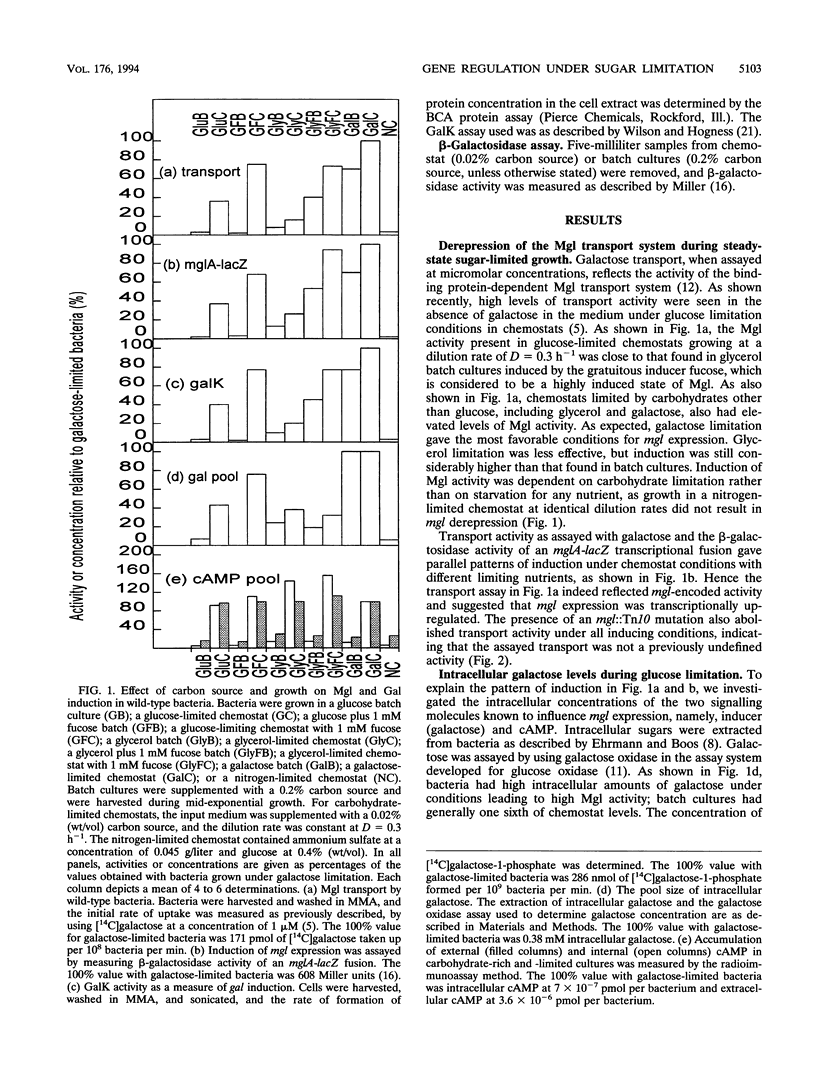
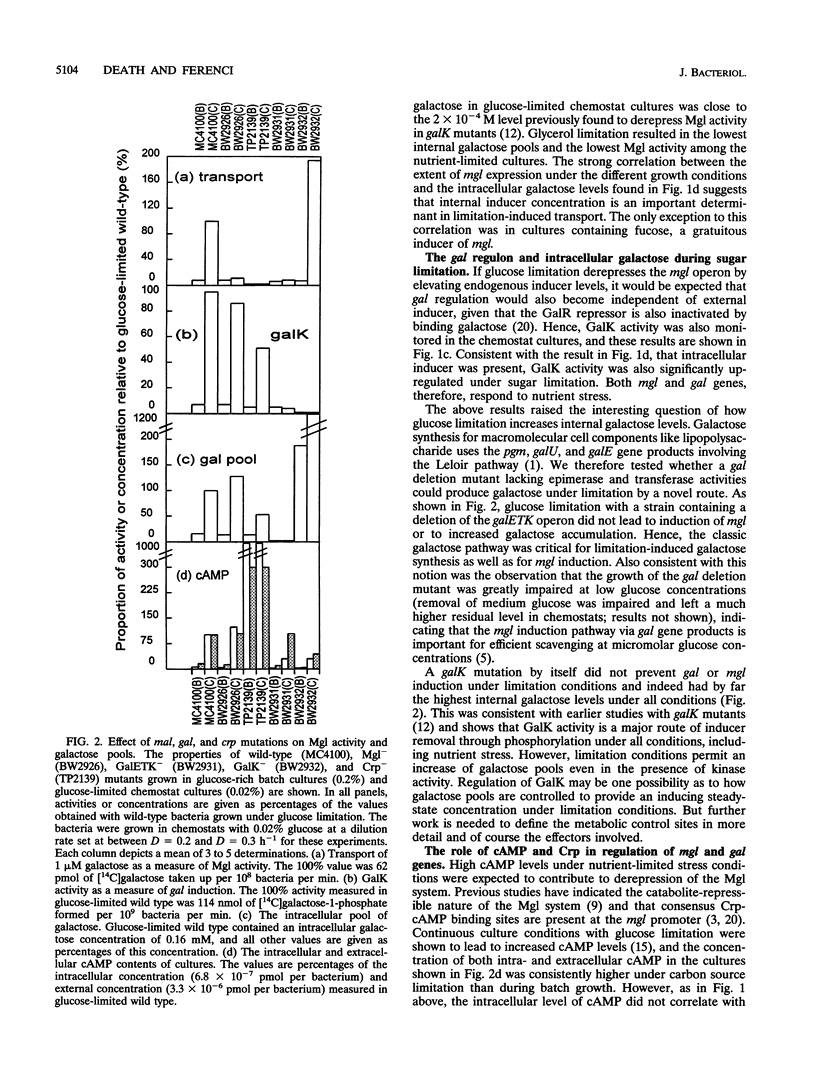
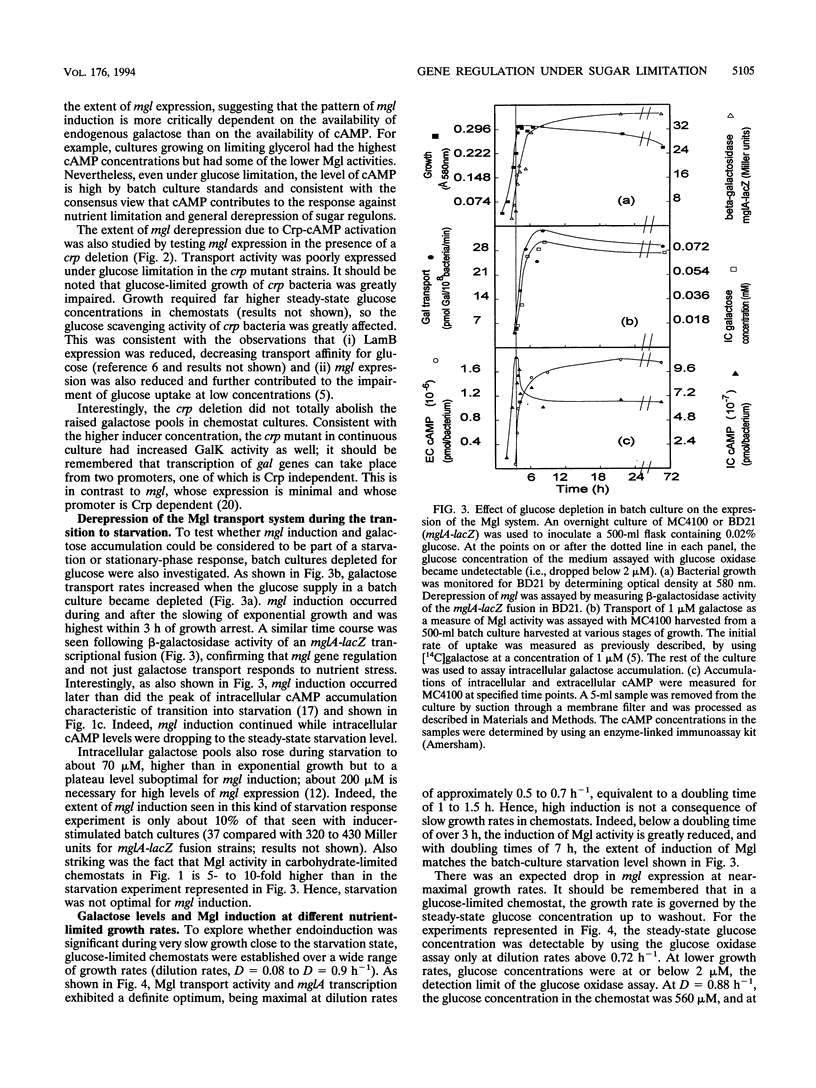
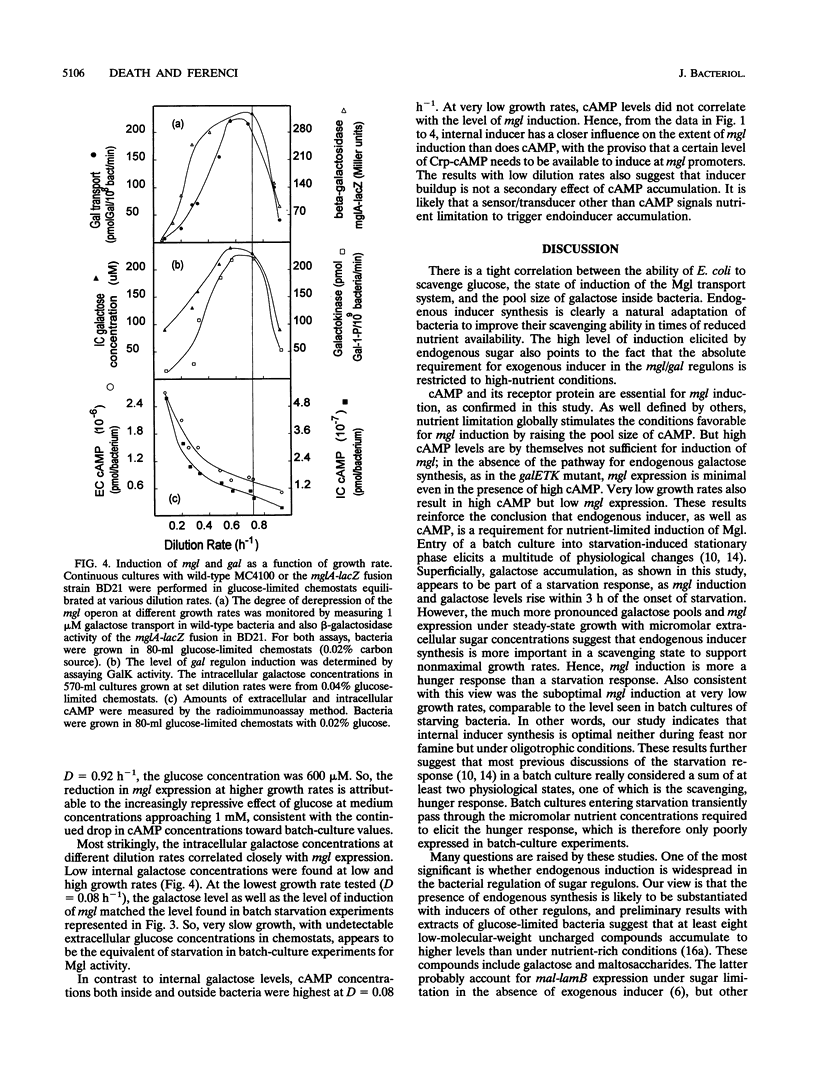
Abstract
Escherichia coli adapted to growth with low carbohydrate concentrations bypassed the requirement for exogenous inducer with at least three well-studied sugar regulons. Induction of mgl and gal genes became independent of added galactose in bacteria approaching stationary phase or during continuous culture with micromolar glucose in the medium. Bacteria became independent of exogenous induction because endogenous galactose and cyclic AMP (cAMP) pools were sufficient for high expression of mgl and gal genes under glucose limitation. Limitation-stimulated induction of mgl was dependent on a functional galETK operon for synthesis of the inducer galactose. Intracellular galactose levels were maximal not during starvation (or slow steady-state growth rates approaching starvation) but at fast growth rates with micromolar glucose. The extent of mgl/gal induction correlated better with inducer availability than with cAMP concentrations under all conditions tested. Endogenous inducer accumulation represents an adaptation to low-nutrient environments, leading to derepression of high-affinity transport systems like Mgl essential for bacterial competitiveness at low nutrient concentrations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed A. Plasmid vectors for positive galactose-resistance selection of cloned DNA in Escherichia coli. Gene. 1984 Apr;28(1):37–43. doi: 10.1016/0378-1119(84)90085-4. [DOI] [PubMed] [Google Scholar]
- Benner-Luger D., Boos W. The mglB sequence of Salmonella typhimurium LT2; promoter analysis by gene fusions and evidence for a divergently oriented gene coding for the mgl repressor. Mol Gen Genet. 1988 Nov;214(3):579–587. doi: 10.1007/BF00330498. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976 Jul 5;104(3):541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- Death A., Ferenci T. The importance of the binding-protein-dependent Mgl system to the transport of glucose in Escherichia coli growing on low sugar concentrations. Res Microbiol. 1993 Sep;144(7):529–537. doi: 10.1016/0923-2508(93)90002-j. [DOI] [PubMed] [Google Scholar]
- Death A., Notley L., Ferenci T. Derepression of LamB protein facilitates outer membrane permeation of carbohydrates into Escherichia coli under conditions of nutrient stress. J Bacteriol. 1993 Mar;175(5):1475–1483. doi: 10.1128/jb.175.5.1475-1483.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker K., Peist R., Reidl J., Kossmann M., Brand B., Boos W. Maltose and maltotriose can be formed endogenously in Escherichia coli from glucose and glucose-1-phosphate independently of enzymes of the maltose system. J Bacteriol. 1993 Sep;175(17):5655–5665. doi: 10.1128/jb.175.17.5655-5665.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrmann M., Boos W. Identification of endogenous inducers of the mal regulon in Escherichia coli. J Bacteriol. 1987 Aug;169(8):3539–3545. doi: 10.1128/jb.169.8.3539-3545.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson P. J. The inter-relationship between proton-coupled and binding-protein-dependent transport systems in bacteria. Biochem Soc Trans. 1980 Dec;8(6):678–679. doi: 10.1042/bst0080678. [DOI] [PubMed] [Google Scholar]
- Hengge-Aronis R. Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell. 1993 Jan 29;72(2):165–168. doi: 10.1016/0092-8674(93)90655-a. [DOI] [PubMed] [Google Scholar]
- Kalckar H. M. The periplasmic galactose binding protein of Escherichia coli. Science. 1971 Nov 5;174(4009):557–565. doi: 10.1126/science.174.4009.557. [DOI] [PubMed] [Google Scholar]
- Kjelleberg S., Albertson N., Flärdh K., Holmquist L., Jouper-Jaan A., Marouga R., Ostling J., Svenblad B., Weichart D. How do non-differentiating bacteria adapt to starvation? Antonie Van Leeuwenhoek. 1993;63(3-4):333–341. doi: 10.1007/BF00871228. [DOI] [PubMed] [Google Scholar]
- Matin A., Matin M. K. Cellular levels, excretion, and synthesis rates of cyclic AMP in Escherichia coli grown in continuous culture. J Bacteriol. 1982 Mar;149(3):801–807. doi: 10.1128/jb.149.3.801-807.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matin A. The molecular basis of carbon-starvation-induced general resistance in Escherichia coli. Mol Microbiol. 1991 Jan;5(1):3–10. doi: 10.1111/j.1365-2958.1991.tb01819.x. [DOI] [PubMed] [Google Scholar]
- Roy A., Glaser P., Danchin A. Aspects of the regulation of adenylate cyclase synthesis in Escherichia coli K12. J Gen Microbiol. 1988 Feb;134(2):359–367. doi: 10.1099/00221287-134-2-359. [DOI] [PubMed] [Google Scholar]
- Schultz J. E., Latter G. I., Matin A. Differential regulation by cyclic AMP of starvation protein synthesis in Escherichia coli. J Bacteriol. 1988 Sep;170(9):3903–3909. doi: 10.1128/jb.170.9.3903-3909.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weickert M. J., Adhya S. The galactose regulon of Escherichia coli. Mol Microbiol. 1993 Oct;10(2):245–251. doi: 10.1111/j.1365-2958.1993.tb01950.x. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Boos W., Kalckar H. M. Role of the galactose transport system in the retention of intracellular galactose in Escherichia coli. J Mol Biol. 1969 Apr 14;41(1):109–120. doi: 10.1016/0022-2836(69)90129-6. [DOI] [PubMed] [Google Scholar]



