Abstract
INTRODUCTION
Laparostomy techniques have advanced since the advent of damage control surgery for the critically injured patient. Numerous methods of temporary abdominal closure (TAC) are described in the literature with most reports focusing on trauma. We describe a modified technique for TAC and report its use in a series of critically ill non-trauma patients.
PATIENTS AND METHODS
Eleven patients under the care of one consultant underwent TAC over a 36-month period. A standardised technique was used in all cases and this is described. Severity of illness at the time of the first laparotomy was assessed using the Portsmouth variant of the Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity (P-POSSUM).
RESULTS
Nineteen TACs were performed in 11 patients with a variety of serious surgical conditions. In-hospital mortality was zero despite seven of the patients having an individual P-POSSUM predicted mortality in excess of 50%. The laparostomy dressing proved simple in construction, facilitated nursing care and was well-tolerated in the critical care environment. All patients underwent definitive fascial closure during the index admission.
CONCLUSIONS
Laparostomy is a useful technique outwith the context of trauma. We have demonstrated the utility of the modified Opsite® sandwich vacuum pack for TAC in a series of critically ill patients with a universally favourable outcome. This small study suggests that selective use of TAC may reduce surgical mortality.
Keywords: Laparostomy, Temporary abdominal closure (TAC), Abdominal compartment syndrome (ACS)
Laparostomy with temporary abdominal closure remains a controversial technique and may be under-utilised in emergency surgery in the UK. The main indications are in the prevention and treatment of abdominal compartment syndrome (ACS) and to facilitate second-look laparotomy in trauma and complex sepsis. The historical unpopularity of laparostomy is multifactorial. Reasons include: (i) under recognition of the potential or actual development of ACS; (ii) a lack of surgical familiarity with the techniques of temporary abdominal closure (TAC); (iii) visceral complications including fistulation; and (iv) the perceived difficulty of obtaining eventual fascial closure.
Most studies on ACS and TAC have focused on trauma patients who have undergone damage control laparotomy for multiple system injury.1–4 These principles can be usefully applied in patients with intra-abdominal sepsis,5,6 severe pancreatitis7,8 and ruptured abdominal aortic aneurysms9,10 in whom similar pathophysiological mechanisms may be operating.11 Regardless of the underlying aetiology, the development of ACS is a common ‘second hit’ in the pathogenesis of multiple organ failure in surgical patients. Established ACS is associated with a grim prognosis.
Many techniques for TAC are described in the literature. These include the ‘Bogota Bag’, towel-clip skin only closure,12 synthetic mesh, both absorbable2,8,13 and non-absorbable,10 the ‘sandwich’ dressing,14 various ‘vacuum pack’ derivatives15–17 and silicone elastomer sheeting.18 The variety of techniques described suggests that each may have limitations in use.
We describe our modification of the Opsite® (Smith & Nephew, Hull, UK) sandwich ‘vacuum pack’ dressing for laparostomy wound management and our experience with it in a small series.
Patients and Methods
Method of construction
Three personnel including the theatre nurse are required.
A medium-sized Opsite® dressing (45 × 55 cm) with the backing removed is placed with the adhesive surface upward on an empty, draped theatre trolley and gently tensioned.
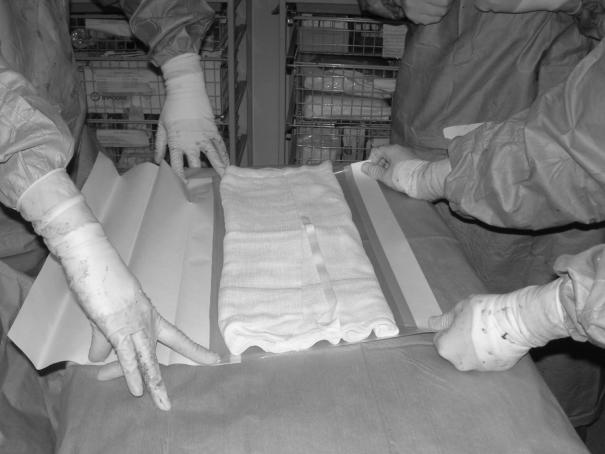
A large abdominal gauze pack (45 × 45 cm) with the sides folded inwards to create a rectangular configuration in the longitudinal axis, is laid onto the Opsite® dressing (Fig. 1).
The Opsite® dressing is then folded over the pack on all four sides leaving some exposed gauze uppermost (Fig. 2). This manoeuvre creates a dressing with both a non-adherent (under) and upper side for laying onto the intestinal surface and insertion beneath the posterior abdominal wall, respectively, while permitting fluid egress. Fenestration of the undersurface is unnecessary.
In this orientation, the dressing is laid on top of the abdominal viscera with the edges gently tucked under the abdominal wall.
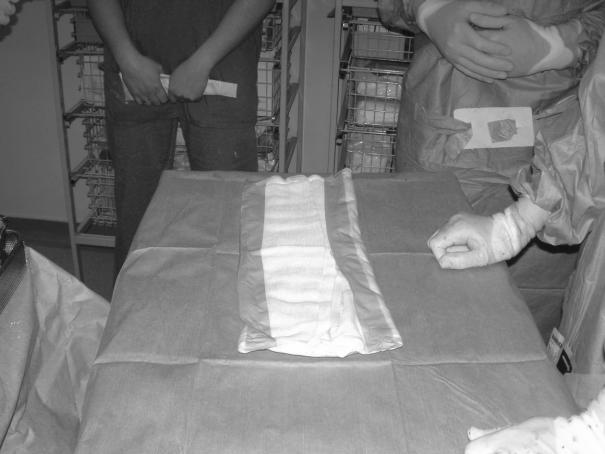
Two medium-sized suction drains are laid on top of the dressing and brought out extrafascially through the skin on the same side (Figs 3 and 4).
The abdominal skin is shaved if necessary, cleaned and dried thoroughly. Two holes are made in a large sized Opsite® dressing (56 × 84 cm) corresponding to the drain exit sites. The backing is then removed and the dressing applied to one side of the abdomen under gentle tension. The drains are picked up with a Mayos' artery forceps and led through the holes in the Opsite®, taking care not to catch the adhesive surface. The Opsite® is then laid onto the skin of the opposite side of the abdomen. This manoeuvre together with the extrafascial, but skin-penetrating, drain pathway is critical in the prevention of leakage of fluid. Feeding jejunostomy tubes and other drains can be treated in a similar fashion.
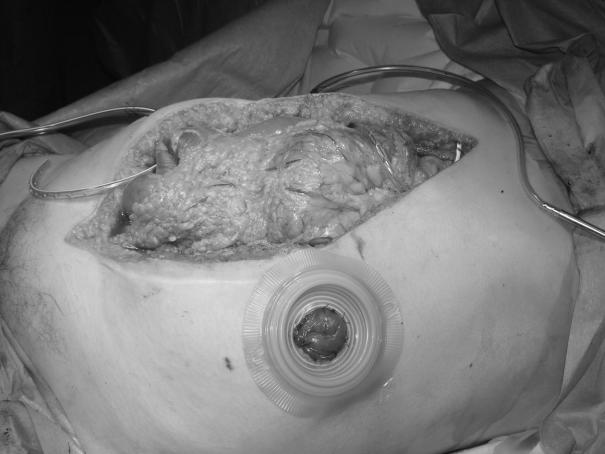
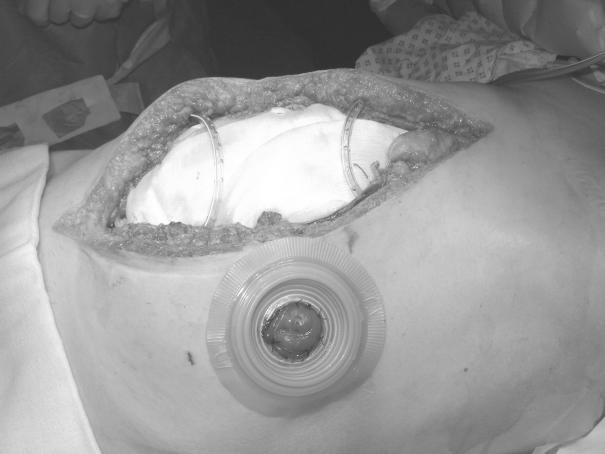
Stomas are accommodated using a two-piece appliance (Fig. 3) The flange is first positioned on the skin and an aperture cut in the Opsite® prior to its application to the skin. Stoma bags can then be attached and later changed, without disturbing the underlying dressing.
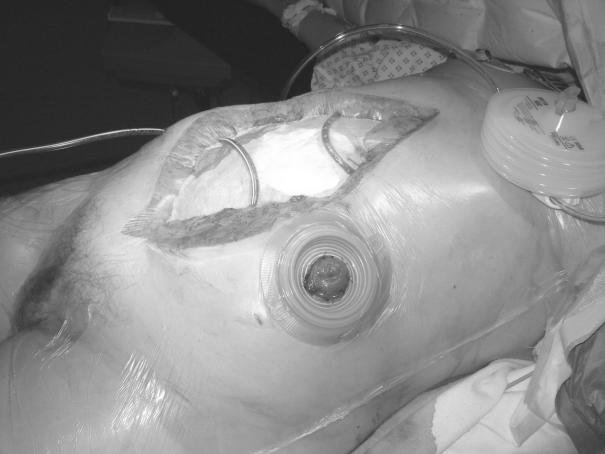
The drains are then attached to the theatre suction unit on low pressure, creating a ‘vacuum pack’ to complete the dressing (Fig. 5). A closed suction system (e.g. Portovac®) is then substituted. If postoperative exudate volumes are large, the drains may be reconnected intermittently to low-pressure wall suction. A loss of vacuum will be readily apparent and any break in the vacuum system (e.g. a tear in the outer Opsite®) should be rectified promptly. A Tagaderm® dressing provides a useful ‘patch’.
If the patient's overall condition permits, postoperative ventilation or paralysis is not required as the dressing is well tolerated.
The dressing is left undisturbed until a planned ‘second-look’ procedure at 24–48 h. If surgical conditions permit, definitive fascial closure may be possible at this time. Close monitoring of vital signs including airway pressures, oxygen requirement and urine output are required during fascial closure and, in the event of significant deterioration occurs, this should be delayed and further TAC is undertaken.
Figure 1.
Gauze packs folded and laid on adhesive surface of tensioned Opsite® dressing.
Figure 2.
Opsite® folded leaving non-adherent undersurface and edges with area of exposed gauze uppermost.
Figure 3.
Placement of flange for stoma appliance and drains following completion of peritoneal toilet. Note extrafascial tunnelling of suction drains.
Figure 4.
Dressing placed onto abdominal contents and deep to posterior sheath with drains positioned on surface of gauze.
Figure 5.
Completed dressing prior to application of stoma appliance. Note ‘vacuum pack’ appearance following application of suction.
Results
Our experience with the modification of the Opsite® sandwich vacuum pack dressing described above extends to a total of 19 dressings in 11 critically ill patients over a 36-month period. The series includes patients with peritonitis, intra-abdominal haemorrhage and ischaemic bowel. The cases, the number of TACs, the time to fascial closure, the use of mesh, maximum airway pressures, ITU and hospital stay and the P-POSSUM predicted mortality at the time of first laparotomy, are presented in Table 1.
Table 1.
Summary of results of 11 patients
| Diagnosis | Age/sex | Number of TACs | Maximum airway pressure | Time to fascial closure | Mesh | ITU stay (days) | Hospital stay (days) | P-POSSUM score at time of first laparotomy | P-POSSUM predicted mortality (%) |
|---|---|---|---|---|---|---|---|---|---|
| Ischaemic bowel | 31F | 1 | 17 | 1 | No | 1 | 16 | 31 | 1.8 |
| Appendicitis, generalised peritonitis | 21F | 2 | 23 | 2 | No | 3 | 9 | 35 | 3.2 |
| Peripancreatic abscess | 63F | 4 | 35 | 8 | No | 6 | 65 | 57 | 58.4 |
| Biliary peritonitis | 74M | 1 | 32 | 5 | Yes | 18 | 81 | 57 | 57.3 |
| Small bowel perforation, generalised peritonitis | 79F | 1 | 21 | 2 | No | 5 | 33 | 59 | 63.1 |
| Ruptured splenic artery, false aneurysm | 33F | 1 | 21 | 2 | No | 1 | 10 | 69 | 88.9 |
| Perforated diverticulitis, faecal peritonitis | 62F | 1 | 27 | 4 | No | 24 | 64 | 61 | 84.2 |
| Colonic anastomotic leak, faecal peritonitis | 45M | 4 | 35 | 10 | No | 11 | 38 | 52 | 34 |
| Ischaemic bowel | 52M | 1 | 23 | 2 | No | 16 | 98 | 48 | 20.3 |
| Biliary peritonitis | 76F | 2 | 21 | 5 | No | 11 | 20 | 63 | 77.8 |
| Sigmoid injury post TAH, faecal peritonitis | 72F | 1 | 21 | 2 | No | 2 | 22 | 54 | 71.9 |
Discussion
The modified Opsite® sandwich vacuum pack dressing which we describe differs from those previously reported in key aspects. First, the gauze pack is wrapped in Opsite® and the undersurface of the dressing in contact with the abdominal viscera is left unfenestrated. Fluid flows freely around the sides of the dressing on to the gauze and thence to the suction drains. Second, the drains are brought out through the skin a short distance from the wound edges and then through the outer layer of Opsite®. This maintains the vacuum, abolishes leakage through the dressing and thus facilitates nursing care.
This system has several advantages over other methods of TAC. The ‘Bogota Bag’ type dressing,12 even with suction applied,17 is not airtight and, therefore, tends to leak. The patient is consequently difficult to nurse, at risk of pressure sores and secondary peritoneal contamination. None of the patients in this series experienced any significant leaks. Efficient, continuous, suction drainage may enhance drainage of infected peritoneal fluid. The risk of secondary visceral injury from adherence to the dressing is less than that associated with meshes. Direct contact between suction drains and bowel with its attendant hazards is also avoided. In contrast to ‘Bogota Bag’ and other prosthetic type closures, there is no damage to the fascia. Intuitively, this must influence final closure rate by preserving tissue integrity. This is particularly relevant when considering the need for multiple dressing applications. It is also conceivable that a vacuum-type dressing prevents fascial regression by splintage. The wound milieu provided may abolish the lag phase of healing when the skin is finally closed in a process akin to delayed primary closure. The robust nature of the dressing was well illustrated in one of the patients who underwent endoscopic retrograde cholangiopancreatography in the prone position. The Opsite® dressing is significantly cheaper than meshes or silicone elastomer sheeting. Finally, the dressing is quick and simple to construct in an unstable patient.
This case series illustrates the effective use of the modified Opsite® sandwich vacuum pack dressing after laparotomy for a wide range of intra-abdominal pathologies. All patients were critically ill and several may have been expected to succumb (Table 1). Most of the literature regarding ACS and TAC concentrates on trauma, particularly damage-control laparotomy. This series deliberately includes no trauma cases although in this time frame we used this form of TAC in two major trauma cases. Six of the 11 patients underwent TAC pre-emptively because they were judged to be high risk for the development of ACS and four to facilitate second-look laparotomy. Only one patient underwent decompressive laparotomy because of high intravesical pressure in the face of worsening organ failure. We believe it is relevant that, despite the severity of the abdominal catastrophe in each case and although systemic upset was present in all cases, only one patient described developed three-system failure and, in this case, complete oliguria was already present preoperatively. None of the remaining nine patients developed more than moderate single system dysfunction.
It is our hypothesis that injudicious closure of the abdomen in such critically ill patients may well contribute to multipleorgan dysfunction with associated systemic inflammatory response syndrome. This concept of an initial mechanical phase, where venous return is impaired along with progressive ventilatory difficulty, leading to a ‘humoral’ phase with potentially irretrievable consequences, is supported by recent evidence from other authors.11 Further support for this belief comes in this series from the fact that no patient undergoing prophylactic TAC developed intravesical or airway pressures greater than 15 cmH2O and 35 cmH2O, respectively. In the case of the single patient requiring decompressive laparostomy, the elevated airway pressures rapidly decreased in association with a sudden and dramatic rise in both urine output and mean arterial blood pressure. We, therefore, advocate laparostomy as a potentially life-saving prophylactic measure in patients at high risk of ACS particularly as the mortality associated with decompressive laparotomy for established ACS is substantial (25–71%).18
In 10 cases, delayed primary fascial closure was achieved during the index admission. None of these required relaxing incisions or skin grafting. In 7 cases, closure was possible at ‘second look’ between 1–5 days. Two cases needed two dressings and two cases, four before final closure. The latest primary closure was achieved at 8 days. One patient required mesh repair at day 10 after four laparostomy dressings. In-hospital mortality was zero. There was one incisional hernia which was subsequently repaired although clearly, follow-up is relatively short and incomplete.
There were two patients with a recovery complicated by enterocutaneous fistula formation but neither appeared directly attributable to the laparostomy management. One patient developed a small bowel fistula related to a feeding jejunostomy tube which resolved rapidly with conservative management. A second patient later developed both gastrocutaneous and colocutaneous fistulae related to an undiagnosed pancreatic malignancy. This patient died 14 months later of carcinomatosis. In the case of the patient with the displaced jejunostomy tube, the absorbable sutures anchoring the catheter to the parietes had cut through the bowel wall creating an enterotomy. We do not feel that jejunostomy placement is contra-indicated in laparostomy cases but, clearly, care is required during dressing changes to avoid traction in the vicinity of the catheter.
Long-term outcomes related to hernia formation require further evaluation although this may prove difficult even within a randomised, controlled trial context, due to the large number of variables and small patient numbers.
A disadvantage of Opsite® is its relative expense. Innovative vacuum type dressings have been described for use in resourceconstrained environments.17 We postulate that the much cheaper alternative of Clingfilm® might be appropriate in these situations. The gauze would require complete wrapping and fenestration and the outer layer would need to encircle the abdomen although it is possible that this could predispose to a secondary ACS as has been described with other forms of TAC.20
Conclusions
A simple, efficient, low-maintenance and relatively costeffective dressing for laparostomy wound management has been described. The universally favourable outcome in this small series of high-risk, seriously ill patients, supports its use. Laparostomy should be considered in patients judged to be high risk for postoperative ACS as we believe this may prove life saving. The availability of our technique may embolden surgeons in its use. We believe there is a strong case for a randomised, controlled trial in such high-risk surgical patients.
References
- 1.Meldrum DR, Moore FA, Moore EE, Franciose RJ, Sauaia A, Burch JM. Prospective characterization and selective management of the abdominal compartment syndrome. Am J Surg. 1997;174:667–72. doi: 10.1016/s0002-9610(97)00201-8. [DOI] [PubMed] [Google Scholar]
- 2.Ivatury RR, Porter JM, Simon RJ, Islam S, John R, Stahl WM. Intraabdominal hypertension after life threatening penetrating abdominal trauma: prophylaxis, incidence and clinical relevance to gastric mucosal pH and abdominal compartment syndrome. J Trauma. 1998;44:1016–21. doi: 10.1097/00005373-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Ertl W, Oberholzer A, Platz A, Stocker R, Trentz O. Incidence and clinical pattern of the abdominal compartment syndrome after ‘damage control’ laparotomy in 311 patients with severe abdominal and/or pelvic trauma. Crit Care Med. 2000;28:1747–53. doi: 10.1097/00003246-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Offner PJ, de Souza AL, Moore EE, Biffl WL, Franciose RJ, Johnson JL, et al. Avoidance of abdominal compartment syndrome in damage control laparotomy after trauma. Arch Surg. 2001;136:676–81. doi: 10.1001/archsurg.136.6.676. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg D. On leaving the peritoneal cavity open in acute generalized suppurative peritonitis. Am J Surg. 1979;137:216–20. doi: 10.1016/0002-9610(79)90148-x. [DOI] [PubMed] [Google Scholar]
- 6.Bailey CMH, Thomson Fawcett MW, Kettlewell MGW, Garrard C, Mortensen NJM. Laparostomy for severe intra-abdominal infection complicating colorectal disease. Dis Colon Rectum. 2000;43:25–30. doi: 10.1007/BF02237239. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Sabrido JL, Tallado JM, Christou NV, Polo JR, Valdecantos E. Treatment of severe intra-abdominal sepsis and/or necrotic foci by an ‘open-abdomen’ approach. Zipper and zipper mesh techniques. Arch Surg. 1988;123:152–6. doi: 10.1001/archsurg.1988.01400260032002. [DOI] [PubMed] [Google Scholar]
- 8.Gentile AT, Feliciano PD, Mullins RJ, Crass RA, Eidemiller LR, Sheppard BC. The utility of polyglycolic acid mesh for abdominal access in patients with necrotising pancreatitis. J Am Coll Surg. 1998;186:313–8. doi: 10.1016/s1072-7515(98)00012-x. [DOI] [PubMed] [Google Scholar]
- 9.Oeschlager BK, Boyle EM, Johansen K, Meissner MH. Delayed abdominal closure in the management of ruptured abdominal aortic aneurysm. Am J Surg. 1997;173:411–5. doi: 10.1016/S0002-9610(97)00081-0. [DOI] [PubMed] [Google Scholar]
- 10.Ciresi DL, Cali RF, Senagore AJ. Abdominal closure using nonabsorbable mesh after massive resuscitation prevents abdominal compartment syndrome and GI fistula. Am Surg. 1999;65:720–4. [PubMed] [Google Scholar]
- 11.Rezende-Neto JB, Moore EE, Melo de Andrade MV, Teixeira MM, Lisboa FA, Arantes RM, et al. Systemic inflammatory response secondary to abdominal compartment syndrome: stage for multiple organ failure. J Trauma. 2002;53:1121–8. doi: 10.1097/00005373-200212000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Feliciano DV, Burch JM. Towel clips, silos and heroic forms of wound closure. In: Maull KI, Cleveland HC, Feliciano DV, et al., editors. Advances in Trauma and Critical Care. vol 16. Chicago: Year Book Medical Publishers; 1991. pp. 231–50. [Google Scholar]
- 13.Mayberry JC, Mullins RJ, Crass RA, Trunkey DD. Prevention of abdominal compartment syndrome by absorbable mesh prosthesis closure. Arch Surg. 1997;132:957–62. doi: 10.1001/archsurg.1997.01430330023003. [DOI] [PubMed] [Google Scholar]
- 14.Schein M, Saadia R, Jamieson JR, Decker GG. The ‘sandwich technique’ in the management of the open abdomen. Br J Surg. 1986;73:369–70. doi: 10.1002/bjs.1800730514. [DOI] [PubMed] [Google Scholar]
- 15.Brock WB, Barker DE, Burns RP. Temporary closure of open abdominal wounds: the vacuum pack. Am Surg. 1995;61:30–5. [PubMed] [Google Scholar]
- 16.Barker DE, Kaufman HJ, Smith LA, Ciraulo DI, Richart CL, Burns RP. Vacuum pack technique of temporary abdominal closure: a 7-year experience with 112 patients. J Trauma. 2000;48:201–6. doi: 10.1097/00005373-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Navsaria PH, Bunting M, Omoshoro-Jones J, Nichol AJ, Kahn D. Temporary closure of open abdominal wounds by the modified sandwich-vacuum pack technique. Br J Surg. 2003;90:718–22. doi: 10.1002/bjs.4101. [DOI] [PubMed] [Google Scholar]
- 18.Foy HM, Nathens AB, Maser B, Mathur S, Jurkovich GJ. Reinforced silicone elastomer sheeting, an improved method of temporary abdominal closure in damage control laparotomy. Am J Surg. 2003;185:498–501. doi: 10.1016/s0002-9610(03)00059-x. [DOI] [PubMed] [Google Scholar]
- 19.Saggi BH, Sugerman HJ, Ivatury RR, Bloomfield GL. Abdominal compartment syndrome. J Trauma. 1998;45:597–609. doi: 10.1097/00005373-199809000-00033. [DOI] [PubMed] [Google Scholar]
- 20.Gracias VH, Braslow B, Johnson JM, Pryor J, Gupta R, Reilly P, et al. Abdominal compartment syndrome in the open abdomen. Arch Surg. 2002;137:1298–300. doi: 10.1001/archsurg.137.11.1298. [DOI] [PubMed] [Google Scholar]