Direct Current (DC) brain polarization can alter the activity of cortical neurons and the excitability of the human cortex (Wassermann and Grafman, 2005). We recently reported that surface-anodal DC current applied to the left prefrontal area enhances verbal fluency in healthy subjects (Iyer et al., 2005). Frontotemporal dementia (FTD) comprises a set of disorders affecting the frontal and/or the anterior temporal lobes of the brain that present with behavioral and cognitive symptoms (Lund/Manchester, 1994), including impaired verbal fluency (the ability to generate words within a specific category) (Perri et al., 2005), which depends substantially on prefrontal cortex function (Stuss et al., 1998). To date, no effective treatment for the cognitive deficits in FTD has been found (Huey et al., 2006). Using letter-cued verbal fluency as the primary therapeutic outcome, we undertook a double-blinded, controlled trial of DC polarization in FTD.
The 10 patients were clinically diagnosed with FTD by published criteria (Lund/Manchester, 1994). Nine had predominantly behavioral, and one language, symptoms. At initial evaluation, the patients had a mean age of 61.3 years (range 46 to 80 years), a mean duration of illness of 3.4 years (range 1.2 to 6 years), and a mean total Mattis Dementia Rating Scale 2 of 109.3 points (range 45 to 131). The patients were taking the following CNS-active medications: Cholinesterase-inhibitors (6 patients), memantine (5 patients), antidepressants (2 patients), atypical antipsychotic medications (2 patients), benzodiazepines (1 patient). We applied 2 mA current through 25 cm2 electrodes (80 μC/cm2) for 40 min in the patients. As in our pilot study in healthy subjects (Iyer et al., 2005), the anode was placed at the F3 International 10-20 electrode position and the cathode over the right supraorbital area. The current was delivered by a Phoresor® II Auto Model PM850 iontophoresis device. In a double-blind, sham-controlled design each patient received separate sessions of active and sham treatment in a randomized and counterbalanced order. In the active condition, the current (2 mA) was delivered for 20 min before and 20 min after the start of testing (40 min). In the sham condition, the device delivered 10 seconds of very low current (0.1 mA) and was then shut off. The device was set by an investigator who neither interacted with the patients nor took part in the analysis of the data. Two target letters, matched for word frequency, were used before and after treatment in each session in a counterbalanced order. A secondary outcome was total score on the Neuropsychiatric Inventory (Cummings et al., 1994), a measure of behavioral symptoms, covering the 24 hours before and after each treatment.
Active polarization produced no improvement in verbal fluency relative to sham (Mean change: 1.4 ± 1.9 vs. 1.3 ± 2.9 words, paired t = −0.10, p = 0.93). There was a significant effect of treatment, independent of type (before treatment: 5.1 ± 5.1 words, after treatment: 6.5 ± 5.6 words, paired t = 2.55, p = 0.02), apparently related to practice (Figure 1). This is similar in magnitude to the practice effect observed in normal control subjects given repeated administrations of a verbal fluency task (Lemay et al., 2004). There was no significant effect of treatment on the Neuropsychiatric Inventory scores.
Figure 1.
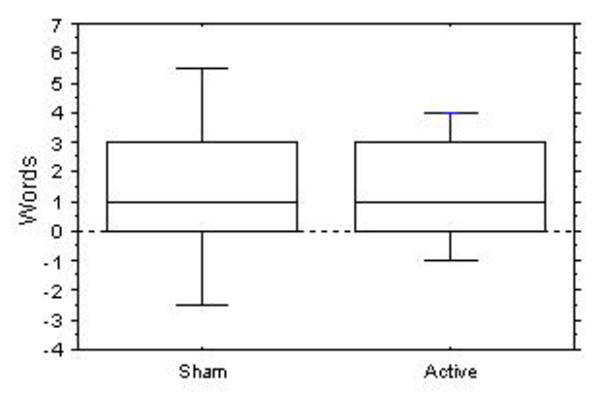
Difference in letter-cued verbal fluencies before and after sham and actual treatment. Bars represent the 90th and 10th, and boxes the 75th and 25th, percentiles. Center line is the median.
Using a treatment that produced significant improvement in verbal fluency in healthy subjects and in a pilot study with FTD patients, we were unable to produce any measurable benefit in FTD. There are several possible reasons for this. First, less current could be reaching the frontal cortex in FTD patients compared to normal controls due to shunting through the increased CSF space left by brain atrophy. Second, depletion of neurons may leave the affected cortex incapable of responding to polarization. Third, some of the more severely affected patients had difficulty cooperating the task and staying in set. A less severely affected patient group might have responded. We do not currently have data to support or refute these theories. While the results from this small trial were negative, there is enough evidence for DC polarization's ability to modulate and enhance local cortical function (Wassermann and Grafman, 2005) to warrant further trials in neurobehavioral disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Huey ED, Putnam KT, Grafman J. A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology. 2006;66:17–22. doi: 10.1212/01.wnl.0000191304.55196.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64:872–875. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- Lemay S, Bedard MA, Rouleau I, Tremblay PL. Practice effect and test-retest reliability of attentional and executive tests in middle-aged to elderly subjects. Clin Neuropsychol. 2004;18:284–302. doi: 10.1080/13854040490501718. [DOI] [PubMed] [Google Scholar]
- Lund/Manchester. Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups. J Neurol Neurosurg Psychiatry. 1994;57:416–418. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri R, Koch G, Carlesimo GA, Serra L, Fadda L, Pasqualetti P, Pettenati C, Caltagirone C. Alzheimer's disease and frontal variant of frontotemporal dementia-- a very brief battery for cognitive and behavioural distinction. J Neurol. 2005;252:1238–1244. doi: 10.1007/s00415-005-0849-1. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Hamer L, Palumbo C, Dempster R, Binns M, Levine B, Izukawa D. The effects of focal anterior and posterior brain lesions on verbal fluency. J Int Neuropsychol Soc. 1998;4:265–278. [PubMed] [Google Scholar]
- Wassermann EM, Grafman J. Recharging cognition with DC brain polarization. Trends Cogn Sci. 2005;9:503–505. doi: 10.1016/j.tics.2005.09.001. [DOI] [PubMed] [Google Scholar]


