was investigated early in the development of tagging techniques.19 Young et al20 found increased twist in HCM, decreased excursion of valve plane toward the apex, and reduced shortening especially in the basal septum. Kramer et al21 found that circumferential shortening was less in patients with HCM than in control subjects in the septal (13% ± 5% vs 24% ± 6%, P = .0002), inferior (13% ± 5% vs 21% ± 4%, P = .001), and anterior (17% ± 5% vs 21% ± 3%, P < .03) regions, but not in the lateral region. Circumferential end-systolic shortening was reduced in patients with HCM compared with control subjects at all levels from apex to base.
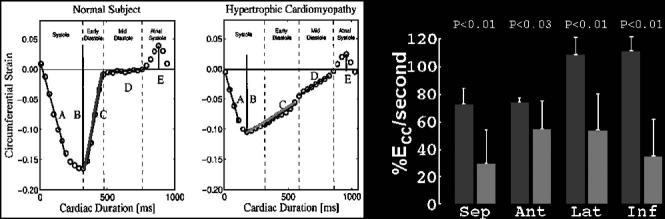
Recent work by Ennis et al22 that focused on creating tag patterns that could be tracked throughout the entire cardiac cycle has shown some interesting differences in the temporal characteristics of strain in HCM vs normals. Using complementary spatial modulation of magnetization23 and extended temporal sampling with cardiac phase to order reconstruction,24 we compared the slow relaxation of the HCM ventricle with normals as shown in Fig. 2.
Fig. 2.
The circumferential strain vs time in a normal volunteer and in a patient with HCM.22 The primary feature that is different is the rapid relaxation of the normal left ventricle during early diastole vs the slow relaxation of the left ventricle with HCM. This feature is quantified in the figure with percentage circumferential strain per second (% Ecc/s). The mean value of this strain rate from 8 patients and 6 normals is shown in the bar graph on the right (normals are the dark gray bars; patients with HCM, light gray bars). Figure adapted from the PhD thesis of Daniel Ennis, Johns Hopkins University, with permission.
Measuring mechanics in DCM (asynchronous activation)
The relationship between asynchronous electrical excitation and the onset of mechanical contraction has been investigated with MRI tagging.4,25-28 These MRI methods are ideal for measuring the nature of mechanical asynchrony found in some patients with DCM, especially those who are candidates for resynchronization therapy.29-31 Fig. 3 shows an example of the evolution of strain in a normal left ventricle vs a patient in end-stage DCM with left bundle branch block (LBBB).
Fig. 3.
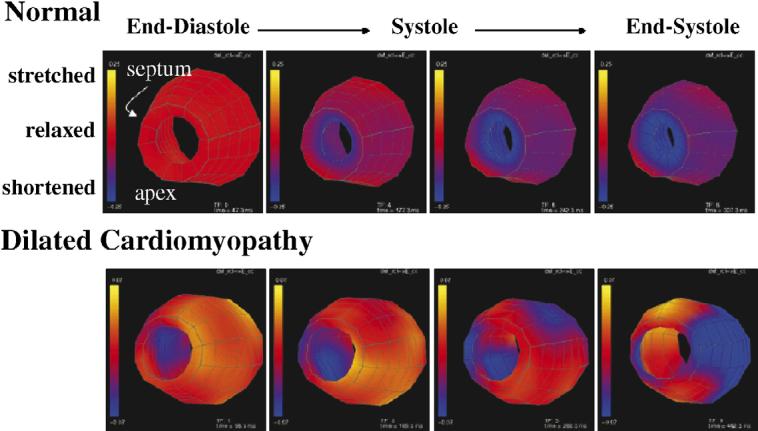
These colorized surfaces show the circumferential stretch (yellow) and contraction (blue) of the midwall of a normal human left ventricle (top) and a left ventricle of a patient with DCM (bottom). The apex is toward the viewer and the free wall is on the right side. Four time frames are shown from the beginning of systole through end-systole. Note the early contraction of the septum and late contraction of the free wall in the patient.
Pacing the left ventricle with either single site or biventricular (BiV) pacing has been shown to improve cardiac function in some patients with DCM.32However, establishing the criteria for which patients will respond best to this therapy is ongoing. Mechanical imaging with magnetic resonance has offered some insight into this problem33 as shown in Fig. 3. Nelson et al34 showed that a mechanical dyssynchrony index which quantified the strain variance at the time of maximal shortening did correlate with response to pacing much better than QRS duration.
The relative efficacy of BiV and left ventricular (LV) pacing has also been studied using MRI tagging techniques by Leclerq et al.35 A similar degree of systolic function improvement was found in a canine model of LBBB-failing hearts, despite radically different electrical activation pattern from the 2 pacing protocols. Epicardial electrical mapping, tagged MRI, and hemodynamics were obtained in dogs with LBBB-failing hearts during right atrial, LV, and BiV stimulation. Biventricular and LV stimulation both significantly improved chamber hemodynamics (eg, 25% increase in dP/dtmax and aortic pulse pressure) compared with atrial pacing-LBBB. The functional improvement correlated with mechanical resynchronization as quantified by MRI tagging techniques. Paradoxically, the electrical dispersion decreased 13% with BiV but increased 23% with LV pacing (P < .01). It was concluded that improved mechanical synchrony and function do not require electrical synchrony. Mechanical coordination was the most important factor for systolic improvement with either BiV- or LV-only pacing.
Discussion
Precise measurements of cardiac mechanics in heart failure have shown that knowledge of the mechanics can predict the efficacy of resynchronization therapy, whereas the QRS interval does not seem to be useful. It should be noted that beyond mechanics, late Gd enhancement is able to accurately differentiate coronary artery disease from non-coronary artery disease etiology of heart failure.36 Also, chronic mechanical asynchrony will have an effect on the myocardial substrate, possibly increasing the probability of lethal arrhythmias.37 Magnetic resonance imaging is an excellent imaging modality to understand heart failure and help guide appropriate therapy.
Acknowledgments
Many of the results and conclusions reported here are from the collaborative efforts of the Cardiac MRI Research Group at Johns Hopkins and the Medical Imaging Group at NHLBI. The authors would especially like to acknowledge the efforts of Michael Guttman, Joni Taylor, Dan Ennis, Owen Faris, Brad Wyman, Greg Nelson, Cecilia Curry, Christoph Leclerq, Andrew Arai, and David Kass.
References
- 1.Ozturk C, Derbyshire JA, McVeigh ER. Estimating motion from MRI data. Proc IEEE. 2003;91:1627. doi: 10.1109/JPROC.2003.817872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozturk C, McVeigh ER. Measurement of cardiac deformations from MRI; physical and mathematical models. Kluwer Academic Publishers; New York: 2001. Motion analysis of the whole heart; p. 91. [Google Scholar]
- 3.Declerck J, Denney TS, Ozturk C, O’Dell W, McVeigh ER. Left ventricular motion reconstruction from planar tagged MR images: a comparison. Phys Med Biol. 2000;45:1611. doi: 10.1088/0031-9155/45/6/315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McVeigh ER, Prinzen FW, Wyman BT, Tsitlik JE, Halperin HR, Hunter WC. Imaging asynchronous mechanical activation of the paced heart with tagged MRI. Magn Reson Med. 1998;39:507. doi: 10.1002/mrm.1910390402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pelc NJ, Herfkens RJ, Shimakawa A, Enzmann DR. Phase contrast cine magnetic resonance imaging [review] Magn Reson Q. 1991;7:229. [PubMed] [Google Scholar]
- 6.Kim RJ, Wu E, Rafael A, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343:1445. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- 7.Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002;47:372. doi: 10.1002/mrm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kellman P, Chung YC, Simonetti OP, McVeigh ER, Arai AE. Multi-contrast delayed enhancement provides improved contrast between myocardial infarction and blood pool. J Magn Reson Imaging. 2005;22:605. doi: 10.1002/jmri.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP. Human heart: tagging with MR imaging—a method for noninvasive assessment of myocardial motion. Radiology. 1988;169:59. doi: 10.1148/radiology.169.1.3420283. [DOI] [PubMed] [Google Scholar]
- 10.Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology. 1989;171:841. doi: 10.1148/radiology.171.3.2717762. [DOI] [PubMed] [Google Scholar]
- 11.McVeigh ER, Atalar E. Cardiac tagging with breath-hold cine MRI. Magn Reson Med. 1992;28:318. doi: 10.1002/mrm.1910280214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Osman NF, Kerwin WS, McVeigh ER, Prince JL. Cardiac motion tracking using CINE harmonic phase (HARP) magnetic resonance imaging. Magn Reson Med. 1999;42:1048. doi: 10.1002/(sici)1522-2594(199912)42:6<1048::aid-mrm9>3.0.co;2-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edvardsen T, Rosen BD, Pan L, et al. Regional diastolic dysfunction in individuals with left ventricular hypertrophy measured by tagged magnetic resonance imaging—the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2006;151:109. doi: 10.1016/j.ahj.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 14.Rosen BD, Saad MF, Shea S, et al. Hypertension and smoking are associated with reduced regional left ventricular function in asymptomatic: individuals the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;47:1150. doi: 10.1016/j.jacc.2005.08.078. [DOI] [PubMed] [Google Scholar]
- 15.Aletras AH, Ding S, Balaban RS, Wen H. DENSE: displacement encoding with stimulated echoes in cardiac functional MRI. J Magn Reson. 1999;137:247. doi: 10.1006/jmre.1998.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aletras AH, Arai AE. meta-DENSE complex acquisition for reduced intravoxel dephasing. J Magn Reson. 2004;169:246. doi: 10.1016/j.jmr.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 17.McVeigh ER. MRI of myocardial function: motion tracking techniques. Magn Reson Imaging. 1996;14:137. doi: 10.1016/0730-725x(95)02009-i. [DOI] [PubMed] [Google Scholar]
- 18.McVeigh E, Faris O, Ennis D, Helm P, Evans F. Electromechanical mapping with MRI tagging and epicardial sock electrodes. J Electrocardiol. 2002;35(Suppl):61. doi: 10.1054/jelc.2002.37156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maier SE, Fischer SE, McKinnon GC, Hess OM, Krayenbuehl HP, Boesiger P. Evaluation of left ventricular segmental wall motion in hypertrophic cardiomyopathy with myocardial tagging. Circulation. 1992;86:1919. doi: 10.1161/01.cir.86.6.1919. [DOI] [PubMed] [Google Scholar]
- 20.Young AA, Kramer CM, Ferrari VA, Axel L, Reichek N. Three-dimensional left ventricular deformation in hypertrophic cardiomyopathy. Circulation. 1994;90:854. doi: 10.1161/01.cir.90.2.854. [DOI] [PubMed] [Google Scholar]
- 21.Kramer CM, Reichek N, Ferrari VA, Theobald T, Dawson J, Axel L. Regional heterogeneity of function in hypertrophic cardiomyopathy. Circulation. 1994;90:186. doi: 10.1161/01.cir.90.1.186. [DOI] [PubMed] [Google Scholar]
- 22.Ennis DB, Epstein FH, Kellman P, Fananapazir L, McVeigh ER, Arai AE. Assessment of regional systolic and diastolic dysfunction in familial hypertrophic cardiomyopathy using MR tagging. Magn Reson Med. 2003;50:638. doi: 10.1002/mrm.10543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer SE, McKinnon GC, Maier SE, Boesiger P. Improved myocardial tagging contrast. Magn Reson Med. 1993;30:191. doi: 10.1002/mrm.1910300207. [DOI] [PubMed] [Google Scholar]
- 24.Feinstein JA, Epstein FH, Arai AE, Foo TK, Hartley MR, Balaban RS, et al. Using cardiac phase to order reconstruction (CAPTOR): a method to improve diastolic images. J Magn Reson Imaging. 1997;7:794. doi: 10.1002/jmri.1880070505. [DOI] [PubMed] [Google Scholar]
- 25.Wyman BT, Hunter WC, Prinzen FW, McVeigh ER. Mapping propagation of mechanical activation in the paced heart with MRI tagging. Am J Physiol. 1999;276:H881. doi: 10.1152/ajpheart.1999.276.3.H881. [DOI] [PubMed] [Google Scholar]
- 26.Hunter GJ, Hamberg LM, Choi N, Jain RK, McCloud T, Fischman AJ. Dynamic T1-weighted magnetic resonance imaging and positron emission tomography in patients with lung cancer: correlating vascular physiology with glucose metabolism. Clin Cancer Res. 1998;4:949. [PubMed] [Google Scholar]
- 27.Wyman BT, Hunter WC, Prinzen FW, Faris OP, McVeigh ER. Effects of single- and biventricular pacing on temporal and spatial dynamics of ventricular contraction. Am J Physiol Heart Circ Physiol. 2002;282:H372. doi: 10.1152/ajpheart.2002.282.1.H372. [DOI] [PubMed] [Google Scholar]
- 28.Faris OP, Evans FJ, Ennis DB, et al. Novel technique for cardiac electromechanical mapping with magnetic resonance imaging tagging and an epicardial electrode sock. Ann Biomed Eng. 2003;31:430. doi: 10.1114/1.1560618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lardo AC, Abraham TP, Kass DA. Magnetic resonance imaging assessment of ventricular dyssynchrony: current and emerging concepts. J Am Coll Cardiol. 2005;46:2223. doi: 10.1016/j.jacc.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Bax JJ, Abraham T, Barold SS, et al. Cardiac resynchronization therapy: Part 2. Issues during and after device implantation and unresolved questions. J Am Coll Cardiol. 2005;46:2168. doi: 10.1016/j.jacc.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 31.Bax JJ, Abraham T, Barold SS, et al. Cardiac resynchronization therapy: Part 1. Issues before device implantation. J Am Coll Cardiol. 2005;46:2153. doi: 10.1016/j.jacc.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 32.Kass DA, Chen CH, Curry C, et al. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999;99:1567. doi: 10.1161/01.cir.99.12.1567. [DOI] [PubMed] [Google Scholar]
- 33.Curry CW, Nelson GS, Wyman BT, et al. Mechanical dyssynchrony in dilated cardiomyopathy with intraventricular conduction delay as depicted by 3D tagged magnetic resonance imaging. Circulation. 2000;101:E2. doi: 10.1161/01.cir.101.1.e2. [DOI] [PubMed] [Google Scholar]
- 34.Nelson GS, Curry CW, Wyman BT, et al. Predictors of systolic augmentation from left ventricular preexcitation in patients with dilated cardiomyopathy and intraventricular conduction delay. Circulation. 2000;101:2703. doi: 10.1161/01.cir.101.23.2703. [DOI] [PubMed] [Google Scholar]
- 35.Leclercq C, Faris O, Tunin R, et al. Systolic improvement and mechanical resynchronization does not require electrical synchrony in the dilated failing heart with LBBB. Circulation. 2002;106:1760. doi: 10.1161/01.cir.0000035037.11968.5c. [DOI] [PubMed] [Google Scholar]
- 36.Casolo G, Minneci S, Manta R, et al. Identification of the ischemic etiology of heart failure by cardiovascular magnetic resonance imaging: diagnostic accuracy of late gadolinium enhancement. Am Heart J. 2006;151:101. doi: 10.1016/j.ahj.2005.03.068. [DOI] [PubMed] [Google Scholar]
- 37.Spragg DD, Leclercq C, Loghmani M, et al. Regional alterations in protein expression in the dyssynchronous failing heart. Circulation. 2003;108:929. doi: 10.1161/01.CIR.0000088782.99568.CA. [DOI] [PubMed] [Google Scholar]