Abstract
INTRODUCTION
The management of bile leaks following laparoscopic cholecystectomy has evolved with increased experience of ERCP and laparoscopy. The purpose of this study was to determine the impact of a minimally invasive management protocol.
PATIENTS AND METHODS
Twenty-four patients with a bile leak following laparoscopic cholecystectomy were recorded consecutively between 1993 and 2003. Between 1993–1998, 10 patients were managed on a case-by-case basis. Between 1998–2003, 14 patients were managed according to a minimally invasive protocol utilising ERC/biliary stenting and re-laparoscopy if indicated.
RESULTS
Bile leaks presented as bile in a drain left in situ post laparoscopic cholecystectomy (8/10 versus 10/14) or biliary peritonitis (2/10 versus 4/14). Prior to 1998, neither ERC nor laparoscopy were utilised routinely. During this period, 4/10 patients recovered with conservative management and 6/10 (60%) underwent laparotomy. There was one postoperative death and median hospital stay post laparoscopic cholecystectomy was 10 days (range, 5–30 days). In the protocol era, ERC ± stenting was performed in 11/14 (P = 0.01 versus pre-protocol) with the main indication being a persistent bile leak. Re-laparoscopy was necessary in 5/14 (P = 0.05 versus preprotocol). No laparotomies were performed (P < 0.01 versus pre-protocol) and there were no postoperative deaths. Median hospital stay was 11 days (range, 5–55 days).
CONCLUSIONS
The introduction of a minimally invasive protocol utilising ERC and re-laparoscopy offers an effective modern algorithm for the management of bile leaks after laparoscopic cholecystectomy.
Keywords: Bile leak, Laparoscopic cholecystectomy
bile leak after laparoscopic cholecystectomy is uncommon but can occur in 0.3–2.7% of patients.1–3 It is defined as the persistent leakage of bile from the biliary tree. This can arise from an injury to the common bile/hepatic duct but it is generally accepted that the vast majority arise from the cystic duct stump or a sub-vesical duct of Luschka.3 A bile leak may result in a biliary fistula, a subhepatic/subphrenic collection and localised or generalised peritonitis. Clearly, this can be associated with significant morbidity and even mortality, particularly if it is not identified and treated at an early stage.4
In the early 1990s when both laparoscopic cholecystectomy and minimally invasive techniques were in their infancy, bile leaks were managed conservatively; if the patient did not improve, a laparotomy was often performed. Management was anecdotal and based upon the experience of the surgeon. However, with the advent of improved radiological percutaneous drainage, therapeutic endoscopic retrograde cholangiography (ERC)5–7 and increased confidence with laparoscopic techniques including suturing,8,9 it became clear that bile leaks could be managed in a minimally invasive manner, potentially reducing morbidity and mortality. Prompt access to the full range of techniques is important, as is a structured approach. In 1998, the hepatobiliary unit at Leicester Royal Infirmary (LRI) introduced a protocol for the minimally invasive management of bile leaks. The purpose of this study is to compare patient outcome before and after introduction of the protocol in order to determine the impact of a structured minimally invasive approach to the management of bile leaks.
Patients and Methods
All bile leaks following laparoscopic cholecystectomy managed at LRI were recorded prospectively between 1993 and 2003. As a tertiary referral centre, these included patients from surrounding district general hospitals. Those who sustained a major bile duct injury were excluded. The case notes were reviewed retrospectively for each patient.
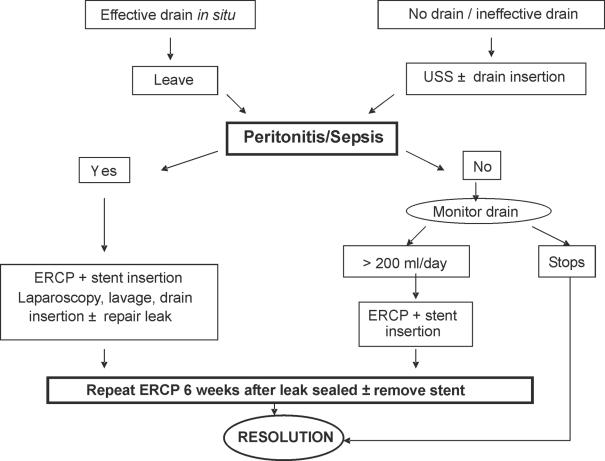
Prior to March 1998, bile leaks were managed on a caseby-case basis. Following this, a minimally invasive protocol was set up in order to provide a clear stepwise approach for the management of bile leaks (Fig. 1). If this is suspected on clinical grounds, the first step is to establish and maintain adequate drainage. If a drain was left in situ at the initial procedure and continued to drain bile, this is left. If not, then an ultrasound scan (USS) is performed and any collection identified drained percutaneously under USS guidance.
Figure 1.
Protocol for the minimally invasive management of bile leaks post laparoscopic cholecystectomy (1998–2003).
If the drainage is successful and adequate then it is unusual for life-threatening peritonitis or sepsis to develop subsequently and management is conservative with antibiotics and a daily assessment of drain output. In our experience, drainage of 200 ml or less of bile per day, and reducing over a period of a few days, is likely to subside on its own. In some cases, this may be all that is necessary and the bile leak will slowly reduce and stop. However, if there is persistent drainage of 200 ml or more of bile per day over a period of a few days, further intervention is necessary and an elective ERC is performed. This may demonstrate the site of bile leak but in any event permits the placement of a (5–7-cm long) internal stent between the common bile duct and the duodenum. Furthermore, any stones/sludge obstructing the free flow of bile down the common bile duct into the duodenum can be identified and removed. If the patient is unwell with significant sepsis despite adequate external drainage of bile, then an ERC and stent insertion is performed urgently. The bile leak invariably reduces and stops, allowing resolution of sepsis with conservative measures. Patients can then be discharged and a second ERC is undertaken 6 weeks later when the leak has completely sealed, in order to remove the stent.
The development of peritonitis is life-threatening and implies that drainage of the bile leak is inadequate. If this develops, an emergency re-laparoscopy is performed. This is performed using the port sites placed at the original laparoscopic cholecystectomy. All bile and any collections are aspirated and the peritoneal cavity thoroughly irrigated until the effluent is clear. At this point, the gall-bladder fossa and cystic duct stump are carefully inspected in order to try to identify the site of the bile leak. If this can be located, a 3.0 vicryl suture is used to close this laparoscopically and an 18-Fr low suction drain is left in the gall bladder fossa.
Data analysis
Data were not normally distributed and results are expressed as raw numbers (%) or median values (range). Continuous variables were analysed using Mann-Whitney U-tests. Categorical data were compared using Fisher's exact test. A P-value < 0.05 was considered to be significant.
Results
A total of 24 patients with a bile leak following laparoscopic cholecystectomy for symptomatic gallstones were managed at LRI between 1993 and 2003. Ten of these were between 1993–1998, before the introduction of the minimally invasive protocol. Fourteen individuals had a bile leak following this and were managed according to the protocol. Pre-operative demographics, indications for surgery and operative details are shown in Table 1. All patients initially had a standard 4-port procedure performed mainly by consultant surgeons. More emergency cholecystectomies were performed and the median operative time was significantly less in the latter group. On table, cholangiography was not routinely undertaken and was unremarkable in the 3 patients in which it was performed. Three procedures required conversion in the pre-protocol group; the anatomy of the biliary tree was unclear in one patient and in two others there were dense adhesions in the right upper quadrant (RUQ). Subsequently, two procedures required conversion; due to bleeding from the cystic artery in one and the presence of an inflammatory phlegmon around the gall-bladder in the other.
Table 1.
Patient demographics, indications for surgery and operative details
| No protocol 1993–1998 (n = 10) | Minimally invasive protocol 1998–2003 (n = 14) | P-value | |
|---|---|---|---|
| Patient age (years) | 51 (25–70) | 63 (28–73) | 0.26 |
| Sex (male/female) | 5/5 | 6/8 | 1.00 |
| Indication for surgery | 0.70 | ||
| Biliary colic/chronic cholecystitis | 4 (40) | 8 (57) | |
| Acute cholecystitis | 5 (50) | 3 (22) | |
| Empyema | 0 | 1(7) | |
| Previous obstructive jaundice | 1 (10) | 1 (7) | |
| Pancreatitis | 0 | 1(7) | |
| Pre-operative ERCP ± stone removal | 5 (50) | 2 (14) | 0.09 |
| ASA grade | 0.13 | ||
| I | 4(40) | 8 (57) | |
| II | 4 (40) | 4 (29) | |
| III | 2 (20) | 2 (14) | |
| Elective/emergency surgery | 10/0 (100/0) | 9/5 (64/36) | 0.05 |
| Grade of surgeon | 0.55 | ||
| Consultant | 8 (80) | 13 (93) | |
| SpR | 2 (20) | 1(7) | |
| Duration procedure (min) | 150 (105–210) | 95 (25–135) | < 0.01 |
| Operative findings | 0.81 | ||
| Quiescent GB | 6 (60) | 9 (64) | |
| Inflamed GB | 4 (40) | 4 (29) | |
| Gangrenous GB | 0 | 1(7) | |
| OTC | 2 (20) | 1 (7) | 0.55 |
| Conversion to open procedure | 3 (30) | 2 (14) | 0.61 |
| Postoperative drain | 10 (100) | 11 (79) | 0.24 |
GB, gall-bladder; OTC, on-table cholangiogram.
Values expressed as raw data (%) or median (range).
The gall-bladder fossa was drained postoperatively in the majority of patients and bile leaks most frequently presented as bile in this drain the day after surgery (8/10 preprotocol, 10/14 protocol). However, biliary peritonitis was the first sign of a bile leak in 2 pre-protocol patients and 4 individuals in the protocol group. Both of those in the former and one patient in the latter group had their drains removed on the first postoperative day as there had been minimal drainage and subsequently developed peritonitis over the next 48 h. The remaining 3 patients post-1998 were not left with a drain postoperatively and presented 2, 3 and 3 days (respectively) after laparoscopic cholecystectomy.
The therapeutic procedures performed are summarised in Table 2. Prior to 1998, ERC was not routinely used in the management of patients with bile leaks. Although this settled with conservative management in 4 (40%) individuals, the remaining 6 (60%) required operative intervention. The indications for surgery were biliary peritonitis following early drain removal in two, persistent drainage of bile (3, 5 and 8 days) in three and the development of a large subphrenic collection in association with a persistent bile leak (20 days) in a septic patient on ITU. All six individuals underwent a laparotomy with on-table cholangiography routinely performed. The site of the leak was an inadequately secured cystic duct (n = 2), a hole just proximal to clips/ligation of the cystic duct (n = 2) or a hole at the junction of the cystic duct and common bile duct (CBD; n = 2). In each case, the site of the leak was sutured and a thorough washout/drainage performed. CBD stones were only identified in one individual who went on to have exploration of the CBD and stone removal with insertion of a T-tube. A postoperative T-tube cholangiogram was unremarkable and this was removed 12 days after insertion.
Table 2.
Management of bile leaks before and after the introduction of a minimally invasive protocol
| No protocol 1993–1998 (n = 10) | Minimally invasive protocol 1998–2003 (n = 14) | P-value | |
|---|---|---|---|
| Percutaneous USS-guided aspiration/drainage | 3 (30) | 4 (29) | 1.00 |
| ERCP | 2 (20) | 11 (79) | 0.01 |
| Stone extracted | 0 | 4(29) | |
| Endoscopic sphincterotomy | 0 | 4(29) | |
| Stent insertion | 1 (10) | 10 (72) | |
| Laparoscopy | 0 | 5(36) | 0.05 |
| Laparotomy | 6 (60) | 0 (0) | < 0.01 |
Values expressed as raw data (%).
Nine of the 10 patients who presented with a bile leak recovered well with no major complications and no need for HDU/ITU care. The median hospital stay was 10 days (range, 5–30 days) post-laparoscopic cholecystectomy and they were discharged from routine review having made a good recovery 37 days (range, 24-90 days) later. One patient was transferred to ITU having developed sepsis and acute renal failure secondary to a subphrenic collection that was drained percutaneously. An ERC and stent insertion failed to control the bile leak and the subphrenic collection reaccumulated necessitating a laparotomy, suture of the cystic duct stump leak and drainage. Although the bile leak was controlled, the patient died of a cardiac event 7 days after this.
Following the introduction of the minimally invasive protocol (Fig. 1), 9/14 (64%) patients were managed non-operatively. Of these 9 cases, one was managed with simple drainage alone and settled spontaneously. However, ERC was employed in the management of the remainder (n = 8; 89%), as well as in 3 individuals who in addition required surgical intervention. The main indication for ERC was a persistent bile leak and this was performed a median of 5 days (range, 2–66 days) after the original laparoscopic cholecystectomy. In 4 individuals, the site of the leak was identified as the cystic duct stump but in the remainder this was unclear. In 4 other patients common bile duct stones were identified and an endoscopic sphincterotomy performed in order to retrieve these and allow any further stone fragments to pass. An internal biliary stent was routinely left in situ in order to promote preferential drainage of bile into the duodenum attenuating the leak and allowing it to stop. These were removed in all patients 6 weeks after discharge. There were no complications directly relating to ERC. However, of those managed conservatively, one patient developed a pneumonia requiring ventilation on ITU and a second developed severe sepsis requiring a prolonged (40-day) ITU admission. Both made a good recovery.
Operative intervention was necessary in 5/14 patients, all of whom were managed laparoscopically. Biliary peritonitis was the initial presentation in four patients (2 of whom had both ERC and stenting and surgery on the same day). In the other, this developed 8 days after laparoscopic cholecystectomy when the bile leak was thought to have resolved and the drain removed (post ERC and stenting). The site of the leak was a subsectoral duct of Luschka in the GB bed in 2 patients and this was sutured. In the remaining 3 individuals, the site of the leak could not be positively identified and they underwent lavage and drainage. Postoperatively, 2 patients required HDU monitoring for 24 h and 48 h, respectively, but made a rapid recovery subsequently. In addition, one individual was re-admitted following discharge home with a pelvic collection that required percutaneous drainage.
The median hospital stay for patients who followed the minimally invasive protocol was 11 days (range, 5–55 days) post-laparoscopic cholecystectomy (P = 0.66 versus pre-protocol) There were no long-term complications but routine follow was slightly longer in this latter group at 49 days (range, 34–180 days; P = 0.04 versus pre-protocol).
Discussion
Laparoscopic cholecystectomy is currently the procedure of choice for symptomatic gallstones. It has evolved from an innovative, but time-consuming, novelty to a routine day-case procedure over the last 20 years.10 Similarly, the management of bile leaks following this procedure has changed. However, the fundamental principles underpinning this have not, i.e. successful drainage of a bile leak is critical. If drainage is inadequate, sepsis and biliary peritonitis develop and this remains a clear indication for surgical intervention. This study documents the changes in technique that have occurred in a specialist unit over the last 10 years and advocates a minimally invasive structured management protocol to treat patients with bile leaks.
The introduction of both ERC and re-laparoscopy as opposed to laparotomy are the two major differences in management established by the protocol. ERC has both a diagnostic and therapeutic role.6 It allows identification of both the site of the leak as well as any residual stones within the bile duct that may be contributing to it. Such stones can be removed and various strategies used to reduce the pressure gradient between the bile duct and the duodenum created by contraction of the sphincter of Oddi.6,7,11 This encourages the preferential flow of bile into the duodenum thus attenuating the bile leak and allowing the site to heal.12 Indeed, this, in effect, means that one no longer has to perform an exploratory laparotomy with the aim of finding and closing the leak. A number of techniques have been proposed including endoscopic sphincterotomy alone, nasobiliary tube drainage and internal biliary-duodenal stents.6,7,11–13 Endoscopic sphincterotomy alone does not appear as effective as the latter two approaches14 and increases the risk of ERC-related complications.15 Furthermore, unless stone extraction is planned, it is unusual for a sphincterotomy to be necessary in order to insert either nasobiliary or internal stents. However, nasobiliary tubes are not particularly well tolerated by patients and can easily become displaced. Thus, our protocol advocates the use of internal biliary duodenal stents. Only 4 sphincterotomies were necessary using this technique, each time for stone extraction.
Prior to 1998, neither diagnostic nor therapeutic ERC were used routinely. Thus, a common indication for surgical intervention was persistence of the bile leak (3/6). In comparison, following introduction of the minimally invasive protocol, this was the main indication for ERC and stenting but was not an indication for surgical intervention. In all but one patient (who developed peritonitis post drain removal), this allowed rapid resolution of the bile leak. ERC and stenting also plays an important role as an adjunct to laparoscopy and washout in patients with biliary peritonitis, helping to reduce or eliminate postoperative bile leakage thus accelerating recovery. However, although the use of ERC has undoubtedly been a major advance, it does have potential drawbacks. The plastic stents inserted into the CBD need removal after 6 weeks, exposing the patient to a second ERC and hospital visit. This explains the longer follow-up for those following the minimally invasive protocol. Furthermore, although there were no ERC-related complications in this study, these are always a concern and can, rarely, be life-threatening.
The second major change advocated by the protocol is the use of laparoscopy rather than laparotomy in the management of patients requiring surgical intervention. Successful ERC and stenting has been accompanied by a shift in the indications for surgery, with biliary peritonitis (5/5) rather than failure of conservative management being the main indication in the protocol era. Interestingly, in both groups, the placement of a drain following laparoscopic cholecystectomy did not prevent the development of biliary peritonitis in a minority of individuals (3/24) presumably either because it became blocked or was removed prematurely. The role of surgical intervention in both groups is primarily to wash out bile from the peritoneal cavity and establish adequate external drainage in order to prevent reaccumulation. If the site of the bile leak can be identified, then an attempt is made to repair this. This was identified in all 6 patients who underwent laparotomy compared to only 2/5 individuals managed laparoscopically. Some authors have been able to identify the majority of bile leaks laparoscopically, advocating the magnification provided by the laparoscope as an important aide.9 However, in our series, 3/5 patients had an ERC and stent before laparoscopy, significantly reducing the leak at the time of surgery. This potentially explains the lower number of leaks identified compared to either laparotomy at our centre or laparoscopy elsewhere. In addition, the anatomical pattern of bile leaks identified varied, with leaks coming from the cystic duct stump itself or its junction with the CBD in those undergoing laparotomy compared with leaks from ducts of Luschka in the laparoscopic group. Once again, the use of ERC and stenting in the latter group may explain this as leaks from the cystic duct stump were effectively sealed in 4 patients without the need for surgical intervention.
Overall morbidity, mortality and hospital stay post laparoscopic cholecystectomy were similar both before and after introduction of a minimally invasive management protocol. It is not surprising that these were similar in view of the relatively small numbers and the pathophysiology of bile leaks (i.e. patients with biliary peritonitis often take several days to get better from the ‘peritonitis’ irrespective of the operative technique). However, anecdotally and bearing in mind that our patients have increasingly high expectations, we feel that the minimally invasive approach is preferable. This minor, but significant, change in the management of bile leaks and saving a laparotomy has a major impact on the patient's perception of the significance of the complication. Furthermore, longer-term problems such as intra-abdominal adhesions and incisional hernias may be reduced.
In our experience, a structured stepwise approach to the management of uncommon complications such as bile leaks is advantageous. In order to run such a protocol there must be the resources and skills available to provide ERC and advanced laparoscopic surgery, 7 days a week. If this is not available, as we have shown, it is perfectly possible, although not preferable, to manage these patients using conventional techniques. Alternatively, they can be transferred to centres where minimally invasive expertise is routinely available. The only death in this series was in a patient who developed severe sepsis and the key point is to prevent this developing by ensuring and maintaining adequate drainage of bile at an early stage followed by prompt preferably minimally invasive management of the underlying leak itself.
Bile leak remains an unusual problem in our practice. Thus it has taken over 10 years to accumulate the relatively small series. Nonetheless, this study provides a useful analysis of the historical and current management of bile leaks.
Acknowledgments
This work was presented, in part, as a poster presentation at the 6th World Congress of the International HepatoPancreato-Biliary Association (IHPBA), Washington, USA, 5 June 2004 and as an oral presentation at the 19th World Congress of the International Society for Digestive Surgery, Yokohama, Japan, 11 December 2004.
References
- 1.Vecchio R, MacFadyen BV, Latteri S. Laparoscopic cholecystectomy: an analysis of 114,005 cases of United States series. Int Surg. 1998;83:215–9. [PubMed] [Google Scholar]
- 2.Merrie AE, Booth MW, Shah A, Pettigrew RA, McCall JL. Bile duct imaging and injury. A regional audit of laparoscopic cholecystectomy. Aust NZ J Surg. 1997;67:706–11. doi: 10.1111/j.1445-2197.1997.tb07114.x. [DOI] [PubMed] [Google Scholar]
- 3.McMahon AJ, Fullarton G, Baxter JN, O'Dwyer PJ. Bile duct injury and bile leakage in laparoscopic cholecystectomy. Br J Surg. 1995;82:307–13. doi: 10.1002/bjs.1800820308. [DOI] [PubMed] [Google Scholar]
- 4.Buanes T, Waage A, Mjaland O, Solheim K. Bile leak after cholecystectomy significance and treatment. Results from the National Norwegian Cholecystectomy Registry. Int Surg. 1996;81:276–9. [PubMed] [Google Scholar]
- 5.De Palma GD, Galloro G, Iuliano G, Puzziello A, Persico F, Masone S, et al. Leaks from laparoscopic cholecystectomy. Hepatogastroenterology. 2002;49:924–5. [PubMed] [Google Scholar]
- 6.Mergener K, Strobel JC, Suhocki P, Jowell PS, Enns RA, Branch MS, et al. The role of ERCP in diagnosis and management of accessory bile duct leaks after cholecystectomy. Gastrointest Endosc. 1999;50:527–31. doi: 10.1016/s0016-5107(99)70077-5. [DOI] [PubMed] [Google Scholar]
- 7.Ryan ME, Geehen JE, Lehman GA, Aliperti G, Freeman ML, Silverman WB, et al. Endoscopic intervention for biliary leaks after laparoscopic cholecystectomy: a multicentre review. Gastrointest Endosc. 1998;47:261–6. doi: 10.1016/s0016-5107(98)70324-4. [DOI] [PubMed] [Google Scholar]
- 8.Azagra JS, DeSimone P, Goergen M. Is there a place for laparoscopy in the management of post-cholecystectomy biliary injuries? World J Surg. 2001;25:1331–4. doi: 10.1007/s00268-001-0119-z. [DOI] [PubMed] [Google Scholar]
- 9.Wills VL, Jorgensen JO, Hunt DR. Role of relaparoscopy in the management of minor bile leakage after laparoscopic cholecystectomy. Br J Surg. 2000;87:176–80. doi: 10.1046/j.1365-2168.2000.01323.x. [DOI] [PubMed] [Google Scholar]
- 10.Leeder PC, Mathews T, Krzeminska K, Dehn TCB. Routine day-case laparoscopic cholecystectomy. Br J Surg. 2004;91:312–7. doi: 10.1002/bjs.4409. [DOI] [PubMed] [Google Scholar]
- 11.Ramesh H. Post cholecystectomy bile leaks and their management. Gastrointest Endosc. 1998;47:564–5. doi: 10.1016/s0016-5107(98)70275-5. [DOI] [PubMed] [Google Scholar]
- 12.Bjorkman DJ, Carr-Locke DL, Lichtenstein DR, Ferrari AP, Slivka A, Van Dam J, et al. Postsurgical bile leaks: endoscopic obliteration of the transpapillary pressure gradient is enough. Am J Gastroenterol. 1995;90:2128–33. [PubMed] [Google Scholar]
- 13.Sugiyama M, Mori T, Atomi Y. Endoscopic nasobiliary drainage for treating bile leak after laparoscopic cholecystectomy. Hepatogastroenterology. 1999;46:762–5. [PubMed] [Google Scholar]
- 14.Marks JM, Ponsky JL, Shillingstad RB, Singh J. Biliary stenting is more effective than sphincterotomy in the resolution of bile leaks. Surg Endosc. 1998;12:327–30. doi: 10.1007/s004649900663. [DOI] [PubMed] [Google Scholar]
- 15.Freeman ML. Complications of endoscopic biliary sphincterotomy: a review. Endoscopy. 1997;29:288–97. doi: 10.1055/s-2007-1004193. [DOI] [PubMed] [Google Scholar]