Abstract
In recent years, considerable progress has been made in the treatment of children with hepatoblastoma largely due to effective pre-operative chemotherapy. Total hepatectomy and liver transplantation has emerged as an effective treatment for the small proportion of children with unresectable hepatoblastoma limited to the liver. A 5-year survival of 70% can be achieved in such cases. In contrast, the results of liver transplantation in children with hepatocellular cancer remain poor because these tumours are usually advanced with evidence of major vascular invasion and/or extrahepatic spread at the time of presentation. An exception is those children in whom the hepatocellular carcinoma is detected during surveillance of chronic liver disease – they typically have smaller tumours and frequently have a good prognosis after liver transplantation. The role of liver transplantation in children with other primary hepatic malignancies remains uncertain because experience is very limited. Liver transplantation is rarely needed in the management of children with benign liver tumours but, if other treatments have failed, it can be a life-saving intervention.
Keywords: Liver transplantation, Paediatric, Liver tumours, Hepatoblastoma
Primary malignant liver tumours make up just over 1% of all childhood cancers, with an incidence of approximately 1.0–1.5 per million children per year in the West.1 Hepatoblastoma and hepatocellular carcinoma account for the vast majority. During the last 30 years, the outcome of children with hepatoblastoma has improved dramatically, largely due to better chemotherapy but also due to surgical advances. In recent years, liver transplantation has played an increasing role in the survival of children with unresectable liver tumours, particularly hepatoblastoma. Table 1 lists those paediatric liver tumours where transplantation may be beneficial in selected cases. This article reviews the indications for liver transplantation in children with a liver tumour. Evidence is derived from a detailed search of the electronic database PubMed (search terms ‘liver transplantation and child’, ‘liver tumour and child’, and specific tumour types) and a hand search of retrieved articles and conference abstracts.
Table 1.
Paediatric liver tumours that may require liver transplantation
| Malignant liver tumours |
| Hepatoblastoma |
| Hepatocellular carcinoma (and fibrolamellar variant) |
| Transitional tumours |
| Epithelioid haemangioendothelioma |
| Undifferentiated embryonal sarcoma |
| Other sarcomas, e.g. angiosarcoma |
| Yolk sac tumour |
| Benign liver tumours |
| Haemangioendothelioma |
| Mesenchymal hamartoma |
| Other liver tumours |
| Inflammatory pseudotumour |
Malignant liver tumours
Hepatoblastoma
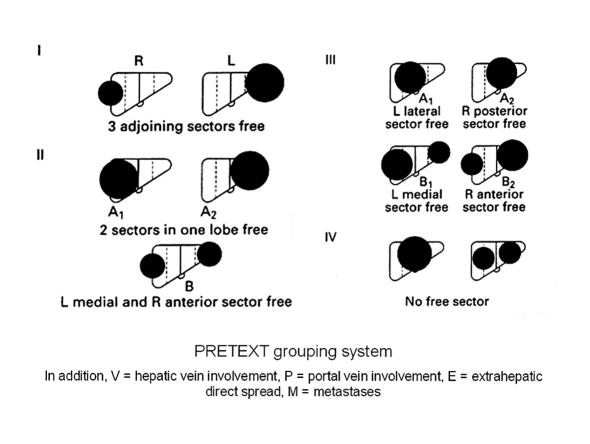
Hepatoblastoma is the commonest primary malignant liver tumour in infants and children, with a peak incidence in the first 3 years of life. In Europe, the staging system adopted by the Societé Internationale d'Oncologie Pediatrique (SIOP) is a pretreatment grouping (PRETEXT) based on the results of imaging studies.2 In the latter, the liver is divided into four sections comprising the left lateral section (Couinaud segments II and III), a left medial section (segment IV and left part of I), a right medial section (segments V and VIII and right part of I) and a right posterior section (segments VI and VII). Sectional involvement by the tumour, and the presence of portal vein, vena caval, metastatic, and extrahepatic tumour spread are noted (Fig. 1). Imaging studies should include either spiral computed tomography (CT) with dual phase contrast and/or magnetic resonance imaging (MRI).
Figure 1.
SIOPEL pretreatment (PRETEXT) grouping system for malignant liver tumours in children.
Effective chemotherapy and complete surgical resection of the tumour are the mainstays of therapy. The European SIOPEL studies have concentrated on pre-operative chemotherapy:3,4 all patients except those with small peripheral tumours are treated by pre-operative chemotherapy, typically cisplatin and doxorubicin (PLADO). Response to chemotherapy is assessed by radiological imaging and serum α-fetoprotein (AFP) levels. Pre-operative chemotherapy renders most tumours smaller, better demarcated from the surrounding liver, and more likely to be completely resected. Hepatoblastoma resectability has improved progressively since the introduction of neoadjuvant chemotherapy; between one and two-thirds of patients initially present with unresectable primary tumours or distant metastases and, after PLADO therapy, up to 85% of these become operable.
The aim of surgery is complete tumour excision. Extended right or left hepatectomies (trisectionectomies) are routinely performed in experienced units.5 Intra-operative ultrasound imaging helps to confirm the extent of the tumour and the proximity of major intrahepatic vascular structures. Resection of the hepatoblastoma must be complete but resection margins do not need to be more than a few millimetres.6
Numerous innovative techniques have been used to treat large central tumours and those involving the hepatic vein confluence or inferior vena cava.7–16 There are few reports of these relatively high-risk approaches in children and their utility is limited. However, these techniques are sometimes useful when dealing with massive benign tumours or when liver transplantation is not an option for malignant lesions. If liver transplantation is available, the risks of a heroic resection must be carefully balanced against those of transplantation.
LIVER TRANSPLANTATION
Hepatoblastoma patients who respond to chemotherapy but have unresectable tumours and no evidence of persistent extrahepatic disease should be considered for orthotopic liver transplantation (OLT). The following criteria are used by SIOPEL to select potential candidates for OLT:
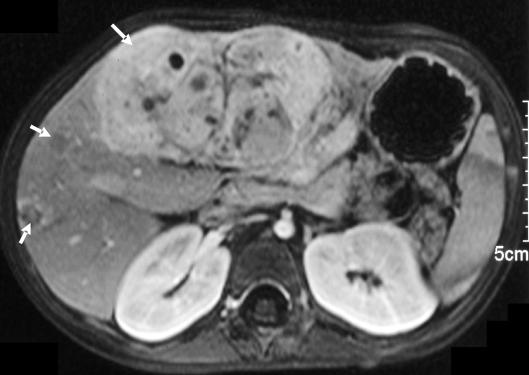
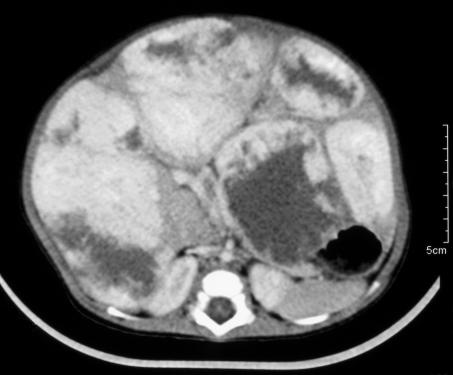
Multifocal PRETEXT IV disease, i.e. tumour in all four liver sections (Fig. 2)
Unifocal PRETEXT IV disease. This is relatively rare but unless pre-operative chemotherapy ‘down-stages’ the tumour to leave a clear margin from the anatomical border of one lateral section (indicating prior compression/displacement rather than tumour invasion), transplantation should be considered
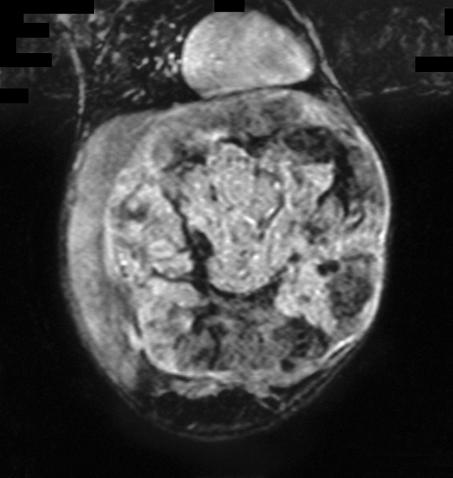
PRETEXT III disease where proximity to major vessels makes adequate tumour clearance doubtful (Fig. 3)
Tumour extension into the vena cava and/or all three hepatic veins
Invasion of the main and/or both left and right branches of the portal vein
Intrahepatic recurrent or residual tumour after previous resection (‘rescue’ transplant).
These are useful guidelines but some uncertainty remains. First, it is recommended that all patients with multifocal PRETEXT IV tumours should undergo primary OLT, even if one of the liver sections is apparently clear after pre-operative chemotherapy.17,18 In support of this, there are anecdotal reports of the presence of viable tumour foci within areas of total hepatectomy specimens despite the apparent disappearance of tumour nodules from these areas after preoperative chemotherapy.19 In addition, the results of primary OLT for multifocal PRETEXT IV hepatoblastoma in SIOPEL-1 were excellent: all 6 of these patients survived.20 However, some PRETEXT IV multifocal tumours can be successfully down-staged by pre-operative chemotherapy allowing long-term survival by partial hepatic resection and chemotherapy. Furthermore, the disappearance of pulmonary metastases with chemotherapy is usually taken to indicate their eradication – so why should similar resolution of hepatic nodules not be regarded as successful treatment? Response to chemotherapy may be critical in deciding the surgical management of children with multifocal PRETEXT IV disease: good responders who are confidently downstaged to PRETEXT III disease probably do well with partial hepatectomy but all others are best treated by total hepatectomy and OLT.
Figure 2.
Unresectable multifocal PRETEXT IV hepatoblastoma in a 3-year-old child treated by liver transplantation. Axial magnetic resonance scan with gadolinium enhancement showing wide-spread multifocal tumour.
Figure 3.
Coronal magnetic resonance scan of a large unresectable PRETEXT III hepatoblastoma in a 7-month-old infant, only partially responsive to chemotherapy. Treated by total hepatectomy and liver transplantation.
Second, the risk of an incomplete resection supports the concept of primary transplantation in some PRETEXT III cases. However, the presence of microscopic residual disease at the resection margin is not a major adverse determinant of survival.4,21,22 None of the 11 patients with positive margins in SIOPEL-1 underwent a second resection and all but one was treated by postoperative chemotherapy alone. None developed recurrent tumour and all the survivors were in complete remission 5 years later.22 In SIOPEL-2, microscopic residual disease was present in 13 children after neoadjuvant chemotherapy and delayed surgery; all survived without recurrent disease.4 It is likely that the chemosensitivity of the tumour is the key to event-free survival in these cases. Despite these observations, it is inappropriate to embark on a resection where tumour clearance is doubtful and these patients are best treated by primary liver transplantation.
Finally, in their important analysis of outcome after transplantation for hepatoblastoma, Otte et al.20 determined that overall survival was 82% in children who received a primary transplant but only 30% in those who had undergone a ‘rescue’ transplant performed after an incomplete resection or after intrahepatic recurrence following partial hepatectomy. For some, the concept of rescue transplantation is controversial in the context of a limited supply of donor organs but it seems unethical to deny a child this chance of survival.
Absolute contra-indications to OLT for unresectable hepatoblastoma include persistent pulmonary metastases and viable extrahepatic tumour not amenable to surgical resection. The tumour should show at least a partial response to chemotherapy (decrease in tumour size and serum AFP); stable or progressive disease is a relative contra-indication to OLT.20,23 Lung secondaries that respond completely to chemotherapy with or without surgical resection do not pose a contra-indication. Similarly, initial involvement of the portal or hepatic veins/vena cava is not a contra-indication; although the outcome is probably worse, long-term survival is possible in 54–77% of cases.20,24
Potential candidates for OLT not only require careful evaluation of their tumour but also their fitness for transplant. Doxorubicin is cardiotoxic and cisplatin is both nephrotoxic and ototoxic. A detailed echocardiogram and assessment of renal function is essential prior to transplant. The child's nutritional status should be optimised. Cyclical chemotherapy must be continued up until the time of transplant to maintain control of the tumour. The interval between the last course of chemotherapy and OLT should not exceed 4 weeks. If the patient is listed for a deceased donor liver, there should be a good chance of obtaining a graft during this period. If not, a live-related donor liver transplant should be considered.
Some authors recommend that the retrohepatic vena cava should be removed en bloc with the liver during the transplant.20 The cava can be reconstructed using either donor iliac vein (deceased donor) or donor jugular vein (live-related transplant).25 Others preserve the native retrohepatic vena cava in selected transplant recipients provided that there is no evidence of direct tumour involvement.24
The potential benefits of OLT must be weighed against the risks of complications and life-long immunosuppression. There is some evidence that less immunosuppression is required after OLT for hepatoblastoma, at least with living-related grafts. Gras et al.26 compared 12 children who had been transplanted for hepatoblastoma with 12 age-matched children transplanted for benign liver disease. Most patients in both groups had received a graft from a living-related donor. Rejection-free survival was 91% in the hepatoblastoma group compared to 58% in the controls who received a similar immunosuppression regimen. The authors concluded that less immunosuppression is required after OLT for hepatoblastoma, possibly because of diminished immunity as a result of pre-operative chemotherapy. If this is confirmed, giving less immunosuppression could help to reduce the combined nephrotoxicity of cisplatin and calcineurin inhibitors in these patients. However, the situation may be different with deceased donor grafts. Tiao et al.27 recorded acute cellular rejection in 4 of 8 children transplanted for hepatoblastoma, most of whom received a deceased donor graft. Mejia et al.28 noted a 70% incidence of rejection among 10 children transplanted for hepatoblastoma, 8 of whom received deceased donor grafts.
The need for post-transplant chemotherapy is currently uncertain. In the world literature review by Otte et al.,20 65 patients received post-transplant chemotherapy and 82 did not. Their respective survival rates were 77% and 70%, not statistically significantly different. The benefits of post-transplant chemotherapy must be balanced against the additional toxicity risks although hepatoblastoma chemotherapy is generally well-tolerated by the transplanted liver.
SURVIVAL AND PROGNOSIS
During the last 30 years, there has been a progressive improvement in the survival of children with hepatoblastoma. Overall 5-year survival in SIOPEL-1, which included 154 children with hepatoblastoma, was 75% [2]. In this study, tumour extent at presentation (PRETEXT grouping) and the presence of distant metastases were the most important prognostic variables. Patients with PRETEXT I tumours had a 100% 5-year survival compared to 57% for those with group IV tumours. The 5-year survival of the 31 patients presenting with lung metastases was 57% compared to 81% for those without metastases.29 Pulmonary metastases can respond well to chemotherapy. In those with persistent lung lesions, surgical resection can result in long-term survival.30,31
In the SIOPEL-1 study, 12 patients, comprising 8% of all the hepatoblastoma cases, were transplanted.20 Of the 7 who received a primary transplant, 6 were long-term survivors and one died from recurrent tumour. However, only two of the five children who underwent a ‘rescue’ transplant after a previous partial hepatectomy survived; one died from tumour recurrence and two from transplantrelated complications. In 2004, Otte et al.20 reviewed the world experience of transplantation for hepatoblastoma. Of the 147 cases identified, the overall survival rate at 6 years after transplant was 82% in the 106 patients who received a primary transplant and 30% in the 41 patients who underwent a rescue transplant. Multivariate analysis of the primary OLT group showed that only macroscopic venous invasion had a significant adverse impact on survival (54% versus 78% survival). Seven of the 12 transplant patients who had pulmonary metastases at presentation survived.20
In a report from Cincinnati, eight of 30 children treated for hepatoblastoma during a 17-year period were transplanted, 5 as a primary procedure and 3 as a rescue procedure; 7 were alive and well and one died from lymphoproliferative disease 7 years after transplant.27 A more recent report from the US analysed 135 children transplanted for hepatoblastoma between 1987 and 2004 identified from the UNOS database.32 Actuarial patient survival at 5 and 10 years was 69% and 66%, respectively. Metastatic/recurrent disease accounted for 54% of deaths. This study did not examine the effect of attempted prior resection on outcome.
Hepatocellular carcinoma
Hepatocellular carcinoma accounts for about one-third of all primary paediatric malignant liver tumours in Western societies but a much higher proportion in countries where hepatitis B is endemic.33 Hepatocellular carcinoma typically occurs in older children (10–14 years) and more commonly affects boys. The tumour may arise as a complication of preexisting liver disease.34 Hepatocellular carcinoma spreads by lymphatic and vascular invasion. Bilobar, multifocal disease is common and at least half of affected children have metastases or extrahepatic spread at presentation.35
Whilst some hepatocellular carcinomas respond to PLADO chemotherapy, the tumour is much more chemoresistant than hepatoblastoma. In the SIOPEL-1 study, partial response to PLADO chemotherapy was observed in only half the cases.36 Complete excision of the tumour offers the only chance of long-term survival but multifocal disease or distant spread frequently precludes successful resection. In the SIOPEL-1 study, 51% of hepatocellular carcinomas never became resectable and complete tumour excision was achieved in only 36%.
In adults with hepatocellular carcinoma, numerous therapies have been used to achieve palliation and, in some cases, render the tumour resectable. These include percutaneous intralesional injection of ethanol, radiofrequency thermal ablation and chemo-embolisation; local recurrence is common but survival rates comparable to those of resection have been achieved in the hands of enthusiasts.
LIVER TRANSPLANTATION
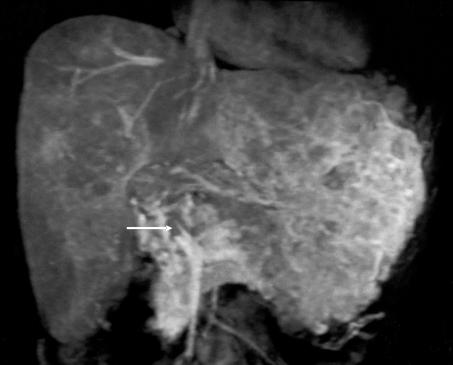
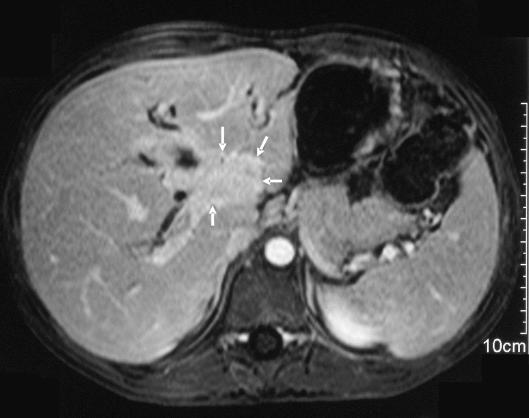
The role of liver transplantation in paediatric hepatocellular carcinoma is controversial. It is not an option in most cases because the disease is too advanced (Fig. 4). However, it should be considered early in patients with unresectable disease without extrahepatic spread and macroscopic vascular invasion. The latter is currently a contra-indication to transplantation for hepatocellular carcinoma in children. In the Pittsburgh series, all four children with non-metastatic hepatocellular carcinoma involving major intrahepatic portal veins died from recurrent tumour after transplant.24 In contrast, three of four patients with no evidence of major venous invasion or metastases were long-term survivors. Lack of response to pre-operative chemotherapy is a relative contra-indication to transplant. There were no longterm survivors in SIOPEL-1 among those who failed to respond to chemotherapy.
Figure 4.
Unresectable hepatocellular carcinoma in a 13-year-old girl without underlying liver disease. PRETEXT IV tumour with extensive vascular invasion. The arrow demonstrates tumour in the main portal vein on this coronal magnetic resonance image.
In an attempt to improve the results of liver transplantation for hepatocellular carcinoma in adults, many units have adopted the Milan criteria. Patients with a low risk of recurrent hepatocellular carcinoma after OLT are selected on the basis of their pre-operative imaging, i.e. those with a single tumour 5 cm or less in diameter or no more than three tumour nodules, each measuring 3 cm or less.37 Adjuvant treatment is given to limit tumour progression whilst awaiting transplant. However, in the original study reported by Mazzaferro et al.,37 13 of the 48 patients who met Milan criteria on pretransplant imaging had tumours that exceeded these dimensions on pathological examination of the explanted liver and their recurrence-free survival was considerably less. Furthermore, although 4-year survival rates of 75% or more can be achieved in adults transplanted according to Milan criteria,38 tumour progression and death on the waiting list result in worse than expected outcomes unless living donor liver transplantation is available. Living-related liver transplantation minimises waiting times and has prompted some to suggest that the criteria should be expanded to include larger tumours when this is an option. The age and size of most patients who require OLT for hepatocellular carcinoma usually mandates the use of a right lobe graft which carries a significant risk to the donor and demands a high level of institutional expertise.
Whether corticosteroid and calcineurin inhibitor immunosuppression increase the likelihood of tumour recurrence after OLT for hepatocellular carcinoma is debatable.39 Some adult liver transplant centres are using sirolimus with or without calcineurin inhibitors as primary immunosuppression after OLT for hepatocellular carcinoma because this immunosuppressive agent has been shown to inhibit the growth of a wide variety of cancers.
SURVIVAL AND PROGNOSIS
Poor prognostic factors for hepatocellular carcinoma include metastatic spread, large tumour size (PRETEXT grouping), lymph node metastases and macroscopic vascular invasion. In SIOPEL-1, there were 37 children with hepatocellular carcinoma and adequate follow-up and only 8 (22%) were alive and disease-free at a median of 75 months.36 None of the 12 patients presenting with lung metastases were long-term survivors. Outcomes were no better in SIOPEL-2 in which chemotherapy was intensified by the addition of carboplatin. In the US, the Pediatric Oncology Group/Children's Cancer Group reported a similar 19%, 5-year, event-free survival in 46 children and adolescents with hepatocellular carcinoma; only 8 (17%) underwent primary complete tumour resection and 7 of these survived.40 A larger study from Taiwan reported only two long-term survivors from a cohort of 55 children with hepatocellular carcinoma, more than two-thirds of whom were cirrhotic; only 18% of tumours were resectable.35
The fibrolamellar variant of hepatocellular carcinoma has traditionally been regarded as having a higher resection rate and a better prognosis compared to typical hepatocellular carcinoma. This has encouraged some to recommend aggressive surgical treatment, either liver resection or transplantation, in patients without evidence of extrahepatic spread and metastases.41 However, a recent analysis of 10 children with fibrolamellar hepatocellular carcinoma from the US showed no difference in outcome compared to typical hepatocellular carcinoma.42
Recent results of liver transplantation for hepatocellular carcinoma in children are shown in Table 2.24,32,36,43–48 One of the largest series24 was an update of a previous report from Pittsburgh.49 In this report, 14 of 19 children with hepatocellular carcinoma had underlying chronic liver disease (tyrosinaemia, progressive familial intrahepatic cholestasis, hepatitis B, etc.). Such children are frequently under regular surveillance allowing detection of their hepatocellular carcinoma at an early stage. Children with cirrhosis from various causes are also included in most other reports of liver transplantation for hepatocellular carcinoma in children. However, if we are to understand the role of transplantation in children with hepatocellular carcinoma, it is essential that subgroups of patients are identified and analysed separately: (i) those who present with an hepatocellular carcinoma (distinguishing those with background liver disease from those with healthy livers); (ii) those in whom an hepatocellular carcinoma is detected during surveillance of chronic liver disease; and (iii) those in whom an ‘incidental’ hepatocellular carcinoma is discovered in the explant when the indication for OLT was the underlying liver disease. The latter generally have an excellent prognosis.50
Table 1.
Recent reports of liver transplantation for hepatocellular carcinoma in children
| Authors | Year | n | Survival |
|---|---|---|---|
| *Yandza et al.43 | 1993 | 2 | Both alive at 2 years |
| Broughan et al.44 | 1994 | 4 | 3(75%) at mean 63 months |
| *Achilleos et al.45 | 1996 | 2 | 0(1 death from recurrence, 1 after OLT) |
| *Otte et al.46 | 1996 | 5 | 3(60%) at median 49 months |
| *Superina & Bilik et al.47 | 1996 | 3 | 3(100%) at 1–5 years |
| *Reyes et al.24 | 2000 | 19 | 79% actuarial survival at 1 year, 63% at 5 years |
| *Tatekawa et al.48 | 2001 | 2 | Both alive at 2.8 and 5 years, respectively |
| *Czauderna et al.36 | 2002 | 2 | Not stated |
| *Austin et al.32 | 2006 | 41 | 63% 5-year actuarial survival |
Includes: (i) patients with hepatocellular carcinoma discovered incidentally within the explanted liver where the indication for OLT was the underlying liver disease rather than the tumour; and/or (ii) hepatocellular carcinoma detected during surveillance of cirrhosis.
If the outcome of children with hepatocellular carcinoma is to improve, the proportion amenable to resection or transplantation must be increased. This may be achieved by greater use of intra-arterial chemotherapy, chemo-embolisation, and/or alternative chemotherapy regimens. The current SIOPEL-5 trial is exploring the utility of the anti-angiogenic agent thalidomide as a supplement to neoadjuvant chemotherapy in children and adolescents with non-cirrhotic hepatocellular carcinoma.
Other primary malignant liver tumours
Liver transplantation has occasionally been performed for other primary malignant liver tumours in children, all of which are rare. Results have generally been poor.
UNDIFFERENTIATED (EMBRYONAL) SARCOMA
This is a highly malignant mesenchymal tumour which usually affects children aged between 5–10 years. Until recently, the prognosis was poor but long-term survival has now been reported after neoadjuvant multi-agent chemotherapy and partial hepatectomy.51,52 The Brussels group described two children who underwent OLT for an unresectable sarcoma; both died within 6 months of the transplant, one from tumour recurrence.46 Dower et al.31 reported a 6-year-old boy with a non-metastatic undifferentiated sarcoma which was successfully treated by chemotherapy and transplantation; the key factor in this patient appears to have been the chemosensitivity of the tumour.
HEPATIC EPITHELIOID HAEMANGIOENDOTHELIOMA (HEHE)
HEHE is a slow growing malignant vascular tumour, distinct from haemangioendothelioma and angiosarcoma. It is most often encountered in young women. It may behave more aggressively in children.12,53 Most HEHE are large and diffuse and unresectable by partial hepatectomy. There is no consistently effective chemotherapy. In a review of five children aged 1–12 years from three European centres both patients treated by liver transplantation died within a year, one from viral infection and the other from recurrent disease.12 In another report, a child with a slow-growing tumour was successfully treated by liver transplantation.54 The role of primary liver transplantation for unresectable HEHE is, therefore, uncertain.
ANGIOSARCOMA
Angiosarcoma in children is often unresectable and lung metastases may be evident at presentation.55 Some cases represent malignant transformation of a pre-existing haemangioendothelioma.55,56 The treatment of this rare, high-grade malignancy has not been standardised. Occasional success after chemotherapy and partial hepatectomy has been reported.56,57 Two children with unresectable hepatic angiosarcoma and no evidence of extrahepatic spread have been treated by liver transplantation: one died from cytomegalovirus infection 4 months later but had no detectable residual tumour55 and the other was alive with recurrent disease 14 months later.58 Adult experience with liver transplantation for angiosarcoma has been dismal.59
YOLK SAC TUMOUR
There is one report of a 2-year-old boy with an unresectable hepatic yolk sac tumour successfully treated by chemotherapy and liver transplantation.60
Benign liver tumours
Mesenchymal hamartoma
Mesenchymal hamartoma of the liver (MHL) is the second commonest benign liver tumour in children. Typically, these are large multicystic masses sometimes reaching 20–30 cm in diameter and weighing up to 3 kg. They usually present during the first 3 years of life. Several reports have described cytogenetic abnormalities in some tumours and highlighted an association between MHL and undifferentiated embryonal sarcoma of the liver.61 They are best treated by complete excision. Very rarely, an MHL is unresectable and liver transplantation may be necessary. Two such children were reported from Pittsburgh.62 Both had undergone previous partial hepatectomies but had residual pain and progressive liver failure; one died from intra-operative bleeding and the other survived. Bejarano et al.63 also described an infant who was successfully transplanted for a recurrent MHL after a previous resection.
Hepatic haemangioendothelioma
Hepatic haemangioendotheliomas (HEs) are among the commonest liver tumours in children. They typically occur in infants and are frequently associated with cutaneous haemangiomas.64 They may present with asymptomatic hepatomegaly but can be associated with high-output cardiac failure from arteriovenous shunting or with platelet sequestration and consumptive coagulopathy. Two histological subtypes are recognised. Type 1 lesions are the commonest and consist of vascular channels lined by a single layer of plump endothelial cells separated by fibrous tissue. Type 2 HEs are composed of multiple layers of larger more pleomorphic endothelial cells with hyperchromatic nuclei forming poorly defined vascular spaces. The latter behave more aggressively and can metastasise.
Many HEs regress spontaneously. Treatment is indicated for problems such as cardiac failure, progressive abdominal distension or respiratory compromise. Corticosteroids, α-interferon or vincristine may induce or accelerate regression in some cases. For bilobar disease, hepatic artery ligation or embolisation can be effective. On rare occasions, when other attempts to control the tumour have failed, liver transplantation is the only therapeutic option. There are several reports of successful transplantation for massive HE (Fig. 5).65–68 Not all cases have survived either because of cerebral bleeding (possibly related to an associated cerebrovascular malformation),62,66 metastases from a type 2 HE,45 or transplant-related complications.66
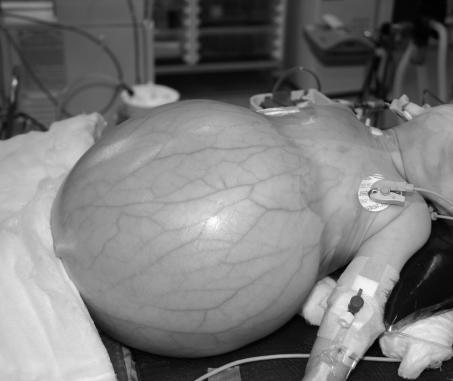
Figure 5.
Massive hepatic haemangioendothelioma in a 7-monthold infant treated by liver transplantation. (A) Clinical; (B) Axial CT scan after intravenous contrast; and (C) operative appearances. The child remains well 2 years later.
Inflammatory myofibroblastic tumours
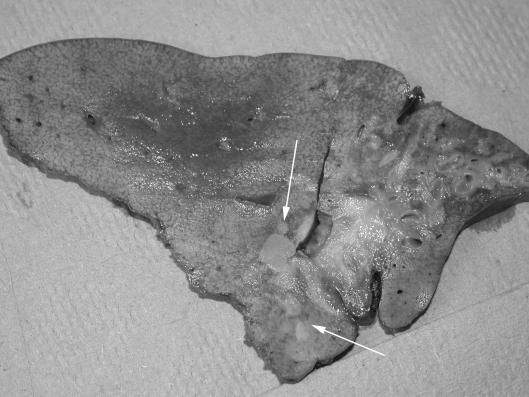
Inflammatory myofibroblastic tumours, also known as inflammatory pseudotumours, are rare proliferative lesions of unknown aetiology which can occur at any age and affect almost any organ system, including the liver. Tumours are hard and solid and composed of myofibroblasts with an admixture of plasma cells, lymphocytes, and histiocytes in a collagen stroma. Their clinical features and imaging characteristics frequently cause confusion with malignancy. Although considered benign, these lesions can have devastating local effects. Most inflammatory myofibroblastic tumours in the liver are solitary. Asymptomatic patients can be managed non-operatively whilst symptomatic lesions are amenable to resection. Some of these tumours occur at the hilum of the liver where they cause biliary obstruction and portal phlebitis.68 Some of these hilar tumours have been successfully resected but others have required treatment by liver transplantation (Fig. 6).62,69–71
Figure 6.
(A) Axial magnetic resonance image of a 7-year-old boy with an aggressive hilar inflammatory myofibroblastic tumour (arrows). (B) Gross appearance of resection specimen after hepatectomy and OLT. Note the dense hilar infiltration, left lobe atrophy and satellite nodules (arrows).69
Conclusions
The results of liver transplantation in children have steadily improved during the past 30 years and 5–10-year survival rates in elective cases are now around 90% in many centres. The challenges posed by long-term immunosuppression remain a concern but there is no doubt that liver transplantation is a lifesaving procedure for selected children with otherwise unresectable liver tumours.
NOTE: An international registry has recently been established to provide a prospective database of children transplanted for an unresectable malignant liver tumour (PLUTO: Pediatric Liver Unresectable Tumor Observatory http://pluto.cineca.org).
References
- 1.Mann JR, Kasthuri N, Raafat F, Pincott JR, Parkes SE, Muir KR, et al. Malignant hepatic tumours in children: incidence, clinical features and aetiology. Paediatr Perinat Epidemiol. 1990;4:276–89. doi: 10.1111/j.1365-3016.1990.tb00651.x. [DOI] [PubMed] [Google Scholar]
- 2.Brown J, Perilongo G, Shafford E, Keeling J, Pritchard J, Brock P, et al. Pretreatment prognostic factors for children with hepatoblastoma – results from the International Society of Paediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer. 2000;36:1418–25. doi: 10.1016/s0959-8049(00)00074-5. [DOI] [PubMed] [Google Scholar]
- 3.Pritchard J, Brown J, Shafford E, Perilongo G, Brock P, Dicks-Mireaux C, et al. Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: a successful approach – results of the first prospective study of the International Society of Paediatric Oncology. J Clin Oncol. 2000;18:3819–28. doi: 10.1200/JCO.2000.18.22.3819. [DOI] [PubMed] [Google Scholar]
- 4.Perilongo G, Shafford E, Maibach R, Aronson D, Brugieres L, Brock P, et al. Risk-adapted treatment for childhood hepatoblastoma. final report of the second study of the International Society of Paediatric Oncology-SIOPEL 2. Eur J Cancer. 2004;40:411–21. doi: 10.1016/j.ejca.2003.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Glick RD, Nadler EP, Blumgart LH, LaQuaglia MP. Extended left hepatectomy (left hepatic trisegmentectomy) in childhood. J Pediatr Surg. 2000;35:303–8. doi: 10.1016/s0022-3468(00)90029-0. [DOI] [PubMed] [Google Scholar]
- 6.Dicken BJ, Bigam DL, Lees GM. Association between surgical margins and long-term outcome in advanced hepatoblastoma. J Pediatr Surg. 2004;39:721–5. doi: 10.1016/j.jpedsurg.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 7.LaQuaglia MP, Shorter NA, Blumgart LH. Central hepatic resection for pediatric tumors. J Pediatr Surg. 2002;37:986–9. doi: 10.1053/jpsu.2002.33825. [DOI] [PubMed] [Google Scholar]
- 8.Takayama T, Makuuchi M, Kosuge T, Yamazaki S, Hasegawa H, Takayama J, et al. A hepatoblastoma originating in the caudate lobe radically resected with the inferior vena cava. Surgery. 1991;109:208–13. [PubMed] [Google Scholar]
- 9.Kaneko K, Ando H, Watanabe Y, Seo T, Nagino M, Kamiya J, et al. Aggressive preoperative management and extended surgery for inflammatory pseudotumor involving the hepatic hilum in a child. Surgery. 2001;129:757–60. doi: 10.1067/msy.2001.112966. [DOI] [PubMed] [Google Scholar]
- 10.Superina RA, Bambini D, Filler RM, Almond PS, Geissler G. A new technique for resecting ‘unresectable’ liver tumors. J Pediatr Surg. 2000;35:1294–9. doi: 10.1053/jpsu.2000.9300. [DOI] [PubMed] [Google Scholar]
- 11.Adam R, Laurent A, Azoulay D, Castaing D, Bismuth H. Two-stage hepatectomy: a planned strategy to treat irresectable liver tumors. Ann Surg. 2000;232:777–85. doi: 10.1097/00000658-200012000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharif K, English M, Ramani P, Alberti D, Otte J-B, McKiernan P, et al. Management of hepatic epithelioid haemangio-endothelioma in children: what option? Br J Cancer. 2004;90:1498–501. doi: 10.1038/sj.bjc.6601720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fusai G, Steinberg R, Prachalias A, Heaton ND, Spitz L, Rela M. Ex vivo liver surgery for extra-adrenal phaeochromocytoma. Pediatr Surg Int. 2006;22:282–5. doi: 10.1007/s00383-005-1413-x. [DOI] [PubMed] [Google Scholar]
- 14.Millar AJW, Hartley P, Khan D, Spearman W, Andronikou S, Rode H. Extended hepatic resection with transplantation back-up for an ‘unresectable’ tumour. Pediatr Surg Int. 2001;17:378–81. doi: 10.1007/s003830000531. [DOI] [PubMed] [Google Scholar]
- 15.Ein SH, Shandling B, Williams WG, Trusler G. Major hepatic tumor resection using profound hypothermia and circulation arrest. J Pediatr Surg. 1981;16:339–42. doi: 10.1016/s0022-3468(81)80691-4. [DOI] [PubMed] [Google Scholar]
- 16.Pichlmayr R, Grosse H, Hauss J, Gubernatis G, Lamesch P, Bretschneider HJ. Technique and preliminary results of extracorporeal liver surgery (bench procedure) and of surgery on the in situ perfused liver. Br J Surg. 1990;77:21–6. doi: 10.1002/bjs.1800770107. [DOI] [PubMed] [Google Scholar]
- 17.Czauderna P, Otte JB, Aronson DC, Gauthier F, Mackinlay G, Roebuck D, et al. Guidelines for surgical treatment of hepatoblastoma in the modern era – recommendations from the childhood liver tumour strategy group of the International Society of Paediatric Oncology (SIOPEL) Eur J Cancer. 2005;41:1031–6. doi: 10.1016/j.ejca.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Otte JB, de Ville de Goyet J, Reding R. Liver transplantation for hepatoblastoma: indications and contraindications in the modern era. Pediatr Transplant. 2005;9:557–65. doi: 10.1111/j.1399-3046.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 19.Dall'Igna P, Cecchetto G, Toffolutti T, Cillo U, Cecchetto A, et al. Multifocal hepatoblastoma: is there a place for partial hepatectomy? Med Pediatr Oncol. 2003;40:113–7. doi: 10.1002/mpo.10107. [DOI] [PubMed] [Google Scholar]
- 20.Otte JB, Pritchard J, Aronson DC, Brown J, Czauderna P, Maibach R, et al. Liver transplantation for hepatoblastoma: results from the International Society of Pediatric Oncology (SIOP) study SIOPEL-1 and review of the world experience. Pediatr Blood Cancer. 2004;42:74–83. doi: 10.1002/pbc.10376. [DOI] [PubMed] [Google Scholar]
- 21.Stringer MD, Hennayake S, Howard ER, Spitz L, Shafford EA, Mieli-Vergani G, et al. Improved outcome for children with hepatoblastoma. Br J Surg. 1995;82:386–91. doi: 10.1002/bjs.1800820334. [DOI] [PubMed] [Google Scholar]
- 22.Schnater JM, Aronson DC, Plaschkes J, Perilongo G, Brown J, Otte J-B, et al. Surgical view of the treatment of patients with hepatoblastoma. Results from the first prospective trial of the International Society of Pediatric Oncology Liver Tumor Study Group (SIOPEL-1) Cancer. 2002;94:1111–20. [PubMed] [Google Scholar]
- 23.Pimpalwar AP, Sharif K, Ramani P, Stevens M, Grundy R, Morland B, et al. Strategy for hepatoblastoma management: transplant versus nontransplant surgery. J Pediatr Surg. 2002;37:240–5. doi: 10.1053/jpsu.2002.30264. [DOI] [PubMed] [Google Scholar]
- 24.Reyes JD, Carr B, Dvorchik I, Kocoshis S, Jaffe R, Gerber D, et al. Liver transplantation and chemotherapy for hepatoblastoma and hepatocellular cancer in childhood and adolescence. J Pediatr. 2000;136:795–804. [PubMed] [Google Scholar]
- 25.Chardot C, Saint Martin C, Gilles A, Brichard B, Janssen M, Sokal E, et al. Living-related liver transplantation and vena cava reconstruction after total hepatectomy including the vena cava for hepatoblastoma. Transplantation. 2002;73:90–2. doi: 10.1097/00007890-200201150-00017. [DOI] [PubMed] [Google Scholar]
- 26.Gras J, Reding R, Brichard B, et al. Optimal therapeutic management of unresectable hepatoblastoma in children: primary liver transplantation with a living related donor graft combined with low immunosuppression. Pediatr Transpl. 2005;9(Suppl 6):114. [Google Scholar]
- 27.Tiao GM, Bobey N, Allen S, Nieves N, Alonso M, Bucuvalas J, et al. The current management of hepatoblastoma: a combination of chemotherapy, conventional resection, and liver transplantation. J Pediatr. 2005;146:204–11. doi: 10.1016/j.jpeds.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Mejia A, Langnas AN, Shaw BW, Torres C, Sudan DL. Living and deceased donor liver transplantation for unresectable hepatoblastoma at a single centre. Clin Transplant. 2005;19:721–5. doi: 10.1111/j.1399-0012.2005.00410.x. [DOI] [PubMed] [Google Scholar]
- 29.Perilongo G, Brown J, Shafford E, Brock P, De Camargo B, Keeling JW, et al. Hepatoblastoma presenting with lung metastases: treatment results of the first cooperative, prospective study of the International Society of Paediatric Oncology on childhood liver tumors. Cancer. 2000;89:1845–53. doi: 10.1002/1097-0142(20001015)89:8<1845::aid-cncr27>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 30.Passmore SJ, Noblett HR, Wisehart JD, Mott MG. Prolonged survival following multiple thoracotomies for metastatic hepatoblastoma. Med Pediatr Oncol. 1995;24:58–60. doi: 10.1002/mpo.2950240113. [DOI] [PubMed] [Google Scholar]
- 31.Dower NA, Smith LJ, Lees G, Kneteman N, Idikio H, Emond J, et al. Experience with aggressive therapy in three children with unresectable malignant liver tumors. Med Pediatr Oncol. 2000;34:132–5. doi: 10.1002/(sici)1096-911x(200002)34:2<132::aid-mpo11>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 32.Austin MT, Leys CM, Feurer ID, Lovvorn HN, 3rd, O'Neill JA, Jr, Pinson CW, et al. Liver transplantation for childhood hepatic malignancy: a review of the United Network for Organ Sharing (UNOS) database. J Pediatr Surg. 2006;41:182–6. doi: 10.1016/j.jpedsurg.2005.10.091. [DOI] [PubMed] [Google Scholar]
- 33.Moore SW, Hesseling PB, Wessels G, Schneider JW. Hepatocellular carcinoma in children. Pediatr Surg Int. 1997;12:266–70. doi: 10.1007/BF01372147. [DOI] [PubMed] [Google Scholar]
- 34.Stringer MD. Liver tumors. Semin Pediatr Surg. 2000;9:196–208. doi: 10.1053/spsu.2000.18844. [DOI] [PubMed] [Google Scholar]
- 35.Chen JC, Chen CC, Chen WJ, Laai HS, Hung WT, Lee PH. Hepatocellular carcinoma in children: clinical review and comparison with adult cases. J Pediatr Surg. 1998;33:1350–4. doi: 10.1016/s0022-3468(98)90005-7. [DOI] [PubMed] [Google Scholar]
- 36.Czauderna P, Mackinlay G, Perilongo G, Brown J, Shafford E, Aronson D, et al. Hepatocellular carcinoma in children: results of the first prospective study of the International Society of Pediatric Oncology Group. J Pediatr Surg. 2002;20:2798–804. doi: 10.1200/JCO.2002.06.102. [DOI] [PubMed] [Google Scholar]
- 37.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–9. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 38.Lo CM, Fan ST. Liver transplantation for hepatocellular carcinoma. Br J Surg. 2004;91:131–3. doi: 10.1002/bjs.4503. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz M, Konstadoulakis M, Roayaie S. Recurrence of hepatocellular carcinoma after liver transplantation: is immunosuppression a factor? Liver Transplant. 2005;11:494–6. doi: 10.1002/lt.20413. [DOI] [PubMed] [Google Scholar]
- 40.Katzenstein HM, Krailo MD, Malogolowkin MH, Ortega JA, Liu-Mares W, Douglass EC, et al. Hepatocellular carcinoma in children and adolescents: results from the Pediatric Oncology Group and the Children's Cancer Group Intergroup study. J Clin Oncol. 2002;20:2789–97. doi: 10.1200/JCO.2002.06.155. [DOI] [PubMed] [Google Scholar]
- 41.El-Gazzaz G, Wong W, El-Hadary MK, Gunson BK, Mirza DF, Mayer AD, et al. Outcome of liver resection and transplantation for fibrolamellar hepatocellular carcinoma. Transplant Int. 2000;13(Suppl 1):S406–9. doi: 10.1007/s001470050372. [DOI] [PubMed] [Google Scholar]
- 42.Katzenstein HM, Krailo MD, Malogolowkin MH, Ortega JA, Qu W, Douglass EC, et al. Fibrolamellar hepatocellular carcinoma in children and adolescents. Cancer. 2003;97:2006–12. doi: 10.1002/cncr.11292. [DOI] [PubMed] [Google Scholar]
- 43.Yandza T, Alvarez F, Laurent J, Gauthier F, Dubousset AM, Valayer J. Pediatric liver transplantation for primary hepatocellular carcinoma associated with hepatitis virus infection. Transplant Int. 1993;6:95–8. doi: 10.1007/BF00336652. [DOI] [PubMed] [Google Scholar]
- 44.Broughan TA, Esquivel CO, Vogt DP, Griffin GC, Norris DG. Pretransplant chemotherapy in pediatric hepatocellular carcinoma. J Pediatr Surg. 1994;29:1319–22. doi: 10.1016/0022-3468(94)90106-6. [DOI] [PubMed] [Google Scholar]
- 45.Achilleos OA, Buist LJ, Kelly DA, Raafat F, McMaster P, Mayer AD, et al. Unresectable hepatic tumors in childhood and the role of liver transplantation. J Pediatr Surg. 1996;31:1563–7. doi: 10.1016/s0022-3468(96)90179-7. [DOI] [PubMed] [Google Scholar]
- 46.Otte JB, Aronson D, Vraux H, de Ville de Goyet J, Reding S, Ninane J, et al. Preoperative chemotherapy, major liver resection, and transplantation for primary malignancies in children. Transplant Proc. 1996;28:2393–4. [PubMed] [Google Scholar]
- 47.Superina R, Bilik R. Results of liver transplantation in children with unresectable liver tumors. J Pediatr Surg. 1996;31:835–9. doi: 10.1016/s0022-3468(96)90147-5. [DOI] [PubMed] [Google Scholar]
- 48.Tatekawa Y, Asonuma K, Uemoto S, Inomata Y, Tanaka K. Liver transplantation for biliary atresia associated with malignant hepatic tumors. J Pediatr Surg. 2001;36:436–9. doi: 10.1053/jpsu.2001.21600. [DOI] [PubMed] [Google Scholar]
- 49.Tagge EP, Tagge DU, Reyes J, Tzakis A, Iwatsuki S, Starzl TE, et al. Resection, including transplantation, for hepatoblastoma and hepatocellular carcinoma: impact on survival. J Pediatr Surg. 1992;27:292–7. doi: 10.1016/0022-3468(92)90849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Esquivel CO, Gutierrez C, Cox KL, Garcia-Kennedy R, Berquist W, Concepcion W. Hepatocellular carcinoma and liver cell dysplasia in children with chronic liver disease. J Pediatr Surg. 1994;29:1465–9. doi: 10.1016/0022-3468(94)90145-7. [DOI] [PubMed] [Google Scholar]
- 51.Bisogno G, Pilz T, Perilongo G, Ferrari A, Harms D, Ninfo V, et al. Undifferentiated sarcoma of the liver in childhood: a curable disease. Cancer. 2002;94:252–7. doi: 10.1002/cncr.10191. [DOI] [PubMed] [Google Scholar]
- 52.Kim DY, Kim KH, Jung SE, Lee SC, Park KW, Kim WK. Undifferentiated (embryonal) sarcoma of the liver: combination treatment by surgery and chemotherapy. J Pediatr Surg. 2002;37:1419–23. doi: 10.1053/jpsu.2002.35404. [DOI] [PubMed] [Google Scholar]
- 53.Makhlouf HR, Ishak KG, Goodman ZD. Epithelioid hemangioendothelioma of the liver: a clinicopathologic study of 137 cases. Cancer. 1999;85:562–82. doi: 10.1002/(sici)1097-0142(19990201)85:3<562::aid-cncr7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 54.Taege C, Holzhausen H, Gunter G, Flemming P, Rodeck B, Rath FW. Malignant epithelioid hemangioendothelioma of the liver – a very rare tumor in children [German] Pathologe. 1999;20:345–50. doi: 10.1007/s002920050369. [DOI] [PubMed] [Google Scholar]
- 55.Awan S, Davenport M, Portmann B, Howard ER. Angiosarcoma of the liver in children. J Pediatr Surg. 1996;31:1729–32. doi: 10.1016/s0022-3468(96)90065-2. [DOI] [PubMed] [Google Scholar]
- 56.Kirchner SG, Heller RM, Kasselberg AG, Greene HL. Infantile hepatic hemangioendothelioma with subsequent malignant degeneration. Pediatr Radiol. 1981;11:42–5. doi: 10.1007/BF00972043. [DOI] [PubMed] [Google Scholar]
- 57.Gunawardena SW, Trautwein LM, Fineglod MJ, Ogden AK. Hepatic angiosarcoma in a child: successful treatment with surgery and adjuvant chemotherapy. Med Pediatr Oncol. 1997;28:139–43. doi: 10.1002/(sici)1096-911x(199702)28:2<139::aid-mpo9>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 58.Dimashkieh HH, Mo JQ, Wyatt-Ashmead J, Collins MH. Pediatric hepatic angiosarcoma: case report and review of the literature. Pediatr Dev Pathol. 2004;7:527–32. doi: 10.1007/s10024-004-4041-x. [DOI] [PubMed] [Google Scholar]
- 59.Penn I. Hepatic transplantation for primary and metastatic cancers of the liver. Surgery. 1991;110:726–35. [PubMed] [Google Scholar]
- 60.Abramson LP, Pillai S, Acton R, Melin-Aldana H, Superina R. Successful orthotopic liver transplantation for treatment of a hepatic yolk sac tumor. J Pediatr Surg. 2005;40:1185–7. doi: 10.1016/j.jpedsurg.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 61.Stringer MD, Alizai NK. Mesenchymal hamartoma of the liver: a systematic review. J Pediatr Surg. 2005;40:1681–90. doi: 10.1016/j.jpedsurg.2005.07.052. [DOI] [PubMed] [Google Scholar]
- 62.Tepetes K, Selby R, Webb M, Madariaga JR, Iwatsuki S, Starzl TE. Orthotopic liver transplantation for benign hepatic neoplasms. Arch Surg. 1995;130:153–6. doi: 10.1001/archsurg.1995.01430020043005. [DOI] [PubMed] [Google Scholar]
- 63.Bejarano PA, Serrano MF, Casillas J, Dehner LP, Kato T, Mittal N, et al. Concurrent infantile hemangioendothelioma and mesenchymal hamartoma in a developmentally arrested liver of an infant requiring hepatic transplantation. Pediatr Dev Pathol. 2003;6:552–7. doi: 10.1007/s10024-003-3024-7. [DOI] [PubMed] [Google Scholar]
- 64.Davenport M. Haemangiomas and other vascular anomalies. In: Howard ER, Stringer MD, Colombani PM, editors. Surgery of the Liver, Bile-Ducts and Pancreas in children. 2nd edn. London: Arnold; 2002. pp. 219–38. [Google Scholar]
- 65.Egawa H, Berquist W, Garcia-Kennedy R, Cox KL, Concepcion W, So SK, et al. Respiratory distress from benign liver tumors: a report of two unusual cases treated with hepatic transplantation. J Pediatr Gastroenterol Nutr. 1994;19:114–7. doi: 10.1097/00005176-199407000-00020. [DOI] [PubMed] [Google Scholar]
- 66.Daller JA, Bueno J, Gutierrez J, Dvorchik I, Towbin RB, Dickman PS, et al. Hepatic hemangioendothelioma: clinical experience and management strategy. J Pediatr Surg. 1999;34:98–106. doi: 10.1016/s0022-3468(99)90237-3. [DOI] [PubMed] [Google Scholar]
- 67.Kasahara M, Kiuchi T, Haga H, Uemoto S, Uryuhara K, Fujimoto Y, et al. Monosegmental living-donor liver transplantation for infantile hepatic hemangioendothelioma. J Pediatr Surg. 2003;38:1108–11. doi: 10.1016/s0022-3468(03)00206-9. [DOI] [PubMed] [Google Scholar]
- 68.Walsh R, Harrington J, Beneck D, Ozkaynak MF. Congenital infantile hepatic hemangioendothelioma type II treated with orthotopic liver transplantation. J Pediatr Hematol Oncol. 2004;26:121–3. doi: 10.1097/00043426-200402000-00014. [DOI] [PubMed] [Google Scholar]
- 69.Dasgupta D, Guthrie A, McClean P, Davison S, Luntley J, Rajwal S, et al. Liver transplantation for a hilar inflammatory myofibroblastic tumor. Pediatr Transplant. 2004;8:517–21. doi: 10.1111/j.1399-3046.2004.00206.x. [DOI] [PubMed] [Google Scholar]
- 70.Heneghan MA, Kaplan CG, Priebe CJ, Jr, Partin JS. Inflammatory pseudotumor of the liver: a rare cause of obstructive jaundice and portal hypertension in a child. Pediatr Radiol. 1984;14:433–5. doi: 10.1007/BF02343436. [DOI] [PubMed] [Google Scholar]
- 71.Kim HB, Maller E, Redd D, Hebra A, Davidoff A, Buzby M, et al. Orthotopic liver transplantation for inflammatory myofibroblastic tumour of the liver hilum. J Pediatr Surg. 1996;31:840–2. doi: 10.1016/s0022-3468(96)90148-7. [DOI] [PubMed] [Google Scholar]