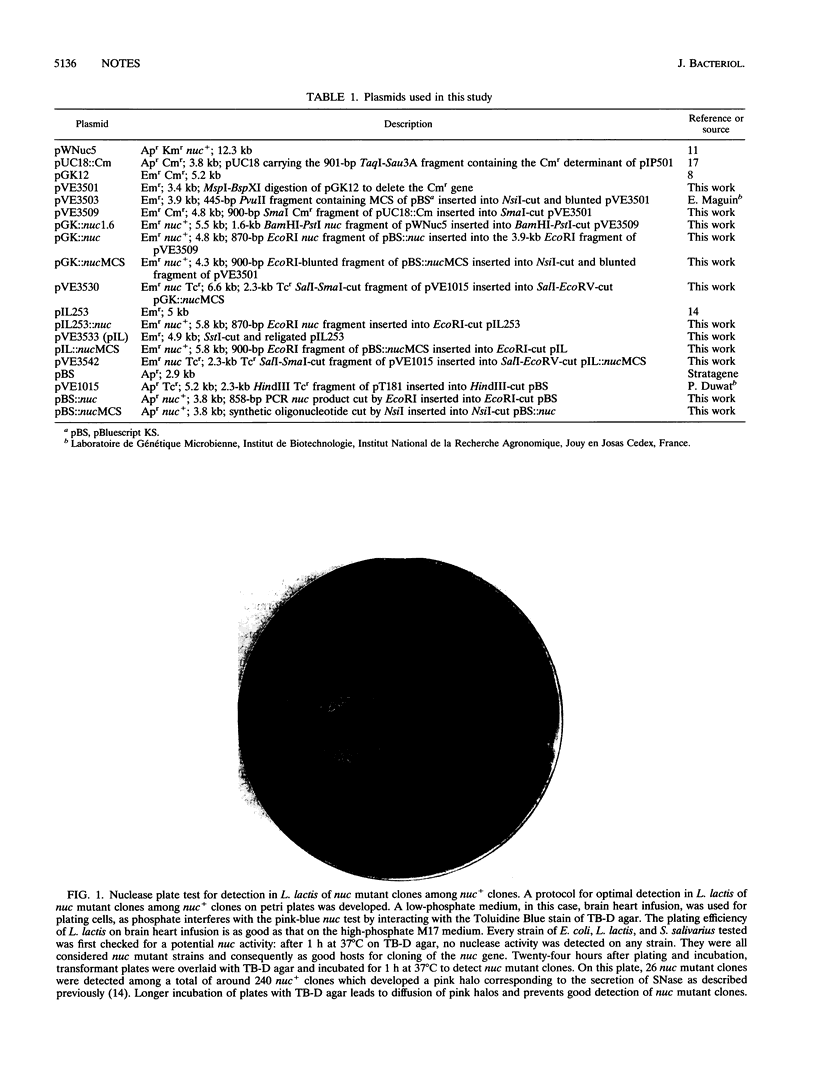
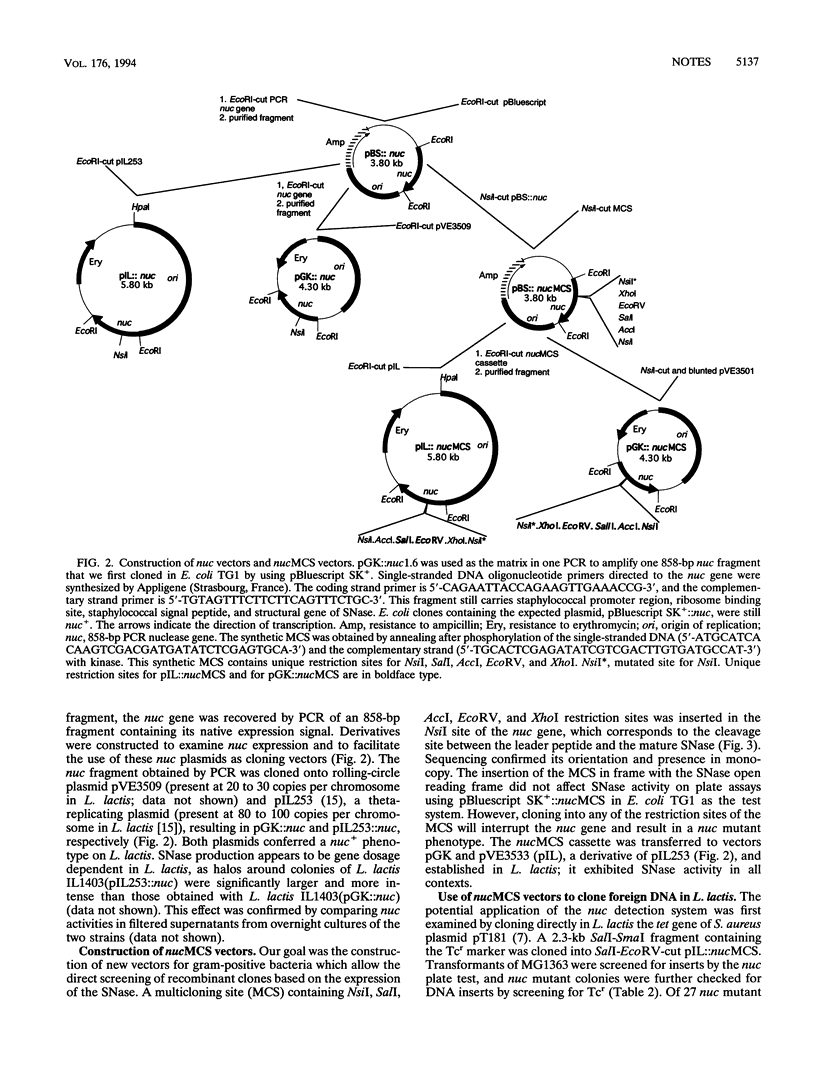
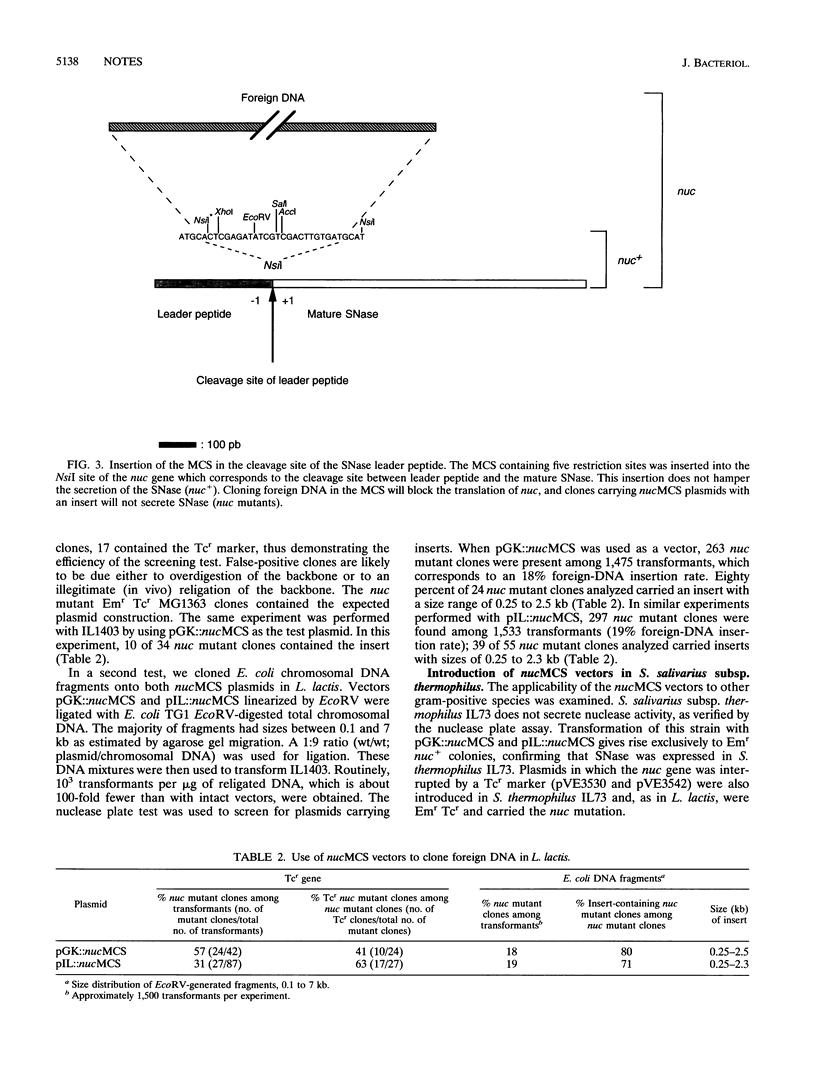
Abstract
A system for direct screening of recombinant clones in Lactococcus lactis, based on secretion of the staphylococcal nuclease (SNase) in the organism, was developed. The nuc gene (encoding SNase) was cloned on both rolling-circle and theta-replicating plasmids. L. lactis strains containing these nuc+ plasmids secrete SNase and are readily detectable by a simple plate test. A multicloning site (MCS) was introduced just after the cleavage site between leader peptide and the mature SNase, without affecting nuclease activity. Cloning foreign DNA fragments into any site of the MCS interrupts nuc and thus results in nuc mutant clones which are easily distinguished fron nuc+ clones on plates. The utility of this system for L. lactis was demonstrated by cloning an antibiotic resistance marker and Escherichia coli chromosomal DNA fragments into the MCS of the nucMCS cassette. Both cloning vectors containing the nucMCS cassette were also introduced into Streptococcus salivarius subsp. thermophilus, in which direct screening of nuc mutant recombinant clones was also achieved. The potential uses of nuc as a secretion reporter system are discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopin A., Chopin M. C., Moillo-Batt A., Langella P. Two plasmid-determined restriction and modification systems in Streptococcus lactis. Plasmid. 1984 May;11(3):260–263. doi: 10.1016/0147-619x(84)90033-7. [DOI] [PubMed] [Google Scholar]
- Gasson M. J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983 Apr;154(1):1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haima P., van Sinderen D., Bron S., Venema G. An improved beta-galactosidase alpha-complementation system for molecular cloning in Bacillus subtilis. Gene. 1990 Sep 1;93(1):41–47. doi: 10.1016/0378-1119(90)90133-c. [DOI] [PubMed] [Google Scholar]
- Khan S. A., Novick R. P. Complete nucleotide sequence of pT181, a tetracycline-resistance plasmid from Staphylococcus aureus. Plasmid. 1983 Nov;10(3):251–259. doi: 10.1016/0147-619x(83)90039-2. [DOI] [PubMed] [Google Scholar]
- Kok J., van der Vossen J. M., Venema G. Construction of plasmid cloning vectors for lactic streptococci which also replicate in Bacillus subtilis and Escherichia coli. Appl Environ Microbiol. 1984 Oct;48(4):726–731. doi: 10.1128/aem.48.4.726-731.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachica R. V., Genigeorgis C., Hoeprich P. D. Metachromatic agar-diffusion methods for detecting staphylococcal nuclease activity. Appl Microbiol. 1971 Apr;21(4):585–587. doi: 10.1128/am.21.4.585-587.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langella P., Le Loir Y., Ehrlich S. D., Gruss A. Efficient plasmid mobilization by pIP501 in Lactococcus lactis subsp. lactis. J Bacteriol. 1993 Sep;175(18):5806–5813. doi: 10.1128/jb.175.18.5806-5813.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl W., Sinskey A. J., Schleifer K. H. Expression, secretion, and processing of staphylococcal nuclease by Corynebacterium glutamicum. J Bacteriol. 1992 Mar;174(6):1854–1861. doi: 10.1128/jb.174.6.1854-1861.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. R., Kovacevic S., Veal L. E. Secretion and processing of staphylococcal nuclease by Bacillus subtilis. J Bacteriol. 1987 Aug;169(8):3508–3514. doi: 10.1128/jb.169.8.3508-3514.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D. A genetic system for analysis of staphylococcal nuclease. Gene. 1983 May-Jun;22(2-3):181–189. doi: 10.1016/0378-1119(83)90102-6. [DOI] [PubMed] [Google Scholar]
- Simon D., Chopin A. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie. 1988 Apr;70(4):559–566. doi: 10.1016/0300-9084(88)90093-4. [DOI] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trieu-Cuot P., de Cespedes G., Horaud T. Nucleotide sequence of the chloramphenicol resistance determinant of the streptococcal plasmid pIP501. Plasmid. 1992 Nov;28(3):272–276. doi: 10.1016/0147-619x(92)90060-n. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]