Abstract
INTRODUCTION
Testicular prostheses produced from various materials have been in use since 1941. The absence of a testicle has been shown to be a psychologically traumatic experience for males of all ages. The indications for insertion of a prosthesis include absence or following orchidectomy from a number of causes such as malignancy, torsion and orchitis. The most common substance used around the world in the manufacture of these implants is silicone; however, in the US, this material is currently banned because of theoretical health risks. This has led to the development of saline-filled prostheses as an alternative.
PATIENTS AND METHODS
A Medline search was carried out on all articles on testicular prosthesis between 1966 and 2006.
CONCLUSIONS
This review highlights the controversies regarding prosthetic materials, the complications of insertion and the potential benefits of this commonly performed procedure.
Keywords: Testicular prostheses, Implants, Silicone
The absence of a testis from the scrotal sac represents a psychologically traumatic experience in males of any age from childhood to the elderly.1 Testicular loss may arise following orchidectomy for torsion, mal-descent, trauma, infection or malignancy. Testicular absence is seen in cryptorchidism from either an undescended or ectopic testis. It may also be a result of testicular agenesis or atrophy following intra-uterine torsion (vanishing testis syndrome). Such patients may, at some stage, request the implantation of an artificial testis for cosmetic or psychological reasons. This is more likely in patients who have lost a testis compared to those born with an absent testis. Female-to-male trans-sexuals may also seek a testicular prosthesis as part of their gender re-alignment surgery.
Literature search
A Medline search was carried out using the search terms ‘testicular prosthesis’ and ‘testicular implants’. The reference lists of key articles were also reviewed.
History and development
The first prosthesis used in 1941 was composed of vitallium (an alloy of cobalt, chromium and molybdenum).2 In 1943, testicular prostheses made of Lucite were available in a range of sizes.3 During the 1950s, numerous other materials, including glass marbles, were used.4 Gelfoam was specifically injected into the tunica albuginea following intracapsular orchidectomy performed on patients with metastatic prostate cancer.5 Plexiglass, Dacron and polyethylene prostheses have also been used without much success. It was then suggested that the ideal testicular prosthesis should be chemically inert and should not elicit any inflammatory or hypersensitivity reactions. The material should also resist mechanical strains, take and hold the desired form, be amenable to sterilisation and be a proven non-carcinogen. As a result, silastic and solid silicone rubber prostheses were developed for use in the 1960s. The demand for more natural-feeling implants led to gel-filled silicone devices appearing in 1972.6 A firmer, silicone-coated product became the standard prosthesis in 1988. However, in the US in 1992, the Food and Drug Administration (FDA) halted the use of gel-filled breast implants due to the theoretical risks of connective tissue and autoimmune disorders, the question of mechanical instability and the remote possibility of tumour development. Indeed, Robinson et al.7 analysed silicone breast implants removed from 300 consecutive patients and found that 64% had some form of device disruption. They suggested that most breast prostheses would lose the integrity of their silicone shell between 8–14 years after implantation. In a study of patients who had previously undergone insertion of penile prostheses, silicone particles were found in 18 of 25 operative tissue specimens and in 4 out of 4 specimens obtained from regional lymph nodes.8 This clearly demonstrated that there had been a leak of silicone into surrounding tissues from the prostheses, albeit in small amounts. This phenomenon of silicone migration is also known as gel bleed. Despite this, no subsequent evidence has been found of a link between usage of penile or testicular prostheses and connective tissue diseases.8,9 This has been further ratified by multispeciality expert panels in the US (Institute of Medicine and the National Science Panel) and the UK. The only cancers attributable to implanted silicone are seen in animal studies and are connective tissue sarcomas in susceptible strains of in-bred rodents.10 Furthermore, there was no reported increase in breast sarcomas in the US during the period of silicone breast implant use11 and there are no reported cases in the world literature of tumours arising from the usage of silicone testicular implants.
As a consequence of the concerns regarding silicone breast implants, there then followed a voluntary withdrawal of silicone-gel filled testicular prostheses in 1995 and replacement with the newly developed saline-filled prosthesis in the US (Mentor Medical Systems).
A prospective study assessing the safety profile of the Mentor saline-filled prosthesis was then undertaken by Turek et al.12 They studied 149 adult and paediatric patients from 18 institutions and assessed various parameters such as connective tissue disorders, complications and quality of life. At one year, none of the patients had developed connective tissue disorders and they concluded that saline-filled prostheses appeared safe and well-tolerated in the short-term.
Current implants in use
There are four companies (Nagor Ltd, Douglas, Isle of Man, UK; Mentor Medical Systems Ltd, Wantage, Oxon UK; Osteotec Plastic Surgery, Dorset, UK; and Silimed, Dieburg, Germany) that supply the majority of testicular prostheses used in the UK. Osteotec Plastic Surgery supplies the Perthese prosthesis. Nagor prostheses are produced as silicone-gel filled and elastomer versions whereas the Silimed implant is only available in the elastomer version, which has a more solid consistency. The Perthese implant is produced in the gel-filled version; however, Mentor Medical Systems provides a re-inforced silicone elastomer version called the Soft-Solid Testicular Prosthesis (SSTP). They also provide a saline-filled prosthesis which has recently received FDA approval and is the only licensed testicular prosthesis available for common usage in the US. The weight, shape and texture of the Mentor SSTP is designed to approximate the normal testicle and is only licensed for investigational purposes in North America. The company is currently conducting a clinical study to evaluate the safety of its SSTP; to date, 60 patients have been enrolled in up to 10 study sites and been followed up for 1 year.
The Mentor, Nagor and Perthese prostheses are produced with a suture loop to aid fixation of the implant in the scrotum's most pendant position and reduce unnecessary movement (Figs 1 and 2).
Figure 1.

Mentor saline-filled prosthesis with suture loop.
Figure 2.
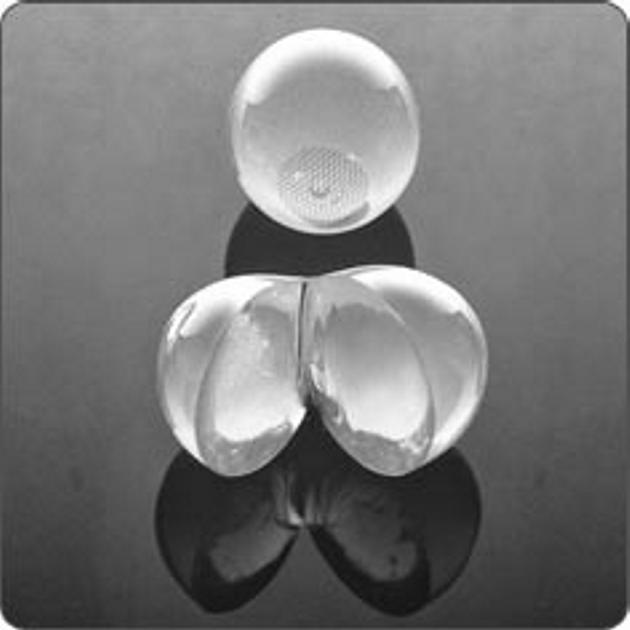
Mentor Semi Solid Testicular Prosthesis (SSTP).
Nagor and Perthese prostheses are produced in small, medium or large sizes. The Mentor saline-filled version is also available in an extra small size whereas its SSTP version is produced in five different sizes as is the Silimed implant.
Indications for insertion
Exploration for a cryptorchid testicle and the finding of testicular agenesis or atrophy is the most common indication for insertion of a testicular prosthesis. Indeed, testicular agenesis or atrophy may be present in up to 8–10% of patients who have an inguinal exploration for cryptorchidism. An implant may also be requested following testicular atrophy arising from damage of the testicular vasculature during orchidopexy, inguinal hernia repair or varicocele ligation. A non-viable testis identified at exploration for testicular torsion or testicular trauma may also be accompanied by the insertion of a prosthesis. Certainly, all men undergoing radical orchidectomy for testicular cancer should be offered simultaneous implantation of a testicular prosthesis. Patients undergoing genital reconstruction for intersex or gender dysphoria are also candidates for testicular prosthesis implantation.
A survey of members of the Western Section of the America Urological Association in 1986 reported the indications for implantation of a testicular prosthesis over a 10-year period13 and is summarised in Table 1. This historical study shows that nearly a fifth of patients undergoing insertion of a testicular prosthesis were in men undergoing bilateral orchidectomy for advanced prostate cancer. However, since the availability in the early 1990s of medical castration with luteinising hormone releasing hormone (LHRH) analogues, the usage of surgical castration in the management of metastatic prostate cancer has fallen dramatically; thus, this indication for implantation has fallen considerably.
Table 1.
Indications for orchidectomy prior to placement of a testicular prosthesis
| Undescended testicle | 35% |
| Testicular torsion | 25% |
| Testicular tumour | 17% |
| Metastatic prostate cancer | 16% |
| Epididymitis/orchitis | 8% |
| Trauma | 1% |
Timing of insertion
The timing of insertion of a testicular prosthesis in a child is not straightforward. The psychological impact of an absent testicle in a child or adolescent is a good reason to consider implantation at the time of the initial surgery for a cryptorchid testis. The problem is that this may necessitate further surgery to insert a larger prosthesis when the child gets older. An alternative strategy is to delay the placement of the definitive prosthesis until the child reaches adolescence. If a child is content with the size of the original prosthesis, further surgery can be avoided.
The underdeveloped scrotum that accompanies an undescended testicle may fail to accommodate the desired sized testicular prosthesis. Methods for increasing scrotal space for the placement of a testicular prosthesis include the use of tissue expanders such as a silicone balloon attached to a filling port14 or a Foley catheter balloon.15 Children and young adults are less likely to request a testicular prosthesis for cryptorchidism compared to acquired testicular loss due to torsion, trauma or tumour.
Counselling and peri-operative management
The main postoperative complications are infection and cosmetic concerns. Pre-operative counselling, peri-operative management and operative technique, therefore, should address these points. To minimise the risk of infection following implantation of a prosthesis, a number of stipulations should be observed which are based on the authors' practice during insertion of penile implants (Table 2).
Table 2.
Conditions for reducing incidence of testicular prosthesis infection
| • Avoid implantation if septic genital skin focus is present |
| • Day-case surgery |
| • Sterile urine |
| • Pre-operative chlorhexidine shower |
| • Pubic hair shave in theatre |
| • Antibiotics – systemic and local |
| • 10-min betadine scrub |
| • Double gloving |
| • Water-proof drapes |
| • Avoid haematoma |
Systemic prophylactic antibiotics against Gram-positive, Gram-negative and anaerobic organisms should be given prior to surgery. We use a single-dose regimen of intravenous cefuroxime, gentamycin and metronidazole. We also irrigate the wound with a gentamycin solution and discharge the patient on oral ciprofloxacin for 5 days. If the prosthesis becomes infected, it will need to be removed. A salvage re-implantation may be carried out at 3–6 months.
Surgical implant techniques
Of historical interest, intracapsular insertion of a testicular prosthesis following subscapsular orchidectomy using a scrotal incision in patients with advanced prostate cancer was first described by Tolson in 1944 and endorsed as recently as 1984.16
In 1972, Abbassian17 described the insertion of a testicular prosthesis in a subcuticular pouch which was said to be useful in patients with extensive atrophy and scarring of the scrotal area. A skin incision is made in the opposite hemi-scrotum ensuring not to cross the midline raphe. Through this incision, a subcuticular pouch is created for the prosthesis in the empty hemi-scrotum. However, this procedure is associated with a high incidence of prosthesis extrusion.
To minimise the risk of extrusion of the prosthesis, Latimmer6 advocated a high scrotal or low inguinal incision, anchoring the prosthesis to the bottom of the scrotum and narrowing the upper scrotum with additional sutures. This technique is difficult to perform in the presence of a contracted or scarred hemi-scrotum. In such circumstances, an appropriate space may be created using a sponge-holding forceps18 or by using the balloon of a Foley catheter.15,19
Currently, most surgeons use a low groin incision whenever possible to implant a testicular prosthesis in the belief that this is associated with a lower risk of infection and extrusion. A finger is then placed into the scrotal sac and the potential space created by inflation of a Foley catheter balloon. The most pendant part of the scrotum is subsequently inverted and the prosthesis secured with a PDS suture placed through its suture loop. During transfixation of the dartos, particular care must be taken to avoid skin penetration and, thereby, promote infection and possible extrusion of the prosthesis.
Complications
Marshall13 reviewed the records of over 2500 testicular prosthetic implantations to establish a list of postoperative complications and their incidence (Table 3).
Table 3.
Complications following testicular prosthesis insertion13
| Extrusion | 3–8% |
| Scrotal contraction | 3–5% |
| Pain | 1–3% |
| Haematoma | 0.3–3% |
| Infection | 0.6–2% |
Prosthesis extrusion, the commonest complication, mainly occurred in patients following orchidectomy for epididymo-orchitis, especially if a scrotal incision had been used to implant the device.
Marshall also noted that previous scrotal surgery and a long lag time between orchidectomy and the insertion of the prosthesis increased the risk of developing complications.
There has been a case report of spontaneous rupture of a silicone testicular prosthesis 11 years after its insertion.20 The spread of silicone to inguinal lymph nodes is also documented in a case report21 but, as mentioned previously, there is no evidence of autoimmune disease or malignancy developing following testicular prosthesis implantation.
In current practice, the most common postoperative complaints concern body image, namely that the device is incorrectly sized/shaped or that it is too high in the hemi-scrotum.22
Benefits of testicular prostheses
In contrast to breast implants, where there have been over 35 articles looking at postoperative patient satisfaction, there have been very few quality of life studies reviewing the outcomes of testicular prosthesis insertion. This is surprising as improvement in body image is the only real indication for insertion of testicular prostheses. A study of 19 patients by Lynch et al.23 suggested that most men were happy with their implants and body image. Two other studies have retrospectively looked at overall patient satisfaction. Adshead et al.22 found that 91% of patients who replied to their questionnaire felt it was extremely important to be offered an implant at the time of an orchidectomy. This study also showed that 73% of those who received a prosthesis felt they had an excellent or good result; however, 23% were dissatisfied because of the shape or position of the prosthesis. Incrocci et al.24 documented that 68% of their patients reported a significant improvement in body appearance with only one patient (5%) dissatisfied.
In the only study to evaluate testicular prosthesis implantation prospectively, Turek et al.12 found, using the Rosenberg Self-Esteem Scale, Body Esteem Scale and the Body Exposure in Sexual Activities Questionnaire (BESAQ), that insertion of a testicular prosthesis led to quantifiable improvements in self-satisfaction, self-esteem, physical attractiveness and positive feelings during sexual activity at 1-year follow-up.
Future developments
Congenital or acquired bilateral anorchia often requires testicular implants and testosterone administration. A group in Boston, MA, USA explored the possibility of creating hormone-releasing testicular prostheses. In animal models, they produced implants that released physiological levels of testosterone over a prolonged period of time; however, no studies have been carried out to date in humans.
Studies have shown that a certain proportion of men who have testicular prostheses inserted are unhappy about the size or shape of their implant.22 One possible reason for this is that as the size of the implant increases, the length-to-width ratio decreases producing a less elliptical implant. The manufacturing companies might address this issue by producing more natural looking implants in the larger sizes required by adults.
Conclusions
Testicular prostheses have been shown to reduce the psychological impact resulting from loss or absence of a testicle. Implantation is technically simple if performed at the time of orchidectomy. A low groin incision and antibiotic usage is associated with low complication rates. The long-term fears associated with silicone implants, namely connective tissue or autoimmune diseases and carcinogenesis, have not been substantiated. Even though silicone implants are still widely used in the UK, only saline-filled implants have FDA approval in the US. Whilst the short-term results of the latter show them to be safe and effective, longer-term quality-of-life results are still pending.
References
- 1.Money J, Sollod R. Body image, plastic surgery (prosthetic testes) and Kallmann's syndrome. Br J Med Psychol. 1978;51:91–4. doi: 10.1111/j.2044-8341.1978.tb02450.x. [DOI] [PubMed] [Google Scholar]
- 2.Girdansky J, Newman HF. Use of a vitallium testicular implant. Am J Surg. 1941;52:514. [Google Scholar]
- 3.Rea CE. The use of testicular prosthesis made of lucite with a note concerning the size of the testis at different ages. J Urol. 1943;49 [Google Scholar]
- 4.Hazzard CT. The development of a new testicular prosthesis. J Urol. 1953;70:959–60. doi: 10.1016/S0022-5347(17)68012-1. [DOI] [PubMed] [Google Scholar]
- 5.Baumrucker GO. Testicular prosthesis for an intracapsular orchiectomy. J Urol. 1957;77:756–8. doi: 10.1016/S0022-5347(17)66627-8. [DOI] [PubMed] [Google Scholar]
- 6.Lattimer JK, Vakili BF, Smith AM, Morishima A. A natural-feeling testicular prosthesis. J Urol. 1973;110:81–3. doi: 10.1016/s0022-5347(17)60122-8. [DOI] [PubMed] [Google Scholar]
- 7.Robinson OG, Jr, Bradley EL, Wilson DS. Analysis of explanted silicone implants: a report of 300 patients. Ann Plast Surg. 1995;34:1–6. doi: 10.1097/00000637-199501000-00001. discussion 6–7. [DOI] [PubMed] [Google Scholar]
- 8.Barrett DM, O'Sullivan DC, Malizia AA, Reiman HM, Abell-Aleff PC. Particle shedding and migration from silicone genitourinary prosthetic devices. J Urol. 1991;146:319–22. doi: 10.1016/s0022-5347(17)37780-7. [DOI] [PubMed] [Google Scholar]
- 9.Peters W, Keystone E, Snow K, Rubin L, Smith D. Is there a relationship between autoantibodies and silicone-gel implants? Ann Plast Surg. 1994;32:1–5. doi: 10.1097/00000637-199401000-00001. discussion 5–7. [DOI] [PubMed] [Google Scholar]
- 10.Oppenheimer BS, Oppenheimer ET, Danishefsky I, Stout AP, Eirich FR. Further studies of polymers as carcinogenic agents in animals. Cancer Res. 1955;15:333–40. [PubMed] [Google Scholar]
- 11.May DS, Stroup NE. The incidence of sarcomas of the breast among women in the United States, 1973–1986. Plast Reconstr Surg. 1991;87:193–4. doi: 10.1097/00006534-199101000-00045. [DOI] [PubMed] [Google Scholar]
- 12.Turek PJ, Master VA. Safety and effectiveness of a new saline filled testicular prosthesis. J Urol. 2004;172:1427–30. doi: 10.1097/01.ju.0000139718.09510.a4. [DOI] [PubMed] [Google Scholar]
- 13.Marshall S. Potential problems with testicular prostheses. Urology. 1986;28:388–90. doi: 10.1016/0090-4295(86)90068-3. [DOI] [PubMed] [Google Scholar]
- 14.Lattimer JK, Stalnecker MC. Tissue expansion of underdeveloped scrotum to accommodate large testicular prosthesis. A technique. Urology. 1989;33:6–9. doi: 10.1016/0090-4295(89)90056-3. [DOI] [PubMed] [Google Scholar]
- 15.Chadha A, Saraiya H. Scrotal reconstruction using Foley catheters as tissue expanders. Ann Plast Surg. 1991;26:291–2. doi: 10.1097/00000637-199103000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Short KL, Howerton LW, Holt H, Amin M. Subcapsular orchiectomy with intracapsular testicular prosthesis for metastatic prostate carcinoma. Urology. 1984;24:38–40. doi: 10.1016/0090-4295(84)90384-4. [DOI] [PubMed] [Google Scholar]
- 17.Abbassian A. A new surgical technique for testicular implantation. J Urol. 1972;107:618. doi: 10.1016/s0022-5347(17)61094-2. [DOI] [PubMed] [Google Scholar]
- 18.Lawrentschuk N, Webb DR. Inserting testicular prostheses: a new surgical technique for difficult cases. BJU Int. 2005;95:1111–4. doi: 10.1111/j.1464-410X.2005.05476.x. [DOI] [PubMed] [Google Scholar]
- 19.Simms MS, Huq S, Mellon JK. Testicular prostheses: a new technique for insertion. BJU Int. 2004;93:179. doi: 10.1111/j.1464-410x.2004.04580.x. [DOI] [PubMed] [Google Scholar]
- 20.John TT, Fordham MV. Spontaneous rupture of testicular prosthesis with external leakage of silicone – a rare event. J Urol. 2003;170:1306. doi: 10.1097/01.ju.0000087615.35085.c9. [DOI] [PubMed] [Google Scholar]
- 21.Doherty AP, Mannion EM, Moss J, Ockrim JL, Christmas TJ. Spread of silicone to inguinal lymph nodes from a leaking testicular prosthesis: a cause for chronic fatigue? BJU Int. 2000;86:1090. doi: 10.1046/j.1464-410x.2000.00961.x. [DOI] [PubMed] [Google Scholar]
- 22.Adshead J, Khoubehi B, Wood J, Rustin G. Testicular implants and patient satisfaction: a questionnaire-based study of men after orchidectomy for testicular cancer. BJU Int. 2001;88:559–62. doi: 10.1046/j.1464-4096.2001.02392.x. [DOI] [PubMed] [Google Scholar]
- 23.Lynch MJ, Pryor JP. Testicular prostheses: the patient's perception. Br J Urol. 1992;70:420–2. doi: 10.1111/j.1464-410x.1992.tb15801.x. [DOI] [PubMed] [Google Scholar]
- 24.Incrocci L, Bosch JL, Slob AK. Testicular prostheses: body image and sexual functioning. BJU Int. 1999;84:1043–5. doi: 10.1046/j.1464-410x.1999.00347.x. [DOI] [PubMed] [Google Scholar]


