Abstract
Minimally invasive cardiac surgery requires arresting and emptying of the heart, which compromises visualization of the surgical field. In this feasibility study a novel surgical procedure is demonstrated in which real-time MRI is used to guide the placement of a prosthetic aortic valve in the beating heart via direct apical access in eight porcine hearts. A clinical stentless bioprosthetic valve affixed to a platinum stent was compressed onto a balloon-tipped catheter. This was fed through a 15-18-mm delivery port inserted into the left ventricular (LV) apex via a minimally invasive subxyphoid incision. Using interactive real-time MRI, the surgeon implanted the prosthetic valve in the correct location at the aortic annulus within 90 s. In four of the animals immediately after implantation, ventricular function, blood flow through the valve, and myocardial perfusion were evaluated with MRI. MRI-guided beating-heart surgery may provide patients with a less morbid and more durable solution to structural heart disease.
Keywords: surgery, valves, imaging, real-time, cardiac
Recently there has been a focus on using minimally invasive approaches in cardiac surgery in an effort to reduce trauma and speed recovery for the patient (1-3). In valve surgery, incisions have been shortened and robotics employed to achieve this goal; however, these approaches still require arresting and emptying of the heart to allow visualization. Real-time MRI techniques previously developed for intravascular guidance (4,5) are immediately applicable to provide vision in the surgical field, even in a beating heart with circulating blood, to guide the surgeon.
A new generation of short (120 cm), wide-bore (70 cm) 1.5T imaging systems (Magnetom Espree, Siemens Medical Solutions) has recently been introduced. This magnet design gives a clearance of up to 30 cm above the chest of the supine patient, and the short design allows a surgeon to directly manipulate thoracoscopic instruments within the chest with ample “attack angles” and degrees of freedom. The more open bore allows better access to the patient for anesthesia when imaging the heart. While MR-guided therapeutic interventions have been pursued extensively (6,7), most MR systems used in these applications to date have not been designed for the high-performance real-time scanning required for guiding cardiac procedures. The imaging gradients and amplifiers of the new systems yield a scanning performance that rivals that of the standard cardiac MR scanners, and therefore high-quality images can be obtained with real-time acquisition speeds. Imaging techniques used for intravascular MR guidance are immediately applicable to minimally invasive surgical procedures. The excellent blood/tissue contrast and the ability to interactively adjust imaging planes to view devices and the beating heart from multiple simultaneous viewpoints makes real-time MR ideal for guiding cardiac surgical interventions.
The key to any interventional treatment is achieving the correct balance between risk and benefit. In the surgical treatment of aortic valve disease, the morbidity of the operation is balanced by the durability of the result. Similarly, the development of percutaneous therapies (8) is driven by the size and shape of the arteries for catheter access, and the miniaturization of devices that may reduce their performance and durability. Interventional MRI has been shown to be successful at guiding percutaneous delivery of prosthetic aortic valves (9). Full exposure of the surgical field provides superior visibility and instrument access, but carries a greater risk of complications and trauma along with increased recovery time. Ideally, one could combine the best device with the best imaging technique, such as MRI, to eliminate the need for a cardiopulmonary bypass and decrease the risk of the procedure without sacrificing the durability of the surgery.
In this paper we describe the first real-time MRI-guided cardiac surgery procedure: the implantation of an aortic valve in the beating heart through direct LV apical access.
MATERIALS AND METHODS
Real-Time Interactive Scanner Control
A fully interactive, real-time MRI system was previously developed for intravascular interventions (10-14). The real-time MRI system consists of an interactive user interface, an in-room display, specialized pulse sequences, and specialized image reconstruction software (10). A custom computer is connected to the commercial scanner through a gigabit Ethernet port. With this system, multiple oblique planes can be imaged and displayed simultaneously at their respective 3D locations. The rendering may be rotated on the in-room display to match the orientation of the patient, and this feature is essential for monitoring the trajectory of a device through the body. Slices may be repositioned and turned on or off as needed. The MRI tissue contrast can be interactively changed by toggling saturation pulses on or off to highlight selected objects. During the valve placement described in this work, a steady-state free precession (SSFP) sequence was used with the following scanning parameters: TR = 3.5 ms, TE = 1.75 ms, imaging flip angle = 35-45°, slice thickness = 7 mm, field of view (FOV) = 340 mm × 255 mm, and matrix = 192 × 108, with 3/4 partial Fourier acquisition in the phase direction. Imaging artifacts were kept to a minimum by the following methods: The temporal resolution was set high enough to avoid significant motion blurring. If a phase-encoding “wrap-around” artifact interfered with the surgical FOV, the orientation was rotated or the posterior coils were turned off. The materials of the valve, delivery device, and stent were tested to ensure that field distortions did not occur.
Surgical Preparation and Devices
All experiments were performed under protocols approved by the NIH Animal Care and Use Committee. With the use of general endotracheal anesthesia, eight domestic Yorkshire swine (45-55 kg) underwent a preprocedure MRI scan to determine the aortic annular diameter, coronary ostial anatomy, and apical location. Via a small subxyphoid incision, the pericardium was opened and the apex of the heart was exposed. Two concentric pursestring sutures were placed around the apex, through which an 15-18 mm trocar was inserted into the left ventricle (LV) to create direct access to the aortic valve.
The delivery device has a 1/4“ central channel for a guidewire-directed NuMed BiB (24 mm OD × 35 mm long) balloon-tipped catheter. Compressed onto the balloon is a 21-25-mm St. Jude Toronto SPV stentless bioprosthetic valve that is affixed to an similarly sized NuMed Cheatham Platinum 28-mm 8-zig platinum-iridium stent (Fig. 1a and b). The valve and its orientation are protected by a sheath that covers the valve, balloon, and catheter (Fig. 1c). A 16-mm stabilizing rod allows the prosthetic valve to be advanced through the sheath (Fig. 1d). During the procedure the animals were monitored for electrocardiographic rhythm, O2 saturation, end tidal CO2, and instantaneous arterial and ventricular blood pressure.
FIG. 1.
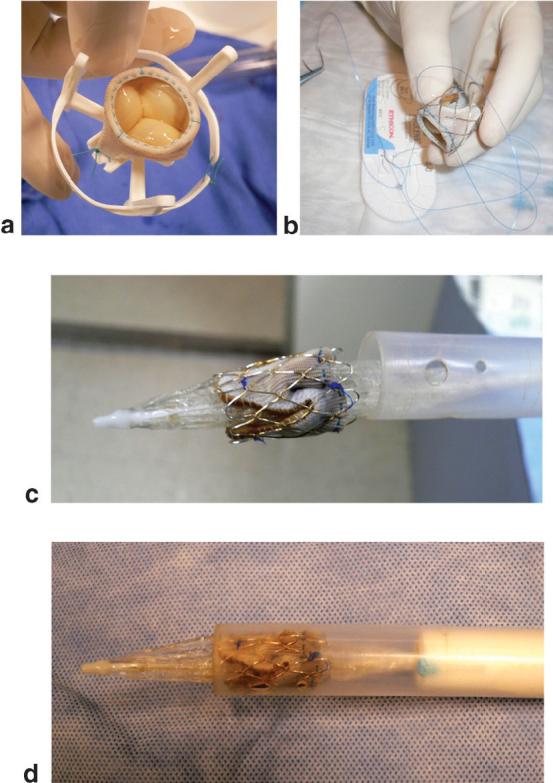
a: Valve before the stent is fastened. b: Valve sewn inside the platinum stent. c: Stent valve on a balloon catheter. d: Prosthetic stent-valve loaded into the delivery device. The blue dot on the shaft of the delivery device is an orientation marker that is visible under MRI.
Preparatory Imaging
Before the stent-valve was inserted, 10-15 min of imaging was performed to determine the orientation of the heart, evaluate ventricular and valve function, locate the native valve annulus and the origin of the coronary arteries, and set up scan planes to be used for the myocardial perfusion and aortic flow imaging performed after the valve was inserted. Once the trocar was in place, the body matrix coil array was placed over the chest and the MR patient table was advanced to move the animal into the imaging volume.
A series of single-shot axial images were acquired in three orthogonal planes to localize the heart and the orientation of the trocar. Using the central sagittal plane through the heart, a series of contiguous axial planes were prescribed. From these axial planes a three-point tool was used to define an imaging plane that passed through the apex, the aortic valve, and the ascending aorta. A cine acquisition in this plane and planes a few millimeters adjacent showed the level of the aortic valve ring as a dark line, and this position was marked as the location for the base of the stent valve (see Fig. 2). An imaging plane orthogonal to this was obtained to view the valve in axial cross section. This view was used to measure blood flow velocity through the valve and determine the location of the coronary arteries, as shown in Fig. 3.
FIG. 2.
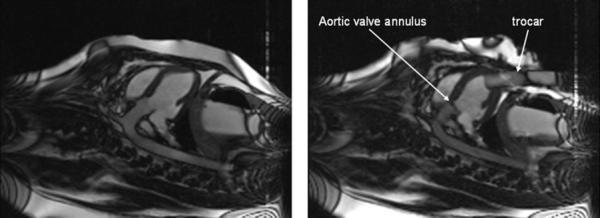
Long-axis view of the porcine heart before (left) and after (right) insertion of the trocar. The position of the aortic valve annulus is obvious as the dark line dividing the LV chamber from the aorta.
FIG. 3.
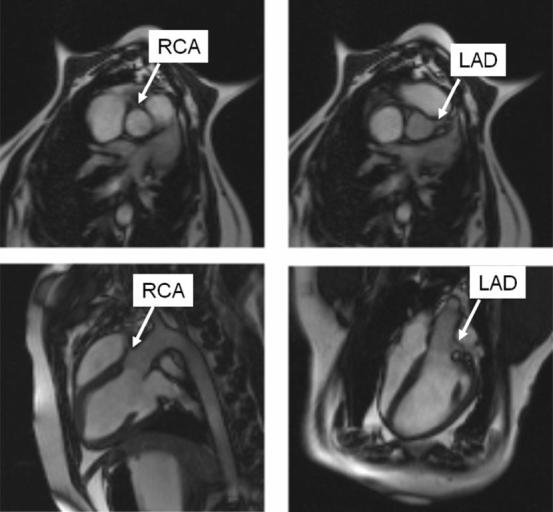
Four views of the root of the aorta used to find the locations of the coronary ostia. The top row shows two parallel short-axis views to obtain the angle of the ostia, and the bottom row shows two long-axis views to obtain the distance of the ostia from the valve plane.
Real-Time Image-Guided Valve Implantation
Figure 4 shows the position of the surgeon at the front edge of the MRI system. He is equipped with headphones and a microphone to talk with the person operating the scanner (Magnacoustics, Atlantic Beach, NY, USA). Figure 5 shows what the surgeon sees during the progress of the valve implantation. The still images in Fig. 5 are single frames extracted from the continuous real-time movie that is playing on the screen shown in Fig. 4. Initially a nitinol guidewire is advanced through the trocar across the native aortic valve. Then the prosthetic stent-valve on the delivery device is advanced through the apical trocar while its location is observed by real-time MRI. The prosthetic valve is then advanced on the balloon along the guidewire, and positioned across the native valve in proper position with respect to the coronary artery ostia. The balloon is inflated using 1% diluted gadopentate dimeglumine (Gd-DTPA, Magnavist; Berlex, Inc.) to implant the prosthetic valve under observation with real-time MRI. The balloon is then deflated and removed through the trocar. At this point the ventricular function resumes and is visualized immediately by real-time MRI.
FIG. 4.
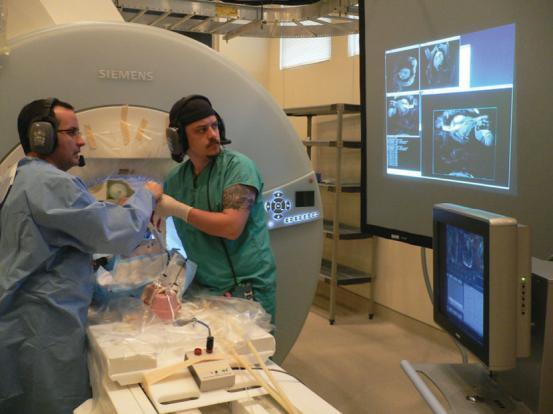
Using real-time MRI on the projection screen, the surgeon advances the delivery device into the LV, just proximal to the aortic root. He then advances the prosthetic stent-valve mounted on the balloon catheter.
FIG. 5.
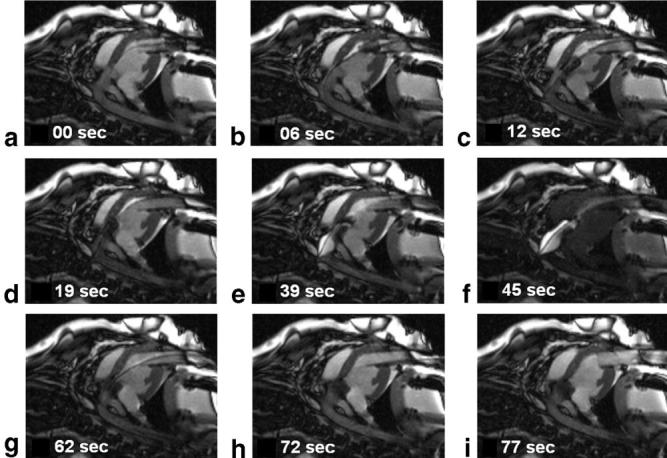
Selected frames from the real-time MRI displayed within the scan room, showing the deployment of the prosthetic valve. a: A guidewire is advanced through the trocar across the native aortic valve. b: The prosthetic valve is advanced to the end of the trocar. c: The prosthetic valve is advanced into position in the LV outflow track. d: The prosthetic valve is inserted across the native valve and aligned with the coronary ostia and the aortic annulus. e: A balloon filled with dilute Gd-DTPA MR contrast agent is used to expand the prosthetic valve. f: Interactive saturation is used to enhance visualization of the extent of balloon inflation. g: The balloon is taken down and pulled back through the trocar. h: The guidewire is removed. i: The delivery device is removed from the trocar. The total time required for this sequence of pictures was 77 s. (Also see the movie in the National Heart, Lung, and Blood Institute [NHLBI] archive: http://imagegallery.nhlbi.nih.gov/mcveighe/mrm1.html)
The velocity of blood flow through the prosthetic valve was measured with a cine phase-contrast imaging sequence (15) in the imaging planes proximal to the valve (just inside the LV), and just distal to the valve. An MR first-pass perfusion scan (16) was performed during intravenous injection of a Gd-DTPA contrast agent to confirm that myocardial blood flow was intact.
The authors had full access to the data and take responsibility for their integrity. All of the authors have read and agreed to the manuscript as written.
RESULTS
A total of eight pigs underwent the acute implantation and imaging procedure. Four animals survived the surgical procedure and valve implantation. Two pigs died during manipulation of the delivery device, and two died after valve implantation, likely due to coronary obstruction. During the procedure some PVCs were observed, but on the whole the heart rhythm was steady.
The location of the aortic valve ring and coronary arteries were obtained in approximately 5 min. Retrospectively gated cine imaging was useful for identifying both of these structures because they move through the scan plane during the heart cycle. The ability to place graphic markers on the real-time images was useful for orienting the valve with respect to the valve ring and the coronaries. In Fig. 5 the timing of the events is illustrated. The valve initially exits the trocar on the balloon and is positioned in 25 s, the balloon is inflated for 30 s, and the balloon is withdrawn in 5 s. The total time elapsed between the exit of the prosthetic valve from the trocar and the removal of the balloon was approximately 1 min. The image quality is superior to that achieved with ultrasound or x-ray fluoroscopy for guiding this procedure.
Immediately after the balloon was removed, the ventricular function was seen to return to normal on the real-time MRI. The phase-contrast cine MR study showed good flow through the valve immediately after valve implantation and apical and chest closure, with no evidence of a paravalvular leak. Figure 6 shows the through-plane blood velocity at both systole and diastole. Good systolic flow can be seen, and there is no diastolic regurgitant flow.
FIG. 6.
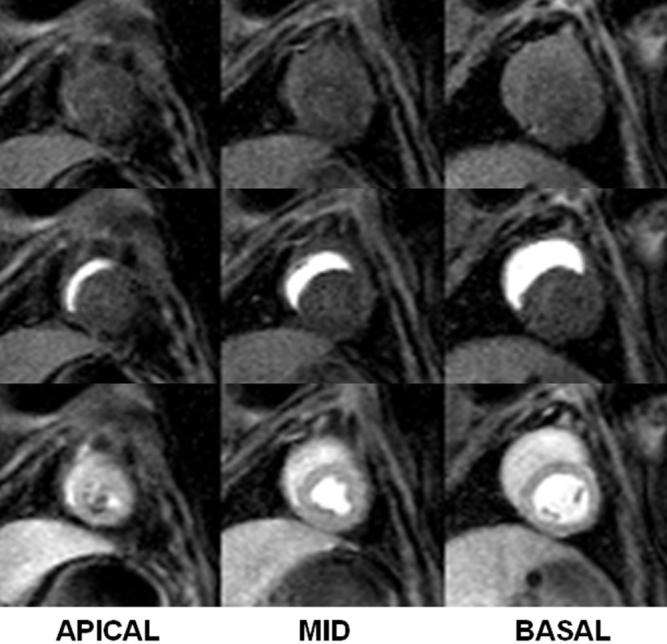
A first-pass perfusion scan in three slices of the heart 3 hr after valve implantation. Each row represents a different time after venous injection of Gd-DTPA. A small area of low perfusion corresponding to the location of the trocar is seen in the endocardium of the most apical slice (7 o’clock position in the bottom left image); however, all of the major coronary territories are perfused, indicating proper valve positioning with respect to the coronary ostia.
Immediately after the valve was placed, the first-pass contrast MRI showed a small apical zone of low flow (data not shown) in the region of the trocar. After removal of the trocar, and apical and chest closure, a subsequent first-pass perfusion study showed uniform enhancement over the LV, as shown in Fig. 7.
FIG. 7.
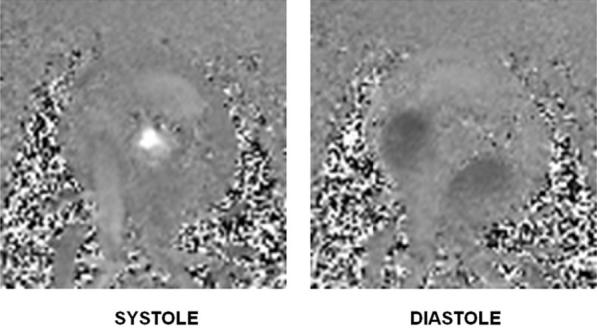
Frames from a cine phase-contrast movie showing the blood flow through the aorta and the atria after trocar removal and chest closure. Note that there is no sign of a paravalvular leak.
Retrospectively gated cine MRI showed excellent myocardial function after valve implantation in both long- and short-axis views (data not shown). The position of the valve was examined postmortem and found to be in the correct location, with the native valve tissue pressed against the aortic wall. In this set of acute procedures there was no evidence of thrombosis, severe bleeding, or pericardial bleeding. In the animals with successful valve implantation, the heart rhythm remained normal postprocedure.
DISCUSSION
Percutaneous valve placement has been under development for many years. The development of this real-time MRI-guided technique for valve delivery may circumvent many of the problems associated with delivering a valve through a relatively small catheter (8). The ability to obtain direct access to the heart opens up opportunities for the placement of more durable, well-tested devices compared to those available through catheter access, since the port is bigger, the distances are smaller, and the force transduction from the surgeon’s hand to the delivery device is more direct. The large port access and real-time visualization of the surgical field also facilitates the development of methods to remove sections of calcified or otherwise compromised valves.
The distinct advantage of MRI for guiding cardiac surgery is that the surgeon can see “through” the blood, and thus all of the morphological landmarks for positioning the device are visible. The short magnet makes it possible to have high-performance, real-time MRI available while the prosthetic valve is being manipulated under image guidance. In addition to aortic valve replacements, other target applications for real-time MRI-guided cardiac surgery include mitral, pulmonary, and tricuspid valve replacements or repairs (17-20).
Although the current stent-valve is made of FDA-approved components, it may have to be modified to improve long-term fixation pending long-term testing for stent-valve migration. Additional MR-visible markers may be beneficial to ensure that the valve is positioned correctly with respect to the location of the coronary arteries.
The ability to measure cardiac function online is also an advantage to performing the surgery within the MR scanner. Myocardial perfusion, blood flow, and valve leaflet motion are all available for the surgeon to observe in the working heart. For revascularization procedures, instant feedback on the impact of the procedure on perfusion is available in addition to anatomic data. For valve repairs, real-time feedback on leaflet function can be used to tailor the repair accordingly. Presently, surgical repairs are done on flaccid, empty hearts, and the ultimate effect of the correction is not apparent until the heart is refilled and beating.
The major limitation of this study is that it entailed a set of acute experiments that were designed to test the feasibility of valve delivery. Many questions remain unanswered, such as those regarding the long-term stability and functionality of the valve, and the response of the heart to the procedure. We are currently designing chronic experiments that we hope will answer important questions about the prevalence of ischemia in the heart and brain, LV aneurysm, and thrombosis in the postoperative period.
Although we have demonstrated here that real-time MRI is an excellent method for guiding valve placement, a number of developments need to occur before this procedure can be evaluated in clinical trials. First, the delivery device must be optimized so that it will have the smallest impact on the ventricle while retaining the ability to deliver a standard valve. The method used to secure the valve needs to be improved to guarantee that the valve will not migrate. Also, a procedure must be developed to extract the calcified diseased valve before the new valve is placed.
Real-time MRI provided better image quality than all competing imaging methods for directing minimally invasive surgery, including x-ray fluoroscopy/angiography (in which some anatomical structures are not visible) and echocardiography (in which the FOV is small and the images are of low quality). The MRI-guided surgery also allowed direct functional assessments to be made prior to, during, and immediately after valve implantation. The combination of real-time noninvasive, noncontrast imaging that can provide both anatomic details and functional assessments will enable the use of minimally invasive approaches that may provide patients with a less morbid and more durable solution to structural heart disease.
Acknowledgments
Grant sponsor: National Heart Lung and Blood Institute (NHLBI), NIH, DHHS.
REFERENCES
- 1.Doty DB, Flores JH, Doty JR. Cardiac valve operations using a partial sternotomy (lower half) technique. J Card Surg. 2000;15:35–42. doi: 10.1111/j.1540-8191.2000.tb00442.x. [DOI] [PubMed] [Google Scholar]
- 2.Vassiliades TA, Jr, Block PC, Cohn LH, Adams DH, Borer JS, Feldman T, Holmes DR, Laskey WK, Lytle BW, Mack MJ, Williams DO. The clinical development of percutaneous heart valve technology: a position statement of the Society of Thoracic Surgeons (STS), the American Association for Thoracic Surgery (AATS), and the Society for Cardiovascular Angiography and Interventions (SCAI) Ann Thorac Surg. 2005;79:1812–1818. doi: 10.1016/j.athoracsur.2005.02.062. [DOI] [PubMed] [Google Scholar]
- 3.Lutter G, Ardehali R, Cremer J, Bonhoeffer P. Percutaneous valve replacement: current state and future prospects. Ann Thorac Surg. 2004;78:2199–2206. doi: 10.1016/j.athoracsur.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Henk CB, Higgins CB, Saeed M. Endovascular interventional MRI. J Magn Reson Imaging. 2005;22:451–460. doi: 10.1002/jmri.20411. [DOI] [PubMed] [Google Scholar]
- 5.Lederman RJ. Cardiovascular interventional magnetic resonance imaging. Circulation. 2005;112:3009–3017. doi: 10.1161/CIRCULATIONAHA.104.531368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulz T, Puccini S, Schneider JP, Kahn T. Interventional and intraoperative MR: review and update of techniques and clinical experience. Eur Radiol. 2004;14:2212–2227. doi: 10.1007/s00330-004-2496-9. [DOI] [PubMed] [Google Scholar]
- 7.Blanco RT, Ojala R, Kariniemi J, Perala J, Niinimaki J, Tervonen O. Interventional and intraoperative MRI at low field scanner—a review. Eur J Radiol. 2005;56:130–142. doi: 10.1016/j.ejrad.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 8.Cribier A, Eltchaninoff H, Tron C, Bauer F, Agatiello C, Nercolini D, Tapiero S, Litzler PY, Bessou JP, Babaliaros V. Treatment of calcific aortic stenosis with the percutaneous heart valve: mid-term follow-up from the initial feasibility studies: the French experience. J Am Coll Cardiol. 2006;47:1214–1223. doi: 10.1016/j.jacc.2006.01.049. [DOI] [PubMed] [Google Scholar]
- 9.Kuehne T, Yilmaz S, Meinus C, Moore P, Saeed M, Weber O, Higgins CB, Blank T, Elsaesser E, Schnackenburg B. Magnetic resonance imaging-guided transcatheter implantation of a prosthetic valve in aortic valve position: feasibility study in swine. J Am Coll Cardiol. 2004;44:2247–2249. doi: 10.1016/j.jacc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Guttman MA, Kellman P, Dick AJ, Lederman RJ, McVeigh ER. Real-time accelerated interactive MRI with adaptive TSENSE and UNFOLD. Magn Reson Med. 2003;50:315–321. doi: 10.1002/mrm.10504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lederman RJ, Guttman MA, Peters DC, Thompson RB, Sorger JM, Dick AJ, Raman VK, McVeigh ER. Catheter-based endomyocardial injection with real-time magnetic resonance imaging. Circulation. 2002;105:1282–1284. doi: 10.1161/01.CIR.0000012425.71261.FC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dick AJ, Guttman MA, Raman VK, Peters DC, Pessanha BS, Hill JM, Smith S, Scott G, McVeigh ER, Lederman RJ. Magnetic resonance fluoroscopy allows targeted delivery of mesenchymal stem cells to infarct borders in swine. Circulation. 2003;108:2899–2904. doi: 10.1161/01.CIR.0000095790.28368.F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raman VK, Karmarkar PV, Guttman MA, Dick AJ, Peters DC, Ozturk C, Pessanha BS, Thompson RB, Raval AN, DeSilva R, Aviles RJ, Atalar E, McVeigh ER, Lederman RJ. Real-time magnetic resonance-guided endovascular repair of experimental abdominal aortic aneurysm in swine. J Am Coll Cardiol. 2005;45:2069–2077. doi: 10.1016/j.jacc.2005.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raval AN, Karmarkar PV, Guttman MA, Ozturk C, Sampath S, DeSilva R, Aviles RJ, Xu M, Wright VJ, Schenke WH, Kocaturk O, Dick AJ, Raman VK, Atalar E, McVeigh ER, Lederman RJ. Real-time magnetic resonance imaging-guided endovascular recanalization of chronic total arterial occlusion in a swine model. Circulation. 2006;113:1101–1107. doi: 10.1161/CIRCULATIONAHA.105.586727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelc NJ, Herfkens RJ, Shimakawa A, Enzmann DR. Phase contrast cine magnetic resonance imaging [Review] Magn Reson Q. 1991;7:229–254. [PubMed] [Google Scholar]
- 16.Kellman P, Derbyshire JA, Agyeman KO, McVeigh ER, Arai AE. Extended coverage first-pass perfusion imaging using slice-interleaved TSENSE. Magn Reson Med. 2004;51:200–204. doi: 10.1002/mrm.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cosgrove DM, III, Sabik JF, Navia JL. Minimally invasive valve operations. Ann Thorac Surg. 1998;65:1535–1538. doi: 10.1016/s0003-4975(98)00300-2. [DOI] [PubMed] [Google Scholar]
- 18.Mihaljevic T, Cohn LH, Unic D, Aranki SF, Couper GS, Byrne JG. One thousand minimally invasive valve operations: early and late results. Ann Surg. 2004;240:529–534. doi: 10.1097/01.sla.0000137141.55267.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boudjemline Y, Agnoletti G, Bonnet D, Behr L, Borenstein N, Sidi D, Bonhoeffer P. Steps toward the percutaneous replacement of atrioventricular valves an experimental study. J Am Coll Cardiol. 2005;46:360–365. doi: 10.1016/j.jacc.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 20.Boudjemline Y, Bonhoeffer P. Images in cardiovascular medicine. Percutaneous aortic valve replacement in animals. Circulation. 2004;109:e161. doi: 10.1161/01.CIR.0000121310.02372.37. [DOI] [PubMed] [Google Scholar]


