Abstract
Acute renal failure can occur following major surgery. Predisposing factors include massive haemorrhage, sepsis, diabetes, hypertension, cardiac disease, peripheral vascular disease, chronic renal impairment and age. Understanding epidemiology, aetiology and pathophysiology can aid effective diagnosis and management. A consensus definition for acute renal failure has recently been developed. It relates to deteriorating urine output, serum creatinine and glomerular filtration rate. In the surgical patient, precipitants are often pre-renal, although intrinsic damage and obstructed urine flow can occur. Worsening renal function results in distal organ damage. Acute renal failure is a marker of disease severity, carrying a poor prognosis if associated with deteriorating respiratory and cardiovascular function. Acute renal failure in the critically ill surgical patient exerts a massive impact on the evolution of complications and prognosis. Management relates to treating life-threatening problems, maintaining effective ventilation and circulation, removal (or reduction) of nephrotoxins and, where appropriate, establishing either renal replacement therapy or palliative care.
Keywords: Acute renal failure, Intensive care, Renal replacement therapy, surgery
Acute renal failure (ARF) can be described as a sudden sustained fall in glomerular filtration rate (GFR) associated with accumulation of metabolic waste products and water. It is a major postoperative complication in surgical patients with a quoted incidence of 10–23%.1,2 Predisposing factors include severity of physiological insult, pre-existing comorbidity, hypovolaemia and sepsis. Despite improvements in recognition and management, e.g. renal replacement therapy (RRT), mortality remains high.3 This high mortality and a variety of definitions warrant further attention if understanding of ARF and improvements in management are to develop. Such attention focuses on definitions, epidemiology, aetiology and pathophysiology.
Definitions
Several definitions exist. The British Renal Association defines ARF as a serum creatinine > 300 μmol/l (> 3.3 mg/dl) and/or a blood urea nitrogen value > 40 mmol/l (240 mg/dl) with previously normal values. The critical illness scoring system, APACHE III, defines ARF as a serum creatinine elevation > 136 μmol/l per day (> 1.5 mg/dl), with a urine output < 410 ml/day and no pre-existing chronic dialysis.4 One review of ARF found 26 different definitions of postoperative ARF in 26 studies.5 This absence of consensus reflects the condition's complexity. Definitions tend to emphasise individual factors such as biochemistry, preexisting impairment, resuscitation measures, nephrotoxic drugs and pathophysiology,4 with most having common elements, e.g. serum creatinine and urine output.
In terms of achieving an easily applicable definition, there is logic in this as they are functions virtually unique to the kidney, easily and routinely measured. Other biochemical criteria such as raised urea, hyperkalaemia and acid–base disturbance are more open to influence from extra-renal factors, e.g. gastrointestinal bleeding, trauma, burns, and endocrine disturbance. This issue is now reflected in a new consensus classification from the Second International Consensus Conference of the Acute Dialysis Quality Initiative.6 The acronym RIFLE stands for Risk of renal dysfunction, Injury to the kidney, Failure of kidney function, Loss of kidney function and End-stage renal disease. It is a multilevel classification (Fig. 1) permitting incorporation of a wide range of progressive disease processes and spectra. It also incorporates factors considered pertinent to ARF (e.g. pre-existing renal disease, rate of functional deterioration) combining them with a stratification based on levels of urine output and serum creatinine. It is readily usable and clinically applicable across patient groups.
Figure 1.
RIFLE classification for acute renal failure.6
Its limitations should, however, be recognised. Serum creatinine can overestimate renal function during the initial development of renal failure – trauma, fever and immobilisation all increase creatinine production and liver dysfunction and decreased muscle mass reduce creatinine production.7 Urine output can vary depending on factors extrinsic to the kidney including post-surgical endocrine changes and post-renal obstruction.
Epidemiology
Lack of consensus definitions, with heterogeneity of patients and disease processes, account for the reported variability in epidemiology. The frequency of ARF among all patients on hospital admission is estimated at 1%.8 ARF associated with surgery being the second most common cause of ARF identified.8 Approximately one-third of patients developing ARF require critical care admission.9 In the largest study to date, patients with ARF on the intensive therapy unit (ITU) had an overall mortality of 62%,1 longer ITU stays, higher APACHE III scores and lower survival rates.10,11
ARF is, therefore, a marker for severity of illness and, with respect to any individual organ system, arguably the strongest denominator for survival in critically ill patients.11 Relative to patients with the same severity of illness (e.g. as judged by APACHE II), it carries between 4.0 (homogeneous critically ill patients) and 7.9 times (cardiac surgery) mortality on intensive care.1,12 Patients with ARF not requiring RRT have a mortality rate of 15%, as opposed to 62% in those requiring RRT.9 Such high mortality rates are, in part, due to evolving trends in patient demographics. e.g. more invasive surgery in older patient groups with significant co-morbidities13 that may conceal improvements in hospital (52% versus 32%) and 1-year (30% versus 21%) survival rates plus renal recovery rates (96% versus 78%) cited over the past 30 years.14
Predisposing factors
Suggested predisposing factors include pre-existing renal impairment, hypertension, cardiac disease, peripheral vascular disease, diabetes mellitus, jaundice, age, massive haemorrhage and severe sepsis.2,8 In the context of surgical patients, absence of uniform definitions and complex interactions between different risk factors has resulted in limited evidence for the relative contribution of such risk factors to ARF.
Pre-existing renal impairment has been implicated as the most significant pre-operative factor contributing to ARF,15 with risk inversely related to creatinine clearance.16 Compared to patients with previously normal renal function, those with pre-existing renal impairment have increased morbidity and mortality manifesting as increased inotrope usage, number of ventilated days, prolonged intensive care and overall hospital stay.17
The incidence of dialysis-dependent ARF depends on the nature of surgery, and ranges from 1% post coronary artery bypass surgery16 to 50% in some series of thoraco-abdominal aortic aneurysm surgery.18 Increasing age has also been identified as a risk factor, although this factor in isolation should not preclude patients from treatment.5 Age-related predispositions include reduced renal reserve, susceptibility to volume depletion due to a ‘thirst deficit’, and reduced ability to conserve salt and concentrate urine.19
Aetiology in the critically ill surgical patient
For diagnostic/aetiological purposes, ARF has historically been divided into three distinct entities determined by basic pathophysiology (Table 1) – pre-renal, renal or intrinsic and post renal or obstructive. These account for 30–60%, 20–40% and 1–10% of cases in the surgical setting, respectively.15 In the critically ill patient, the initial insult is usually pre-renal with renal hypoperfusion from either vasodilation in severe sepsis, or hypovolaemia, e.g. following massive haemorrhage. This is in contrast to intrinsic damage from renal disease (e.g. glomerulonephritis) or chemical/pharmacological injury. Clinical history often indicates this basic pathophysiology but urinary biochemistry can also be used to distinguish between pre-renal and intrinsic damage, with the pre-renal damage reflecting hypoperfusion and the attempt to conserve water and electrolytes, particularly sodium (Table 2). With respect to pre-renal failure however cellular damage (and possible tubular obstruction) may subsequently occur.
Table 1.
Causes (with examples) of acute renal failure in the critically ill surgical population
Pre-renal
|
Renal
|
Post-renal
|
Table 2.
Differentiation between pre-renal and intrinsic renal damage
| Investigation | Pre-renal | Intrinsic renal |
|---|---|---|
| Urinary specific gravity | >1,020 | <1.010 |
| Urinary sodium (mmol/l) | >500 | <350 |
| Urinary osmolality (mosmol/l) | <20 | >40 |
| Urine/Plasma osmolality | >2 | <1.1 |
| Urine/Plasma urea ratio | >20 | <10 |
| Urine/Plasma creatinine ratio | >40 | <20 |
| Fractional sodium excretion | <1 | >1 |
| Renal failure index | <1 | >1 |
Notes:
In critical illness per se, it is also increasingly accepted that initial aetiologies can determine the course of a disease process, e.g. acute respiratory distress syndrome (ARDS) from extrapulmonary causes such as sepsis behave differently to those with an intrapulmonary cause such as aspiration or lung contusion.20 The analogy is potentially applicable in ARF, and supported by the fact that the mortality in patients with ARF secondary to acute tubular necrosis (ATN) has been reported as 64%, compared with 16% for those patients with ARF as part of a multisystem disease.3 This stresses the importance of not only identifying, but effectively treating the causes of ARF.
Pathophysiology
In the surgical patient, hypoperfusion is frequently the pre-renal precipitant, e.g. hypovolaemia, severe sepsis, cardiogenic shock, cardiac tamponade and hepatorenal syndrome (HRS). Total renal blood flow (1–1.5 l/min) normally exceeds the organ's oxygen requirements but only 10% of it passes through the renal medulla, where PaO2 is 8–15 mmHg (1.2–2.2 kPa). Metabolically active medullary cells are, therefore, susceptible to hypoperfusion and subsequent hypoxia.21 Hypoperfusion initially results in afferent arteriole dilatation, efferent arteriole vasoconstriction and expansion of intravascular volume with the aim of maintaining glomerular filtration. Neural and hormonal factors (including the renin–angiotensin–aldosterone system) mediate this response. Left unabated, vasoconstriction results in ischaemia. HRS is a classic example of the consequence of this cascade of events; intense vasoconstriction of the renal circulation occurs in response to extreme under filling of the arterial circulation, due to arterial vasodilatation in the splanchnic vascular bed.22
Ischaemia-reperfusion injury23 can arise either after circulatory restoration from global hypoperfusion or in isolation, e.g. aortic cross-clamping in vascular surgery. Whilst mechanisms remain poorly understood, ischaemia results in vasoconstriction, tubular swelling and endothelial activation. In the post-ischaemic reperfusion period, activated neutrophils generate and release reactive oxygen species and cytotoxic compounds. Surrounding tissues are infiltrated, circulating polymorphs and the arachidonic acid cascade are activated23 worsening the inflammatory response, supporting the ‘injured kidney’ theory of the kidney as a pro-inflammatory mediator.
Sepsis is another major contributor to ARF in the surgical patient.24 Bacterial pro-inflammatory factors and resultant cytokine release not only cause local tissue injury, but aggravate haemodynamic instability. Thus in the context of the critically ill these mechanisms are interlinked in their propagation and effect.
With respect to the histological picture in the critically ill, there is limited information.25 It has been postulated that, in sepsis, malfunction arises through immune-mediated cellular apoptosis.25 Post mortem evidence does not support this.25 The hypothesis is also countered by patients who recover normal function in a number of days, far quicker than the regenerative capacity of renal tubular cells. Rather than ‘programmed cell death’, an alternative mechanism may revolve around ‘cellular shutdown’ termed cytopathic hypoxia.26 Here, outcome correlates with mitochondrial genotype.27 The inherited inability to utilise oxygen at the mitochondrial level may exert a protective effect, and has been termed ‘aestivation’. This concept fits well with the apparent paucity of histological changes seen at a renal tubular level, and accounts for disparities between renal biopsy results and clinical severity.25 It may also account for the rapid restoration of renal function in some critically ill patients.
Irrespective of mechanisms involved and the absence/presence of abnormal histopathology, renal failure has systemic effects, playing a crucial role in inflammation and the evolution of distal organ injury (Table 3). If ARF develops a vicious cycle of renal dysfunction, cytokine release, dysfunction of distal organs, failure of urinary cytokine clearance and further distal organ dysfunction ensue.24
Table 3.
Systemic effects of acute renal failure
Uraemic intoxication
|
The ‘injured’ kidney: a pro-inflammatory mediator
|
Such pathophysiology creates 10% mortality from ARF alone, rising to 45–50% if associated with respiratory failure.28 If three or more organ failure ensue (one of the organs being ARF), it can increase to 95%. Nevertheless 70% of survivors recover to normal renal function and less than 10% require long-term renal replacement therapy (30% if patients had pre-existing renal disease).9,29
Management of ARF
Initial resuscitation – ‘ABC’
Initial management rests with managing life-threatening problems and treating the cause. On-going management relates to maintaining adequate ventilation with effective circulating volume and cardiac output in order to restore renal perfusion pressure. Early transfer to critical care for invasive cardiovascular monitoring is likely. Frequent monitoring of urea and electrolytes is also necessary to gauge both the effectiveness of management and the evolution of the ARF. Obstruction to the lower urinary tract should always be excluded and, in postoperative abdominal surgical patients, intra-abdominal hypertension must be considered a contributing factor.30,31 Effective, early management may minimise severity, allowing for natural reversal of pathophysiology. Examples include aggressive surgical control of bleeding, radiological or surgical drainage of a septic focus, and abdominal decompression to prevent abdominal compartment syndrome.
Aside from treating the immediately life-threatening causes of ARF (e.g. massive haemorrhage), immediate life-threatening consequences of ARF also require attention, i.e. hyperkalaemia (K+ > 6.5 mmol/l). This may compound acute renal failure in major burns, critical ischaemia with rhabdomyolysis and in crush injuries.32 Due to life-threatening cardiac compromise, immediate treatment of hyperkalaemia should be instituted. In certain circumstances, renal replacement therapy although indicated may not be readily available, or establishing it can waste valuable time. It is therefore essential that the resident surgeon is aware of the initial pharmacological options (and mechanisms of action) for hyperkalaemia involving calcium, dextrose, insulin, etc. (Table 4).
Table 4.
Emergency management of hyperkalaemia (K+ > 6.5 mmol/l)
Treatment
|
| Notes |
Recognise effects
|
Identify surgical causes
|
Treatment modalities
To date, with the possible exception of the use of N-acetylcysteine in radiocontrast-induced renal failure,33,34 there is no pharmacological ‘magic bullet’ preventing or reversing acute renal failure. Drugs such as dopexamine augment cardiac output and may have a role in restoring perfusion, although its renoprotective qualities remain uncertain.35,36 Low-dose dopamine has no renoprotective effect; data suggest it neither maintains nor improves renal function.37 Loop diuretics are commonly used in volume control, and have a theoretical benefit of reducing metabolic demand, hence protecting against ischaemic injury. Beyond experimental models, renal protection has never been demonstrated and their use may be associated with increased mortality in ITU patients.38
Nephrotoxic agents
The role of nephrotoxic substances compounding the initial renal insult must also be considered. Effects include direct cardiovascular effects, tubular damage, tubular obstruction, interstitial nephritis and glomerulonephritis. Of particular relevance to surgical patients are hypotensive agents, nonsteroidal anti-inflammatory drugs, aminoglycosides and radiocontrast media. Wherever possible, such substances should be avoided. If they are necessary, plasma levels may have to be monitored and advice sought on their administration.
Renal replacement therapy
Of ITU patients with ARF, 50–70% require renal replacement therapy (RRT).11 It simplifies fluid and nutritional management and early use may improve outcome.14 Indications (Table 5) for commencing renal replacement therapy can border on the subjective as no consensus currently exists6 although application of the RIFLE criteria may in future aid development of specific guidelines.7
Table 5.
Indications for renal replacement therapy
| Indication | Proposed value |
|---|---|
| Anuria/oliguria | Urine < 200 ml per 12 h |
| Hyperkalaemia | K+ > 6.5 mmol/l |
| Severe acidaemia | pH < 7.1 |
| Increased creatinine | Creatinine > 300 μmol/l |
| Uraemia | Urea > 30 mmol/l |
| Fluid overload | Respiratory impairment |
| Temperature control | Control of hypo- and hyperthermia |
| Sepsis | Potential: overall mechanism uncertain |
| Acute poisoning | Seek toxicology advice, e.g. salicylates |
Considerations relate to intermittent versus continuous (Table 6). Continuous therapies predominate in the UK where ‘intermittent’ usually means haemodialysis (HD) and ‘continuous’ continuous veno-venous haemofiltration (CVVH). Few randomised, controlled trials exist comparing the two. In the absence of haemodynamic instability, the two modalities are probably equivalent.39 CVVH causes less haemodynamic instability as time is allowed for fluid to reequilibrate between body compartments.40 This is likely to be of relevance in a variety of surgical patients, in particular those with vasodilatory shock and capillary leak where sudden, large volume fluid movement between body compartments can create life-threatening haemodynamic changes, e.g. septic shock, acute pancreatitis, and fulminant liver failure. CVVH has also been advocated in sepsis due to its immunomodulatory effects by removing endotoxins and inflammatory mediators.39–41 Its overall role in this context is uncertain.
Table 6.
Continuous veno-venous haemofiltration and haemodialysis
| Continuous veno-venous haemofiltration | Haemodialysis |
|---|---|
| Convection: water pushed across membrane. and solute follows | Diffusion: solutes follow concentration gradient. Fluids: counter-current mechanism |
| ‘Continuous’: interruptions for surgery and other procedures | Intermittent |
| Cardiovascular stability | Less cardiovascular stability |
| Less complement activation | |
| Less white cell sequestration | |
| ‘Middle’ molecule clearance (500–5000 Da) | Better clearance of urea/creatinine |
PRINCIPLE OF CVVH
Solutes (e.g. urea, creatinine) are removed from the blood by convection across a semi-permeable membrane in the artificial filter (Fig. 2). Water is pushed across the membrane due to an established pressure gradient, carrying dissolved solutes with it (‘solvent drag’). Solutes are cleared slowly, but efficiently, over a prolonged period. A specialised, sterile replacement fluid replaces the large volume of ultrafiltrate removed. Studies have shown that filtration rates of 35 ml/kg/h result in better survival rates in critically ill patients.41 The process is continuous except when the filter clots or a procedure needs to be performed.
Figure 2.
Continuous veno-venous haemofiltration.
PRINCIPLE OF HAEMODIALYSIS
Solutes are removed by diffusion across the semi-permeable membrane down a concentration gradient. Blood and dialysate fluid are circulated in a ‘counter-current’ fashion (Fig. 3) but, unlike CVVH, no replacement fluid is re-infused. The treatment is intermittent38 with large volumes of fluid being dialysed in a period of a few hours.
Figure 3.
Haemodialysis.
Anticoagulation
All patients requiring renal replacement therapy need suitable anticoagulation (usually intravenous heparin) with regular monitoring. Prostacyclin is useful in cases of heparin-induced thrombocytopenia, or patients with coagulopathies and active bleeding. Citrate has also been used as an anti-coagulant. Pathologically/pharmacologically coagulopathic patients may not require any anti-coagulant.
Vascular access
Most current RRT is veno-venous in nature. Vascular access is via a central vein. The insertion site is, in effect, determined by the location of other central venous catheters already in situ. Subclavian lines are associated with higher stenosis rates of up to 90%.38 This may be significant if arterio-venous fistula formation is being considered for long-term renal replacement. The internal jugular route permits greater patient mobility but may be associated with increased immediate complications. The femoral route is useful for short-term renal replacement but is associated with increased rates of infection.38
Conclusions
Acute renal failure can be defined as an abrupt collapse of kidney functions, occurring in series or simultaneously. It exerts a massive impact on the evolution of complications (frequently infectious) and prognosis in the critically ill surgical patient. It carries a high mortality initially because, although often a ‘victim’ of disease processes, it progressively becomes the ‘offender’ in terms of systemic effects.
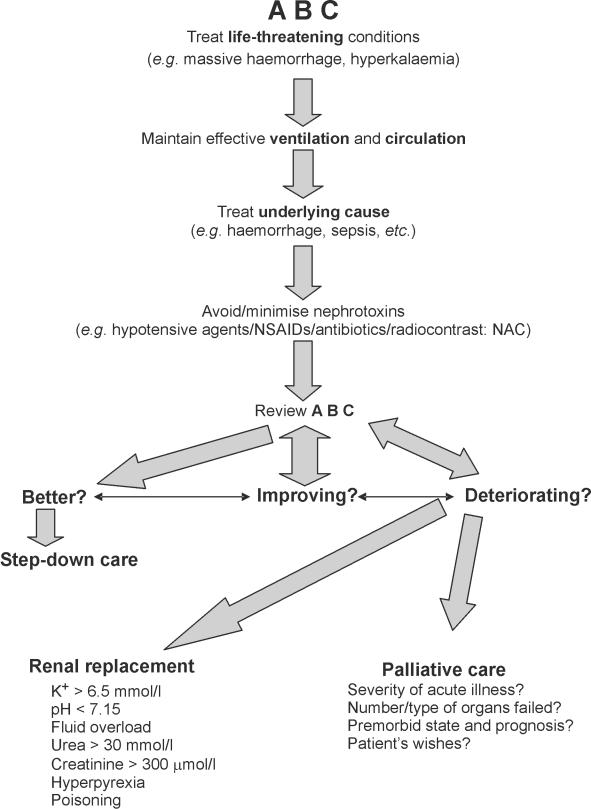
The ideal goal in management is primary prevention, although this may be unrealistic given the complex, multifactorial nature of the condition. Early identification of the ‘at-risk’ patient, maintenance of euvolaemia, avoidance of nephrotoxic agents, and the pre-emptive use of N-acetylcysteine in patients at risk of radiocontrast-induced nephropathy are arguably the only modalities available in this context. Once ARF is established, management centres on the treatment of life-threatening problems, maintaining effective ventilation and circulation (pharmacologically aided if necessary), removal (or reduction) of nephrotoxins, and, where appropriate, establishing renal replacement therapy. These measures should form part of a step-wise treatment algorithm when assessing and determining the management surgical patients with ARF (Fig. 4).
Figure 4.
Algorithm for the management of acute renal failure (ARF)
Acknowledgments
The review is a statement of the authors' opinion supported by selective references. Selected content has been presented in a ‘Critical Care Update’ at the Annual Scientific Meeting of the Association of Surgeons of Great Britain and Ireland, April 2004 (Harrogate) and April 2005 (Glasgow.)
References
- 1.Metnitz PGH, Krenn CG, Stelzer H, Lang T, Ploder J, Lenz K, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;28:2051–8. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Hoste EAJ, Lameire NH, Vanholder RC, Benoit DD, Decruyenaere JMA, Colardyn FA. Acute renal failure in patients with sepsis in a surgical ICU; predictive factors, incidence, comorbidity and outcome. J Am Soc Nephrol. 2003;14:1022–30. doi: 10.1097/01.asn.0000059863.48590.e9. [DOI] [PubMed] [Google Scholar]
- 3.Butkus DE. Persistent high mortality in acute renal failure. Are we asking the right questions? Arch Intern Med. 1983;143:209–12. [PubMed] [Google Scholar]
- 4.Knaus WA, Wagner DP, Draper EA. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalised adults. Chest. 1991;1100:1619–36. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 5.Tillyard A, Keays R, Soni N. The diagnosis of acute renal failure in intensive care: mongrel or pedigree? Anaesthesia. 2005;60:903–14. doi: 10.1111/j.1365-2044.2005.04278.x. [DOI] [PubMed] [Google Scholar]
- 6.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R202–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellomo R, Kellum JA, Ronco C. Defining acute renal failure: physiological principles. Intensive Care Med. 2004;30:33–7. doi: 10.1007/s00134-003-2078-3. [DOI] [PubMed] [Google Scholar]
- 8.Shusterman N, Strom BL, Murray TG, Morrison G, West SL, Maislin G. Risk factors and outcome of hospital acquired acute renal failure. Clinical epidemiologic study. Am J Med. 1987;83:65–71. doi: 10.1016/0002-9343(87)90498-0. [DOI] [PubMed] [Google Scholar]
- 9.Liano F, Pascaul J. The Madrid acute renal failure study group. Epidemiology of acute renal failure. A prospective multi-centre, community-based study. Kidney Int. 1996;50:811–8. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 10.Clermont G, Acker CG, Angus DC, Sirio CA, Pinsky MR, Johnson JP. Renal failure in the ICU. Comparison of the impact of acute renal failure and end-stage disease on ICU outcomes. Kidney Int. 2002;62:986–96. doi: 10.1046/j.1523-1755.2002.00509.x. [DOI] [PubMed] [Google Scholar]
- 11.Kellum JA, Angus DC, Johnson JP. Continuous versus intermittent renal replacement therapy: a meta-analysis. Intensive Care Med. 2002;28:29–37. doi: 10.1007/s00134-001-1159-4. [DOI] [PubMed] [Google Scholar]
- 12.Chertow GM, Levy EM, Hammermiester KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–8. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 13.Paganini EP, Tapolyai M, Goormastic M. Establishing a dialysis therapy/patient outcome link in intensive care unit acute dialysis for patients with acute renal failure. Am J Kidney Dis. 1996;28(Suppl 3):S81–9. [Google Scholar]
- 14.Gettings LG, Reynolds HN, Scalea T. Outcome in post-traumatic acute renal failure when continuous renal replacement therapy is applied early versus late. Intensive Care Med. 1999;25:805–13. doi: 10.1007/s001340050956. [DOI] [PubMed] [Google Scholar]
- 15.Carmichael P, Carmichael AR. Acute renal failure in the surgical setting. Aust NZJ Surg. 2003;73:144–53. doi: 10.1046/j.1445-2197.2003.02640.x. [DOI] [PubMed] [Google Scholar]
- 16.Chertow GM, Lazarus JM, Christiansen CL. Pre-operative renal risk stratification. Circulation. 1997;95:878–84. doi: 10.1161/01.cir.95.4.878. [DOI] [PubMed] [Google Scholar]
- 17.Deutsch E, Bernstein RC, Addonizio P, Kussmaul WG. Coronary artery bypass surgery in patients on chronic haemodialysis. A case-control study. Ann Intern Med. 1989;110:369–72. doi: 10.7326/0003-4819-110-5-369. [DOI] [PubMed] [Google Scholar]
- 18.Svensson LG, Crawford ES, Hess KR, Coseli JS, Safi HJ. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg. 1993;17:357–68. [PubMed] [Google Scholar]
- 19.Phillips PA, Rolls BJ, Ledingham JG. Reduced thirst after water deprivation in healthy elderly men. N Engl J Med. 1984;311:753–9. doi: 10.1056/NEJM198409203111202. [DOI] [PubMed] [Google Scholar]
- 20.Callister MEJ, Evans TW. Pulmonary versus extra-pulmonary acute respiratory distress syndrome: different diseases or just a useful concept. Curr Opin Crit Care. 2002;8:21–5. doi: 10.1097/00075198-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Brezis M, Epstein FH. Cellular mechanisms of acute ischaemic injury in the kidney. Annu Rev Med. 1993;44:27–37. doi: 10.1146/annurev.me.44.020193.000331. [DOI] [PubMed] [Google Scholar]
- 22.Gines P, Guevara M, Arroyo V, Rodes J. Hepatorenal syndrome. Lancet. 2003;362:1819–27. doi: 10.1016/S0140-6736(03)14903-3. [DOI] [PubMed] [Google Scholar]
- 23.Kerrigan CL, Stotland MA. Ischaemia reperfusion injury: a review. Microsurgery. 1993;14:165–75. doi: 10.1002/micr.1920140307. [DOI] [PubMed] [Google Scholar]
- 24.Druml W. Acute renal failure is not a ‘cute’ renal failure. Intensive Care Med. 2004;30:1886–90. doi: 10.1007/s00134-004-2344-z. [DOI] [PubMed] [Google Scholar]
- 25.Wan L, Bellomo R, Giantomasso DD, Ronco C. The pathogenesis of septic acute renal failure. Curr Opin Crit Care. 2003;9:496–502. doi: 10.1097/00075198-200312000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Fink MP. Cytopathic hypoxia. Is oxygen use impaired in sepsis as a result of an acquired intrinsic derangement in cellular respiration? Crit Care Clin. 2002;18:165–75. doi: 10.1016/s0749-0704(03)00071-x. [DOI] [PubMed] [Google Scholar]
- 27.Brealy D, Brand M, Hargreaves I. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–23. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 28.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA. 1996;275:1489–94. [PubMed] [Google Scholar]
- 29.Metnitz PGH, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, et al. Effect of acute renal failure requiring renal replacement therapy on outcome of critically ill patients. Crit Care Med. 2002;30:2051–8. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Doty JM, Saggi BH, Blocher CR, Fakhry I, Gehr T, Sica D, et al. Effects of increased renal parenchymal pressure on renal function. J Trauma. 2000;48:874–7. doi: 10.1097/00005373-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 31.McNelis J, Marini CP, Simms HH. Abdominal compartment syndrome: clinical manifestations and predictive factors. Curr Opin Crit Care. 2003;9:133–6. doi: 10.1097/00075198-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Hollander-Rodriguez JC, Calvert JF. Hyperkalaemia. Am Fam Phys. 2006;73:283–90. [PubMed] [Google Scholar]
- 33.Diaz-Sandoval LJ, Kosowsky BD, Losordo DW. Acetylcysteine to prevent angiography-related renal tissue injury (the APART trial.) Am J Cardiol. 2002;89:356–8. doi: 10.1016/s0002-9149(01)02243-3. [DOI] [PubMed] [Google Scholar]
- 34.Kshirsagar AV, Poole C, Mottl A, et al. Acetylcysteine for the prevention of radiocontrast induced nephropathy. A meta-analysis of prospective controlled trials. J Am Soc Nephrol. 2004;15:761–9. doi: 10.1097/01.asn.0000116241.47678.49. [DOI] [PubMed] [Google Scholar]
- 35.Welch M, Newstead CG, Smyth JV, Dodd PD, Walker MG. Evaluation of dopexamine hydrochloride as a renoprotective agent during aortic surgery. Ann Vasc Surg. 1995;9:488–92. doi: 10.1007/BF02143865. [DOI] [PubMed] [Google Scholar]
- 36.Berndes E, Mollhoff T, Van Aken H, et al. Effects of dopexamine on creatinine clearance, systemic inflammation and splanchnic oxygenation in patients undergoing coronary artery bypass grafting. Anesth Analg. 1997;84:950–7. doi: 10.1097/00000539-199705000-00002. [DOI] [PubMed] [Google Scholar]
- 37.Bellomo R, Chapman M, Finfer S. Low-dose dopamine in patients with early renal dysfunction: A placebo-controlled randomised trial. Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Lancet. 2000;356:2139–43. doi: 10.1016/s0140-6736(00)03495-4. [DOI] [PubMed] [Google Scholar]
- 38.Intensive Care Society. Critical Care Focus: Renal Failure. 1st edn. London: BMJ Books; 1999. [Google Scholar]
- 39.Waldrop J, Ciraulo DL, Milner TP, Gregori D, Kendrick AS, Richart CM, et al. A comparison of continuous renal replacement therapy to intermittent dialysis in the management of renal insufficiency in the acutely ill surgical patient. Am Surg. 2005;71:36–9. [PubMed] [Google Scholar]
- 40.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2004;30:536–55. doi: 10.1007/s00134-004-2210-z. [DOI] [PubMed] [Google Scholar]
- 41.Ronco C, Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcome of acute renal failure: a prospective randomised trial. Lancet. 2000;356:26–30. doi: 10.1016/S0140-6736(00)02430-2. [DOI] [PubMed] [Google Scholar]