Abstract
The discovery of epigenetic silencing as a key mechanism of tumor suppressor gene inactivation in human cancer has led to great interest in utilizing epigenetic modulatory drugs as cancer therapeutics. It is less appreciated that medically important tumor-associated antigens, particularly the Cancer Testis or Cancer/Germ-line family of antigens (CG antigens), which are being actively tested as cancer vaccine targets, are epigenetically activated in many human cancers. However, a major limitation to the therapeutic value of CG antigen-directed vaccines is the limited and heterogeneous expression of CG antigens in tumors. Recent work has begun to dissect the specific epigenetic mechanisms controlling differential expression of CG antigen genes in human cancers. From a clinical perspective, convincing data indicate that epigenetic modulatory agents, including DNA methyltransferase (DNMT) and histone deacetylase (HDAC) inhibitors, robustly promote the expression of CG antigens, as well as class I major histocompatibility complex (MHC I) and other immune costimulatory molecules, in tumors. Importantly, the effects of these agents on CG antigen gene expression often show marked specificity for tumor cells as compared to normal cells. Taken together, these data encourage clinical evaluation of combination therapies involving epigenetic modulatory drugs and CG antigen-directed tumor vaccines for the treatment of human malignancies.
Keywords: cancer-testis antigens, cancer/germ-line antigens, epigenetics, DNA methylation, 5-aza-2′-deoxycytidine, immune response, MAGE-A1, NY-ESO-1, HDAC inhibitors, DNMTs
Epigenetic Regulation of CG Antigens
CG antigens are a large family of genes of mostly unknown function that show highly restricted expression, limited to germ cells of the testis and ovary, trophoblast, and a variety of human cancers.1 CG antigen genes have been grouped based on their chromosomal localization, with approximately half of these genes encoded on the X chromosome (CG-X genes), and half on autosomes (non-X CG antigens).1 The founding member of the gene family, MAGE-1 (later renamed MAGE-A1)2 was discovered in 1991 by Boon and coworkers, who identified it as a gene encoding an MHC I-restricted antigen recognized specifically by cytotoxic T lymphocytes (CTL) from a melanoma patient.3 Since that time, over 80 additional members of the gene family have been identified, by either cytotoxic T-lymphocyte (CTL) or seroreactivity studies, and/or by virtue of their expression pattern in somatic, gametogeneic, and cancer tissues.1,4
The first evidence that CG antigen genes are epigenetically regulated was provided by the observation that treatment with 5-aza-2′-deoxycytidine (DAC), a classical DNA methyltransferase inhibitor,5 activates MAGE-A1 expression in human melanoma cell lines.6 Additionally, hypomethylation of specific CpG sites in the MAGE-A1 gene was found to correlate with gene expression.7,8 A number of other CG antigen genes, including MAGE-A2, -A3, -A4, NY-ESO-1, LAGE-1 and XAGE-1 were also found to be regulated by promoter DNA methylation,9-11 suggesting that DNA methylation may be a primary regulator of expression of this gene class.10 Compared with tumor suppressor genes (TSGs), CG antigen genes show the opposite methylation pattern in normal somatic tissues and tumors. TSGs are typically unmethylated and expressed in normal tissues but can become silenced in association with DNA methylation in cancer.12 In contrast, CG antigen genes are methylated and silent in normal tissues but become hypomethylated and activated in certain human cancers. Notably, CG antigen promoters, which often contain CpG islands,13 show a similar change in methylation (hypomethylation) as does global genomic DNA in many human cancers.14,15 An early study has investigated the relationship between CG antigen gene expression and global hypomethylation in cancer.8 Using a panel of cancer cell lines showing variable levels of MAGE-A1 expression, De Smet et al. found a general correlation between MAGE-A1 expression and genomic DNA hypomethylation.8 Additional investigations utilizing clinical tumor sample isolates and multiple CG antigen genes are required to firmly establish this relationship.
A number of recent studies have provided additional information about the epigenetic mechanisms controlling CG antigen gene expression (Fig. 1). There are three enzymatically active DNMT enzymes in mammalian cells, DNMT1, DNMT3a, and DNMT3b16 and any combination of these enzymes could theoretically be involved in CG antigen gene regulation. Kozlowski et al. reported that in HCT116 colon cancer cells, which normally do not express CG antigen genes, genetic knockout of both DNMT1 and DNMT3b, but not of either enzyme alone, induces CG antigen gene expression.17 In addition, two recent studies have found that DNMT1 may play a more prominent independent role in directing CG antigen gene repression in other cancer cell types.18,19 There may also be important distinctions with regard to DNMTs involvement in CG antigen gene methylation, based on the specific CG antigen gene under study. For example, we have recently shown that methylation of the MAGE-A1 and NY-ESO-1 promoters is cooperatively maintained by DNMT1 and DNMT3b in HCT116 cells (functional redundancy), while methylation at the XAGE-1 promoter requires the activity of both enzymes.19
Figure 1.
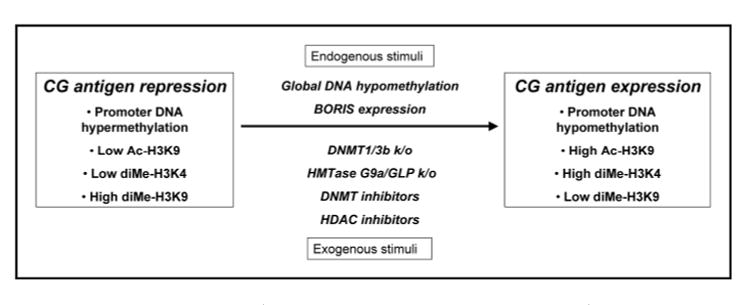
Epigenetic regulation of CG antigen gene expression. Repression of CG antigen genes in somatic tissues and human cancer cells is associated with DNA hypermethylation, low levels of acetylated lysine 9 and dimethylated lysine 4 of histone H3 (Ac-H3K9 and diMeH3K4), and high levels of diMe-H3K9. In contrast, activation of CG antigen genes, seen in germ cells of the testis and ovary and in some human cancers, is associated with promoter hypomethylation and the opposite pattern of histone code modifications. Endogenous stimuli proposed to lead to CG antigen gene expression include global genomic DNA hypomethylation and expression of the CTCF paralog BORIS. Exogenous stimuli that promote CG antigen gene expression include siRNA knockdown of DNMT1, or genetic knockout of both DNMT1 and DNMT3b in human cancer cells. In murine ES cells, genetic knockout of the euchromatic histone methyltransferase enzymes G9a or GLP causes CG antigen gene activation. Finally, treatment of human cancer cells with epigenetic modulatory drugs, including DNMT and HDAC inhibitors, activates CG antigen gene expression.
Histone modifications also appear to play a critical role in epigenetic regulation of CG antigen gene expression. An intriguing observation in this context was provided by the work of Shinkai and colleagues, who performed an oligonucleotide microarray screen for genes upregulated in murine ES cells sustaining a genetic knockout of the euchromatic histone methyltransferase enzyme G9a.20 These authors reported that MAGE-A2 expression was activated in G9a-/- ES cells, and that this correlated with changes in the histone modification pattern at the MAGE-A2 promoter, including loss of dimethylated H3K9 (diMe-H3K9), and gains in dimethylated H3K4 (diMe-H3K4) and acetylated H3K9/K14.20 Additionally, genetic knockout of the highly related histone methyltransferase GLP, also in mouse ES cells, showed a similar phenotype with respect to MAGE-A2 expression and histone code modifications.21 Whether other CG antigen genes besides MAGE-A2 are affected by G9a/GLP loss in this manner, and whether G9a/GLP are involved in CG antigen gene regulation in humans is unknown. With regard to histone modifications, our recent work, using HCT116 cells, has shown that loss of diMe-H3K9 is not sufficient for activation of CG antigen genes, including MAGE-A1, NY-ESO-1 and XAGE-1, because the levels of this modification are reduced in DNMT1 or DNMT3b knockout cells that do not express significant levels of these genes.19 However, we observe a strong association between increased levels of diMe-H3K4 and Ac-H3K9 and CG antigen gene activation, which is only seen in DNMT1/3b double knockout HCT116 cells.19
Recently, Lobanenkov and coworkers have provided an important conceptual advance in our understanding of the mechanism of regulation of CG antigen genes.22,23 These studies investigated the role of the Brother of Regulator of Imprinted Sites (BORIS), a germ-cell specific CCCTC-binding factor (CTCF) paralog,24,25 in the regulation of various CG antigen genes, including MAGE-A1 and NY-ESO-1.22,23 CTCF is a epigenetic modulatory protein with a well-established role in reading gene-imprinting marks in soma.26 CTCF and BORIS share a conserved central 11 Zinc Finger DNA binding domain (11ZF DBB) but have divergent N and C termini.25 Conditional expression of BORIS was shown to activate expression of CG antigen genes, and kinetic studies showed that DAC treatment activates BORIS expression prior to inducing the expression of other CG antigen genes.22 Furthermore, downregulation of BORIS by RNA interference prior to DAC treatment reduced the capacity of DAC to activate MAGE-A1 expression.22 BORIS was also shown to bind directly to the MAGE-A1 and NY-ESO-1 promoters and to displace CTCF at these loci.22,23 These data provide intriguing evidence that aberrant expression of BORIS in human cancers could play a role in the initiation of CG antigen gene expression. Interestingly, BORIS is coexpressed in testicular germ cells with CG antigen genes, suggesting that it may regulate the expression of CG antigen genes during normal development.24,25 The relationship between BORIS, CTCF, and other epigenetic control mechanisms in the regulation of CG antigen gene expression in human cancer is an important area for continued investigation.
Activation of CG Antigen Expression and Immune Recognition Using Epigenetic Modulatory Drugs
As described above, the expression of CG antigens is activated by treatment with the classical DNA methyltransferase inhibitor DAC. We have recently shown, using a microarray approach, that DAC treatment simultaneously activates many CG antigens, and the level of induction is more robust than other types of genes induced by this agent.27 Other epigenetic modulatory drugs have also been shown to activate CG antigen gene expression. Treatment of cancer cell lines with zebularine, a second generation DNMT inhibitor and cytidine analog, also leads to robust CG antigen gene activation.28 Additionally, combination treatment of DAC and depsipeptide, an HDAC inhibitor, leads to enhanced expression of the CG antigen gene NY-ESO-1 in thoracic cancer cells.29 It was also recently shown that combination treatments utilizing DAC and the classical HDAC inhibitor Trichostatin A synergistically activate the expression of MAGE-A class genes in human cancer cell lines.30 A summary of the published data reporting CG antigen gene activation by epigenetic modulators is shown in Table 1.
Table 1. CG antigen gene induction using epigenetic modulatory agents.
| Epigenetic Modulator | Induced CG Antigen and/or Costimulatory Molecule | Tumor Cell Type | Enhanced Tumor Cell Lysis by CTL | Ref. |
|---|---|---|---|---|
| DACa | MAGE-A1 | Melanoma | Yes | 6 |
| DAC | MAGE-A1, MHC I, ICAM-1, LFA-3 | Melanoma | N/d | 34 |
| DAC | MHC I | Colon | N/d | 32 |
| DAC/Depsipeptide | NY-ESO-1 | Thoracic | Yes | 29 |
| DAC | Numerous CG antigens | Renal Cell | Yes | 43 |
| DAC | Numerous CG antigens | Bladder | N/d | 44 |
| DAC | Numerous CG antigens | Mesothelioma | N/d | 45 |
| DACb | MAGE-A1, NY-ESO-1, SSX | MDS, AML | N/d | 39 |
| Zebularine | Numerous CG antigens | Bladder, Pancreas, Colon | N/d | 28 |
| DAC | Numerous CG antigens, MHC I, β-2-microglobulin | Colon | N/d | 27 |
| DAC | MAGE-A3 | Melanoma | Yes | 46 |
| DAC | XAGE-1 | Gastric, Colon | N/d | 11 |
| DAC | P1A | Multiple typesc | Yes | 37 |
| DAC/Trichostatin A | MAGE-A1, -A2, -A3, -A12 | Multiple types | N/d | 30 |
5-aza-2′-deoxycytidine.
in vivo experiment involving treated patients.
includes in vivo activation of P1A in treated mice.
N/d, not determined.
Perhaps most importantly, both DAC and zebularine appear to activate CG antigen gene expression specifically in tumor-derived cells.27,28 In our studies, we performed cDNA microarray analyses of three carcinoma and one normal epithelial cell line treated with DAC, and observed robust induction of >16 distinct CG antigen genes in all three carcinoma cell lines but not in normal epithelial cells.27 Differential inhibition of DNMT enzymes did not appear to account for this phenomenon as covalent DNMT1 adduct formation and global genomic DNA methylation occurred in both the tumor and normal cell lines.27 Similarly, a microarray study by Peter Jones and colleagues found that zebularine treatment induced eight different CG antigens in three different tumor cell lines, with no induction in four different normal fibroblast cell lines.28
Immune recognition of CG antigens is dependent on proper display of CG antigen-derived peptides in the context of MHC I molecules on the surface of tumor cells.31 Thus, it is critical to note that DAC treatment also induces MHC I expression in cancer cells27,32-34 (Table 1). MHC I downregulation is a common mechanism of immune evasion in human cancer,35 and MHC I and MHC II gene expression are repressed by DNA methylation in various malignancies and possibly also during development.36 In addition to MHC, other immune costimulatory molecules, including ICAM-1 and LFA-3, are induced in tumor cells by DAC treatment.34 CG antigen peptide/MHC cell surface complexes are recognized by cytotoxic T-lymphocytes (CTLs). Thus, it would be predicted that treatment with epigenetic modulators such as DAC would enhance CTL-directed killing of tumor cells following induction of CG antigen genes. In fact, this has been observed in numerous cell-based studies (Table 1). These data provide a critical validation that CG antigen gene activation by treatment with epigenetic modulators not only activates CG antigen gene expression, but that it also leads to functional CG antigen presentation and recognition by immune cells. Remarkably, Guo et al recently reported a novel strategy in which tumor cell lines were treated with DAC to induce the expression of a specific CG antigen (P1A) de novo.37 One of these cell lines, the mammary tumor line 4T1, was used to challenge mice which received adoptive transfer of P1A-specific CTL cells.37 Sequential DAC treatment and adoptive transfer in syngeneic BALB/c mice resulted in pronounced regression of 4T1 lung tumor metastases, as compared to control animals receiving adoptive transfer but not DAC treatment.37 This “chemoimmunotherapy” study suggests that it is possible to activate immune responses to previously CG antigen-negative tumors by treatment with epigenetic modulators prior to, or concurrent with, vaccination. This strategy could make possible the clinical use of CG antigen-directed vaccines for classes of tumors not previously known to express CG antigens.
We have recently shown, in a Phase I clinical trial for the treatment of solid tumors, that low dose infusion of DAC (Decitabine) elicits hypomethylation of the MAGE-A1 promoter in vivo, in peripheral blood mononuclear cells (PBMCs).38 Because we did not obtain tumor tissue in this study, it remains unclear whether similarly favorable pharmacodynamics would also be observed in solid tumors, although one would anticipate similar results in liquid tumor tissues. We did not measure MAGE-A1 expression in this clinical study; however, in vitro experiments indicate that normal cells have a reduced capacity to express MAGE-A1, even if the promoter is hypomethylated.27 Thus, while specificity remains a paramount issue, DNA methyltransferase inhibitors may yield high therapeutic indices, with regard to activation of CG antigen expression in vivo. In this context, Sigalotti et al reported that peripheral blood and bone marrow cells from DAC-treated myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) patients showed increased expression of multiple CG antigen genes at the mRNA level.39 This important finding further highlights the potential clinical utility of epigenetic modulators for activating CG antigen-directed immune responses.
Concluding Remarks
CG antigen vaccines are under clinical development, and formulations based on specific peptides, full-length proteins, and DNA vaccination strategies are under active investigation.1 A recent placebo-controlled clinical trial using recombinant NY-ESO-1 protein and a novel adjuvant generated antibody, CD4+, and CD8+ T-cell immune responses, as well as superior clinical outcomes in melanoma patients.40 Thus, CG antigen vaccines are both safe and have the capacity to be highly immunogenic. However, heterogeneous expression of CG antigens, both within individual tumors and throughout the patient population, presents a significant barrier to the clinical utility of the single agent vaccine approach. Recent clinical experience with DAC, and the related compound 5-azacytidine, have demonstrated that low dose treatments with these agents show low toxicity, along with high response rates in hematopoetic malignancies.41,42 Taken together, data showing robust CG antigen and MHC I gene activation by epigenetic modulators in vitro and in vivo, along with the generally low in vivo toxicity of both CG antigen vaccines and epigenetic modulators, suggests that prior or concurrent administration of epigenetic modulatory drugs could significantly increase immunological and clinical responses to CG antigen-directed vaccines.
Acknowledgments
I thank Drs. Kunle Odunsi and Dominic Smiraglia of Roswell Park Cancer Institute for critical reading of the manuscript and helpful discussions. Work in the author's laboratory is supported by grant RO1CA116674 from the National Cancer Institute, and by a grant from Phi Beta Psi.
Abbreviations
- CG antigen
cancer/germ-line antigen
- DAC
5-aza-2′-deoxycytidine or decitabine
- DNMT
DNA methyltransferase
- HDAC
histone deacetylase
- MHC
major histocompatability complex
- CTL
cytotoxic T lymphocytes
- TSG
tumor suppressor genes
- BORIS
brother of the the regulator of imprinted sites
- CTCF
CCCTC-binding factor
- diMe-H3K9
dimethylated histone H3 lysine 9
- diMe-H3K4
dimethylated histone H3 lysine 4
- Ac-H3K9
acetylated histone H3K9
- MDS
myelodysplastic syndrome
- AML
acute myeloid leukemia
References
- 1.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, game-togenesis and cancer. Nat Rev Cancer. 2005;5:615–25. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 2.Chomez P, De Backer O, Bertrand M, De Plaen E, Boon T, Lucas S. An overview of the MAGE gene family with the identification of all human members of the family. Cancer Res. 2001;61:5544–51. [PubMed] [Google Scholar]
- 3.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 4.Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: Review, standardization, and commentary. Cancer Immun. 2004;4:1. [PubMed] [Google Scholar]
- 5.Christman JK. 5-Azacytidine and 5-aza-2′-deoxycytidine as inhibitors of DNA methylation: Mechanistic studies and their implications for cancer therapy. Oncogene. 2002;21:5483–95. doi: 10.1038/sj.onc.1205699. [DOI] [PubMed] [Google Scholar]
- 6.Weber J, Salgaller M, Samid D, Johnson B, Herlyn M, Lassam N, Treisman J, Rosenberg SA. Expression of the MAGE-1 tumor antigen is upregulated by the demethylating agent 5-aza-2′-deoxycytidine. Cancer Res. 1994;54:1766–71. [PubMed] [Google Scholar]
- 7.Serrano A, Garcia A, Abril E, Garrido F, Ruiz-Cabello F. Methylated CpG points identified within MAGE-1 promoter are involved in gene repression. Int J Cancer. 1996;68:464–70. doi: 10.1002/(SICI)1097-0215(19961115)68:4<464::AID-IJC11>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.De Smet C, De Backer O, Faraoni I, Lurquin C, Brasseur F, Boon T. The activation of human gene MAGE-1 in tumor cells is correlated with genome-wide demethylation. Proc Natl Acad Sci USA. 1996;93:7149–53. doi: 10.1073/pnas.93.14.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigalotti L, Coral S, Nardi G, Spessotto A, Cortini E, Cattarossi I, Colizzi F, Altomonte M, Maio M. Promoter methylation controls the expression of MAGE2, 3 and 4 genes in human cutaneous melanoma. J Immunother. 2002;25:16–26. doi: 10.1097/00002371-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 10.De Smet C, Lurquin C, Lethe B, Martelange V, Boon T. DNA methylation is the primary silencing mechanism for a set of germ line- and tumor-specific genes with a CpG-rich promoter. Mol Cell Biol. 1999;19:7327–35. doi: 10.1128/mcb.19.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lim JH, Kim SP, Gabrielson E, Park YB, Park JW, Kwon TK. Activation of human cancer/testis antigen gene, XAGE-1, in tumor cells is correlated with CpG island hypomethylation. Int J Cancer. 2005;116:200–6. doi: 10.1002/ijc.21007. [DOI] [PubMed] [Google Scholar]
- 12.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3:415–28. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 13.Bird A. The essentials of DNA methylation. Cell. 1992;70:5–8. doi: 10.1016/0092-8674(92)90526-i. [DOI] [PubMed] [Google Scholar]
- 14.Ehrlich M. DNA methylation in cancer: Too much, but also too little. Oncogene. 2002;21:5400–13. doi: 10.1038/sj.onc.1205651. [DOI] [PubMed] [Google Scholar]
- 15.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4:143–53. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 16.Robertson KD. DNA methylation, methyltransferases, and cancer. Oncogene. 2001;20:3139–55. doi: 10.1038/sj.onc.1204341. [DOI] [PubMed] [Google Scholar]
- 17.Koslowski M, Bell C, Seitz G, Lehr HA, Roemer K, Muntefering H, Huber C, Sahin U, Tureci O. Frequent nonrandom activation of germ-line genes in human cancer. Cancer Res. 2004;64:5988–93. doi: 10.1158/0008-5472.CAN-04-1187. [DOI] [PubMed] [Google Scholar]
- 18.Loriot A, De Plaen E, Boon T, De Smet C. Transient down-regulation of DNMT1 methyltransferase leads to activation and stable hypomethylation of MAGE-A1 in melanoma cells. J Biol Chem. 2006 doi: 10.1074/jbc.M510469200. In press. [DOI] [PubMed] [Google Scholar]
- 19.James SR, Link PA, Karpf AR. Epigenetic regulation of X-linked cancer/germline antigen genes by DNMT1 and DNMT3b. Oncogene. 2006 doi: 10.1038/sj.onc.1209678. [DOI] [PubMed] [Google Scholar]
- 20.Tachibana M, Sugimoto K, Nozaki M, Ueda J, Ohta T, Ohki M, Fukuda M, Takeda N, Niida H, Kato H, Shinkai Y. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002;16:1779–91. doi: 10.1101/gad.989402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tachibana M, Ueda J, Fukuda M, Takeda N, Ohta T, Iwanari H, Sakihama T, Kodama T, Hamakubo T, Shinkai Y. Histone methyltransferases G9a and GLP form heteromeric complexes and are both crucial for methylation of euchromatin at H3-K9. Genes Dev. 2005;19:815–26. doi: 10.1101/gad.1284005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vatolin S, Abdullaev Z, Pack SD, Flanagan PT, Custer M, Loukinov DI, Pugacheva E, Hong JA, Morse H, IIIrd, Schrump DS, Risinger JI, Barrett JC, Lobanenkov VV. Conditional expression of the CTCF-paralogous transcriptional factor BORIS in normal cells results in demethylation and derepression of MAGE-A1 and reactivation of other cancer-testis genes. Cancer Res. 2005;65:7751–62. doi: 10.1158/0008-5472.CAN-05-0858. [DOI] [PubMed] [Google Scholar]
- 23.Hong JA, Kang Y, Abdullaev Z, Flanagan PT, Pack SD, Fischette MR, Adnani MT, Loukinov DI, Vatolin S, Risinger JI, Custer M, Chen GA, Zhao M, Nguyen DM, Barrett JC, Lobanenkov VV, Schrump DS. Reciprocal binding of CTCF and BORIS to the NY-ESO-1 promoter coincides with derepression of this cancer-testis gene in lung cancer cells. Cancer Res. 2005;65:7763–74. doi: 10.1158/0008-5472.CAN-05-0823. [DOI] [PubMed] [Google Scholar]
- 24.Loukinov DI, Pugacheva E, Vatolin S, Pack SD, Moon H, Chernukhin I, Mannan P, Larsson E, Kanduri C, Vostrov AA, Cui H, Niemitz EL, Rasko JE, Docquier FM, Kistler M, Breen JJ, Zhuang Z, Quitschke WW, Renkawitz R, Klenova EM, Feinberg AP, Ohlsson R, Morse HC, IIIrd, Lobanenkov VV. BORIS, a novel male germ-line-specific protein associated with epigenetic reprogramming events, shares the same 11-zinc-finger domain with CTCF, the insulator protein involved in reading imprinting marks in the soma. Proc Natl Acad Sci USA. 2002;99:6806–11. doi: 10.1073/pnas.092123699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klenova EM, Morse HC, IIIrd, Ohlsson R, Lobanenkov VV. The novel BORIS + CTCF gene family is uniquely involved in the epigenetics of normal biology and cancer. Semin Cancer Biol. 2002;12:399–414. doi: 10.1016/s1044-579x(02)00060-3. [DOI] [PubMed] [Google Scholar]
- 26.Ohlsson R, Renkawitz R, Lobanenkov V. CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends Genet. 2001;17:520–7. doi: 10.1016/s0168-9525(01)02366-6. [DOI] [PubMed] [Google Scholar]
- 27.Karpf AR, Lasek AW, Ririe TO, Hanks AN, Grossman D, Jones DA. Limited gene activation in tumor and normal epithelial cell lines treated with the DNA methyltransferase inhibitor 5-aza-2′-deoxycytidine. Mol Pharmacol. 2004;65:18–27. doi: 10.1124/mol.65.1.18. [DOI] [PubMed] [Google Scholar]
- 28.Cheng JC, Yoo CB, Weisenberger DJ, Chuang J, Wozniak C, Liang G, Marquez VE, Greer S, Orntoft TF, Thykjaer T, Jones PA. Preferential response of cancer cells to zebularine. Cancer Cell. 2004;6:151–8. doi: 10.1016/j.ccr.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 29.Weiser TS, Guo ZS, Ohnmacht GA, Parkhurst ML, Tong-On P, Marincola FM, Fischette MR, Yu X, Chen GA, Hong JA, Stewart JH, Nguyen DM, Rosenberg SA, Schrump DS. Sequential 5-Aza-2 deoxycytidine-depsipeptide FR901228 treatment induces apoptosis preferentially in cancer cells and facilitates their recognition by cytolytic T lymphocytes specific for NY-ESO-1. J Immunother. 2001;24:151–61. doi: 10.1097/00002371-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Wischnewski F, Pantel K, Schwarzenbach H. Promoter demethylation and histone acetylation mediate gene expression of MAGE-A1, -A2, -A3, and -A12 in human cancer cells. Mol Cancer Res. 2006;4:339–49. doi: 10.1158/1541-7786.MCR-05-0229. [DOI] [PubMed] [Google Scholar]
- 31.Gillespie AM, Coleman RE. The potential of melanoma antigen expression in cancer therapy. Cancer Treatment Reviews. 1999;25:219–27. doi: 10.1053/ctrv.1999.0126. [DOI] [PubMed] [Google Scholar]
- 32.Karpf AR, Peterson PW, Rawlins JT, Dalley BK, Yang Q, Albertsen H, Jones DA. Inhibition of DNA methyltransferase stimulates the expression of signal transducer and activator of transcription 1, 2, and 3 genes in colon tumor cells. Proc Natl Acad Sci USA. 1999;96:14007–12. doi: 10.1073/pnas.96.24.14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fonsatti E, Sigalotti L, Coral S, Colizzi F, Altomonte M, Maio M. Methylation-regulated expression of HLA class I antigens in melanoma. Int J Cancer. 2003;105:430–1. doi: 10.1002/ijc.11077. (author reply 432-3) [DOI] [PubMed] [Google Scholar]
- 34.Coral S, Sigalotti L, Gasparollo A, Cattarossi I, Visintin A, Cattelan A, Altomonte M, Maio M. Prolonged upregulation of the expression of HLA class I antigens and costimulatory molecules on melanoma cells treated with 5-aza-2′-deoxycytidine (5-AZA-CdR) J Immunother. 1999;22:16–24. doi: 10.1097/00002371-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Algarra I, Garcia-Lora A, Cabrera T, Ruiz-Cabello F, Garrido F. The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: Implications for tumor immune escape. Cancer Immunol Immunother. 2004;53:904–10. doi: 10.1007/s00262-004-0517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Elsen PJ, Holling TM, van der Stoep N, Boss JM. DNA methylation and expression of major histocompatibility complex class I and class II transactivator genes in human developmental tumor cells and in T cell malignancies. Clin Immunol. 2003;109:46–52. doi: 10.1016/s1521-6616(03)00200-6. [DOI] [PubMed] [Google Scholar]
- 37.Guo ZS, Hong JA, Irvine KR, Chen GA, Spiess PJ, Liu Y, Zeng G, Wunderlich JR, Nguyen DM, Restifo NP, Schrump DS. De novo induction of a cancer/testis antigen by 5-aza-2′-deoxycytidine augments adoptive immunotherapy in a murine tumor model. Cancer Res. 2006;66:1105–13. doi: 10.1158/0008-5472.CAN-05-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samlowski WE, Leachman SA, Wade M, Cassidy P, Porter-Gill P, Busby L, Wheeler R, Boucher K, Fitzpatrick F, Jones DA, Karpf AR. Evaluation of a 7-day continuous intravenous infusion of decitabine: Inhibition of promoter-specific and global genomic DNA methylation. J Clin Oncol. 2005;23:3897–905. doi: 10.1200/JCO.2005.06.118. [DOI] [PubMed] [Google Scholar]
- 39.Sigalotti L, Altomonte M, Colizzi F, Degan M, Rupolo M, Zagonel V, Pinto A, Gattei V, Maio M. 5-Aza-2′-deoxycytidine (decitabine) treatment of hematopoietic malignancies: A multimechanism therapeutic approach? Blood. 2003;101:4644–4646. doi: 10.1182/blood-2002-11-3458. (discussion 4645-6) [DOI] [PubMed] [Google Scholar]
- 40.Davis ID, Chen W, Jackson H, Parente P, Shackleton M, Hopkins W, Chen Q, Dimopoulos N, Luke T, Murphy R, Scott AM, Maraskovsky E, McArthur G, MacGregor D, Sturrock S, Tai TY, Green S, Cuthbertson A, Maher D, Miloradovic L, Mitchell SV, Ritter G, Jungbluth AA, Chen YT, Gnjatic S, Hoffman EW, Old LJ, Cebon JS. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc Natl Acad Sci USA. 2004;101:10697–702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaminskas E, Farrell A, Abraham S, Baird A, Hsieh LS, Lee SL, Leighton JK, Patel H, Rahman A, Sridhara R, Wang YC, Pazdur R. Approval summary: Azacitidine for treatment of myelodysplastic syndrome subtypes. Clin Cancer Res. 2005;11:3604–8. doi: 10.1158/1078-0432.CCR-04-2135. [DOI] [PubMed] [Google Scholar]
- 42.Issa JP. Optimizing therapy with methylation inhibitors in myelodysplastic syndromes: Dose, duration, and patient selection. Nat Clin Pract Oncol. 2005;2 1:S24–9. doi: 10.1038/ncponc0355. [DOI] [PubMed] [Google Scholar]
- 43.Coral S, Sigalotti L, Altomonte M, Engelsberg A, Colizzi F, Cattarossi I, Maraskovsky E, Jager E, Seliger B, Maio M. 5-aza-2′-deoxycytidine-induced expression of functional cancer testis antigens in human renal cell carcinoma: Immunotherapeutic implications. Clin Cancer Res. 2002;8:2690–5. [PubMed] [Google Scholar]
- 44.Liang G, Gonzales FA, Jones PA, Orntoft TF, Thykjaer T. Analysis of gene induction in human fibroblasts and bladder cancer cells exposed to the methylation inhibitor b5-aza-2′-deoxycytidineb. Cancer Res. 2002;62:961–6. [PubMed] [Google Scholar]
- 45.Sigalotti L, Coral S, Altomonte M, Natali L, Gaudino G, Cacciotti P, Libener R, Colizzi F, Vianale G, Martini F, Tognon M, Jungbluth A, Cebon J, Maraskovsky E, Mutti L, Maio M. Cancer testis antigens expression in mesothelioma: Role of DNA methylation and bioimmunotherapeutic implications. Br J Cancer. 2002;86:979–82. doi: 10.1038/sj.bjc.6600174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sigalotti L, Fratta E, Coral S, Tanzarella S, Danielli R, Colizzi F, Fonsatti E, Traversari C, Altomonte M, Maio M. Intratumor heterogeneity of cancer/testis antigens expression in human cutaneous melanoma is methylation-regulated and functionally reverted by 5-aza-2′-deoxycytidine. Cancer Res. 2004;64:9167–71. doi: 10.1158/0008-5472.CAN-04-1442. [DOI] [PubMed] [Google Scholar]


