Abstract
INTRODUCTION
We monitored image intensifier use by orthopaedic trainees to assess their exposure to ionising radiation and to investigate the influence of sub-specialty training.
MATERIALS AND METHODS
Five different orthopaedic registrars recorded their monthly image intensifier screening times and exposure doses for all cases (trauma and elective), for a combined total of 12 non-consecutive months. Radiation exposure was monitored using shoulder and waist film badges worn both by surgeons and radiographers screening their cases.
RESULTS
Registrars in spinal sub-specialties were exposed to significantly higher doses per case and cumulative doses per month than non-spinal trainees (P < 0.05), but significantly lower screening times per case (P < 0.05). There were no significant differences in cumulative screening times per month (P > 0.05). Regression analysis for all surgeons showed a significant relationship between shoulder film badge reading and cumulative dose exposed per month (P < 0.05), but not for cumulative screening time. Shoulder film badge recordings were significantly higher for spinal compared with non-spinal registrars (P < 0.05), although all badges were below the level for radiation reporting. Only one radiographer badge recorded a dose above threshold.
CONCLUSIONS
Whilst the long-term effects of sub-reporting doses of radiation are not fully understood, we consider that this study demonstrates that trainees should not be complacent in accepting inadequate radiation protection. The higher doses encountered with spinal imaging means that sub-specialty trainees should be alerted to the risk of their increased exposure. The principle of minimising radiation exposure must be maintained by all trainees at all times.
Keywords: Radiation, Orthopaedics, Spine surgery
Natural sources, such as radon gas and cosmic rays, account for about 85% of background radiation (2.4 milliSieverts per year (mSv/year)).1–3 Man-made sources derived from industrial output and the military contribute to less than 1% of background radiation. The remaining proportion comes from medical practice. The potential clinical and biological harmful effects of ionising radiation exposure are well documented.3–5 However, there are deficiencies in the understanding of the risks in clinical practice and in approaches to reducing radiation exposure.6,7 The range of operative procedures requiring image intensifier screening has led to an interest by orthopaedic surgeons in their exposure to ionising radiation.8–11 Studies have considered exposure during experimental operation set-ups on phantom patients,12 during common trauma operations,13–15 for different locations and personnel in the operating theatre,16–18 for different parts of the body,19 and also investigating methods to reduce exposure.20,21 There is evidence, however, that actual practice of basic ionising radiation protection varies widely.22
This study sought to investigate actual practice in a non-experimental, non-selective orthopaedic theatre environment in a medium-sized district general hospital (DGH). We used the most commonly available monitoring systems (dosimeter film badges) to assess radiation exposure. The specific aims were to: (i) monitor the monthly operative total case load requiring image intensifier screening undertaken by orthopaedic registrars in our department, using film badges to monitor their radiation exposure; (ii) compare the surgeons' and radiographers' radiation exposure for the cases performed; and (iii) assess the effect of sub-specialisation in orthopaedic training on radiation exposure.
Materials and Methods
For the purpose of the study, a Theatre Screening Unit (TSU) was defined as one orthopaedic registrar and a radiographer screening for that surgeon's cases over one calendar month. In practical terms, five different orthopaedic registrars monitored their theatre image intensifier usage during all cases (elective in-patient, day-surgery and trauma; surgeon or assistant) during 4 non-consecutive months between August 2003 and April 2004. Image intensifiers available for theatre screening consisted of two identical Siemens Siremobil Compact and one Siemens Siremobil 2000. The image intensifiers set the potential difference in kilovolts (kV) and current in milli-amps (mA) automatically. The details of the cases performed and the image intensifier output were recorded. The TSU consisted of each registrar during a 1-month period who controlled four film badges, each with the ability to record a radiation exposure above a threshold of 0.1 mSv. The surgeon wore two film badges, one underneath a 0.25-mm thick lead apron at waist level, and one outside the apron on the shoulder closest to the image intensifier. Each surgeon also gave a collar and waist badge pair to the radiographer controlling the image intensifier during screening, who wore them in addition to their own personal badges. Between cases, each surgeon stored all four film badges in theatre lockers away from the image intensifier. A control film badge was kept in the same locker area to control for background radiation. All film badges were sent for reading at the Personal Dosimetry Service in the regional clinical physics department.
Operation data collected included case type, screening factors (kV and mA), screening time (min) and dose output from the image intensifier in centiGrays.square centimetres (cGy.cm2). Operations were assigned to one of twelve summary categories (Table 1). The grouped spinal category included the use of the image intensifier for all spinal cases consisting of spinal facet joint and nerve root injections and localising spinal levels for discectomy, and instrumented fusion and prosthesis insertion. Category ‘Miscellaneous’ consisted of infrequent and varied non-spinal screening cases, such as locating metal for removal and arthrography. No attempt was made to measure the surgeon-radiographer-source distances, since these can vary significantly during the course of a case.
Table 1.
Case summary and image intensifier dose outputs and screening times
| Operation | Cases (n) | Median dose (range) (cGy.cm2) | Median time (range) (min) |
|---|---|---|---|
| MUA upper limb | 18 | 4.50 (0.10–100.00) | 0.20 (0.10–0.90) |
| MUA lower limb | 4 | 23.00 (5.00–58.00) | 0.20 (0.20–0.60) |
| Reduction dislocation | 2 | 16.00 (10.00–22.00) | 0.10 |
| ORIF upper limb | 14 | 5.00 (1.00–78.00) | 0.20 (0.10–1.80) |
| ORIF lower limb | 17 | 10.00 (0.10–65.00) | 0.30 (0.10–1.60) |
| ORIF hip | 20 | 245.00 (27.00–1103.00) | 0.80 (0.10–2.70) |
| K-wire upper limb | 15 | 7.00 (1.00–48.00) | 0.50 (0.10–1.40) |
| External fixator | 1 | 23.00 | 0.70 |
| IM nail femur | 5 | 641.00 (551.00–945.00) | 3.70 (1.50–3.80) |
| IM nail tibia | 6 | 63.50 (6.00–159.00) | 1.25 (0.70–4.10) |
| Spinal | 95 | 131.00 (19.00–865.00) | 0.20 (0.10–0.70) |
| Miscellaneous | 13 | 3.00 (0.10–262.00) | 0.12 (0.10–0.20) |
MUA, manipulation under anaesthesia; K-wire, Kirschner wire fixation.
Data were analysed using SPSS for Windows v. 10.1. Data were tested for normality using the Kolmogorov-Smirnov test. A Kruskal-Wallis test and pair wise Mann-Whitney U-tests were performed to determine differences in the case comparisons and between surgeons. A Mann-Whitney U-test was used to test between cumulative data and film badge reading. Regression analysis was tested for significance using Spearman's correlation coefficient. Differences were considered significant at the P < 0.05 level.
Results
A total of 210 cases required screening during the 4 months studied. The five different registrars contributed to 12 TSU months (assigned names ‘Surgeon 1–12’), although filmbadge readings were processed for only 11 months, and the potential 8 other TSU months were not used for the analysis due to incomplete records, badge loss or contamination. Surgeons 2, 6, 8 and 11 were spinal sub-specialty registrars who also participated in the trauma rota. Overall, 98.6 % of cases (207/210) were screened using the two identical Siemens Siremobil Compact image intensifiers and 1.4% (3/210) used the Siemens Siremobil 2000.
All data were non-normally distributed (Kolmogorov-Smirnov P < 0.05). The median (range) for the voltage and current for the image intensifier were 68.00 kV (44.00–110.00 kV) and 2.45 mA (0.30–12.10 mA), respectively. The categories of operations performed during the study period and the median (range) for the dose and time per case are shown in Table 1. The data per surgeon for cases performed are shown in Table 2.
Table 2.
Summary of image intensifier dose outputs, screening times, and shoulder film badge readings per TSU
| Surgeon | Cases (n) | Median dose (range) (cGy.cm2) | Cumulative dose (cGy.cm2) | Median time (range) (min) | Cumulative time (min) | Shoulder film badge (surgeon/radiographer) (mSv) |
|---|---|---|---|---|---|---|
| 1 | 9 | 4.00 (0.10–11.00) | 37.30 | 0.20 (0.10–1.20) | 2.60 | 0.00/0.00 |
| 2 (S) | 33 | 57.00 (0.10–945.00) | 2924.00 | 0.10 (0.10–3.80) | 11.70 | 0.29/0.00 |
| 3 | 13 | 65.00 (3.00–945.00) | 2736.00 | 0.40 (0.10–4.10) | 14.00 | 0.28/0.00 |
| 4 | 17 | 17.00 (0.10–641.00) | 1968.00 | 0.40 (0.10–3.30) | 11.90 | 0.56/0.00 |
| 5 | 16 | 22.50 (4.00–628.00) | 1445.00 | 0.55 (0.10–3.00) | 12.00 | 0.00/0.00 |
| 6 (S) | 28 | 180.00 (1.00–865.00) | 5913.00 | 0.20 (0.10–0.70) | 6.20 | 0.96/0.00 |
| 7 | 2 | 398.50 (10.0–787.00) | 797.00 | 0.75 (0.30–1.20) | 1.50 | 0.16/0/00 |
| 8 (S) | 32 | 101.50 (1.00–608.00) | 4275.00 | 0.30 (0.10–1.80) | 12.10 | 0.46/0.11 |
| 9 | 6 | 17.50 (4.00–209.00) | 400.00 | 0.45 (0.10–0.70) | 2.50 | 0.00/0.00 |
| 10 | 7 | 20.00 (1.00–563.00) | 845.00 | 0.30 (0.10–3.70) | 6.20 | −/− |
| 11 (S) | 33 | 106.00 (1.00–1103.00) | 6041.00 | 0.30 (0.10–2.70) | 12.40 | 0.21/0.00 |
| 12 | 11 | 9.00 (1.00–633.00) | 1493.00 | 0.10 (0.10–1.30) | 4.60 | 0.16/0.00 |
(S) Spinal sub-specialty.
The Kruskal-Wallis test showed that between different surgeons, both the dose output and the screening times were not from the same population distribution (P < 0.05). Pair-wise Mann-Whitney U-tests showed that open reduction and internal fixation (ORIF) of hip fractures, femoral and tibial intramedullary nailing (IM nail), and grouped spinal cases all had significantly higher median screening doses compared with the remaining groups from the same population distribution (P < 0.05). Hip fracture fixation and both femoral and tibial intramedullary nailing had significantly longer screening times compared with the remaining groups from the same population (P < 0.05).
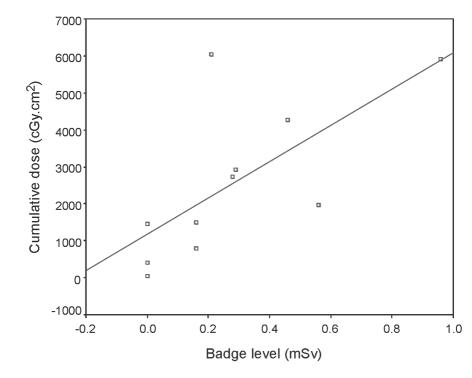
The film badges for the surgeons' shoulder were the only site which consistently recorded a dose reading above the detection threshold of 0.1 mSv (Table 2). The only waist badges to record levels above the detection threshold were those of Surgeon 4 (0.21 mSv), and Surgeon 11 (0.10 mSv). The only radiographer badge to record a level was the radiographer shoulder badge for Surgeon 8 (0.11 mSv). Regression analysis showed that there was a significant relationship between surgeon shoulder badge level and cumulative dose output screened per month (P < 0.05; Fig. 1), but not for the cumulative screening time (P > 0.05).
Figure 1.
Scatter plot showing cumulative dose output versus surgeon shoulder film badge reading.
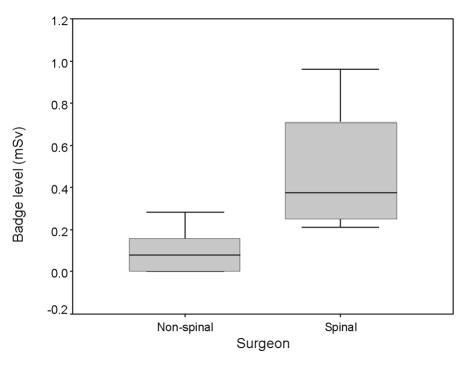
Registrars in spinal sub-specialties (Surgeons 2, 6, 8, 11) screened per month at significantly higher median [range] doses per case (100.00 cGy.cm2 [0.10–1103.00 cGy.cm2]), and were exposed to a significantly higher median (range) cumulative dose per month (5094.00 cGy.cm2 [2924.00–6041.00 cGy.cm2]) than registrars in non-spinal specialties (12.00 cGy.cm2 [0.10–945.00 cGy.cm2] and 1145.00 cGy.cm2 [37.30–2736.00 cGy.cm2], respectively; P < 0.05). The median [range] time per case was significantly lower for spinal sub-specialty registrars (0.20 min [0.10–3.80 min] versus 0.35 min [0.10–4.10 min]; P < 0.05). There were no significant differences for the cumulative time per month between the spinal (11.90 min [6.20–12.40 min]) and non-spinal registrars (5.40 min [1.50–14.00 min]; P > 0.05). The median surgeon shoulder film badge readings were significantly higher for the spinal registrars (0.38 mSv) compared with non-spinal registrars (0.16 mSv) for badge readings within the 95% confidence intervals (P < 0.05; Fig. 2).
Figure 2.
Box and whiskers plot showing median, inter-quartile range, and extremes (95% confidence interval) for surgeon shoulder film badge reading. *P < 0.05 Mann-Whitney U-test.
Discussion
This study investigated actual theatre practice and ionising radiation exposure in a DGH for 5 registrars over a 12 TSU month screening period. No attempt was made to restrict the cases screened or select radiographer or image intensifier. Herscovici and Sanders3 considered that to limit radiation exposure, three variables could be controlled – mechanical (amount, duration and direction of beam), barriers (protective devices), and span (working distance between surgeon and image intensifier).
The standard local radiation protection available consisted of off-the-shelf 0.25-mm lead aprons of various styles, thyroid lead shields if selected by the surgeon and application of the ‘as low as reasonably achievable’ (ALARA) principle for dose reduction.1,7 Barry23 measured his annual total exposure to ‘gonads and internal organs as close to zero’ and considered the lead apron to be effective in limiting the dose to the trunk, as well as shielding approximately 82% of the active bone marrow in the adult. In this study, two surgeons did record exposure at waist level below the lead apron, a finding we feel may reflect the different styles of apron available (side fastening versus wrap-round). Maruthainar et al.22 examined the availability and use of thyroid shields by orthopaedic registrars in UK hospitals and showed that only 14% of trainees routinely used protection whilst screening in theatre, and that 16% of hospitals did not provide shields for use.
The standard radiation exposure monitors in our hospital are film badges and they were used in this study. Film badges work by the exposure of photographic film to radiation,10 have a threshold for detection of 0.1 mSv and for screening p.d. settings of less than 100 kV are accurate to within 20%.15 For the purposes of statistical analysis in this study, a below-detection threshold badge (< 0.1 mSv) was given a zero value, the limitation of which is recognised. Some studies have employed more sensitive thermoluminescent dosimeters (TLD) and electronic personal dosimeters (EPD), with the latter having a sensitivity for detection of 1 µSv).18 Despite their limitations, film badges have been employed successfully in the majority of contemporary studies in this field and remain the ‘gold standard for personal radiation monitoring in hospitals’.17
No consistent exposure level was detected with the radiographers' film badges which concurs with work by Alonso et al.18 who showed that for hip fracture fixation, the scattered dose outside a 2-m zone is less than 1 μSv. Although they question the necessity of personal lead protection outside this area, they do recommend that lead aprons and thyroid shields be worn by surgeon and assistants.
Rampersaud et al.24 studied radiation exposure to spinal surgeons during in vitro pedicle screw insertion in six cadavers. They concluded that the dose rates were up to 10–12 times greater than for other non-spinal musculoskeletal procedures requiring screening. In this study, registrars in training in spinal sub-specialties were exposed to significantly higher median doses per case, cumulative doses per month and recorded significantly higher shoulder film badge readings compared with other non-spinal registrars. We have shown a statistically significant relationship between the cumulative screening dose output from the image intensifier and the detection of ionising radiation using a film badge. However, all badges fell below the monthly reporting limit for this badge (providing an estimate of eye dose) of 1.25 mSv. The annual equivalent dose (eye lens) is 150 mSv, and the annual total body effective dose is 20 mSv in accordance with the Ionising Radiation Regulations 1999.25 Use of a lead apron, thyroid shield and radiation attenuating glasses would result in exposure to skin would being the dose limiting factor, with a monthly reporting level of 4.17 mSv and an annual equivalent dose of 500 mSv.
Since the image intensifier sets the dose output automatically according to the density of the tissue being penetrated, the exposure per case will, therefore, depend on both the duration of screening, and also on the nature of the tissue being imaged. It is not surprising that imaging of the spine through the trunk is associated with an exposure higher than that for extremity imaging. It has already been described that whilst hip fracture fixation and tibial and femoral nailing were also associated with screening doses significantly higher than the rest of the population of cases by group, they were also associated with significantly higher screening times, and this differentiates them from the shorter screening times noted for the spinal cases.
We have not considered the effect of surgeon grade on exposure but appreciate that this is a factor which might affect screening times. A further study would be required to investigate this variable.
Conclusions
We consider that whilst the long-term effects of sub-reporting doses of ionising radiation are not fully understood, although the radiation doses recorded in this study were within legal requirements for doctors using ionising radiation, sub-specialty trainees should be aware of their increased exposure compared with other trainees who are only exposed during trauma cases. Inadequate personal radiation protection should not be accepted, and thyroid shields and lead attenuating glasses should be more readily available; however, trainees should equally strive to keep screening to a minimum, applying the ALARA principle at all times.
Acknowledgments
The authors thank the Personal Dosimetry Service in the Department of Clinical Physics at St Bartholomew's Hospital, London.
References
- 1.Picano E. Sustainability of medical imaging. BMJ. 2004;328:578–80. doi: 10.1136/bmj.328.7439.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Radiation Protection Board. Ionising radiation and how we are exposed to it. < http://www.nrpb.org/radiation> (Accessed 19 June 2004)
- 3.Herscovici D, Sanders RW. The effects, risks, and guidelines for radiation use in orthopaedic surgery. Clin Orthop. 2000;375:126–32. doi: 10.1097/00003086-200006000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Hanford JM, Quimby EH, Frantz VK. Cancer arising many years after radiation therapy. JAMA. 1962;181:404–10. [PubMed] [Google Scholar]
- 5.Hall P, Adami H-O, Trichopoulos D, Pedersen NL, Lagiou P, Ekbom A, et al. Effect of low doses of ionising radiation in infancy on cognitive function in adulthood: Swedish population based cohort study. BMJ. 2004;328:19–21. doi: 10.1136/bmj.328.7430.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shiralkar S, Rennie A, Snow M, Galland RB, Lewis MH, Gower-Thomas K. Doctors' knowledge of radiation exposure: questionnaire study. BMJ. 2003;327:371–2. doi: 10.1136/bmj.327.7411.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoker DJ. Ionising radiation and the orthopaedic patient. J Bone Joint Surg Br. 1993;75:4–5. doi: 10.1302/0301-620X.75B1.8421031. [DOI] [PubMed] [Google Scholar]
- 8.Riley SA. Radiation exposure from fluoroscopy during orthopaedic surgical procedures. Clin Orthop. 1989;249:257–60. [PubMed] [Google Scholar]
- 9.Jones DW, Stoddart J. Radiation use in the orthopaedic theatre: a prospective audit. Aust NZ J Surg. 1998;68:782–4. doi: 10.1111/j.1445-2197.1998.tb04676.x. [DOI] [PubMed] [Google Scholar]
- 10.Smith GL, Briggs TWR, Lavy CBD, Nordeen H. Ionising radiation: are orthopaedic surgeons at risk. Ann R Coll Surg Engl. 1992;74:326–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Hynes DE, Conere T, Mee MB, Cashman WF. Ionising radiation and the orthopaedic surgeon. J Bone Joint Surg Br. 1992;74:332–4. doi: 10.1302/0301-620X.74B3.1587871. [DOI] [PubMed] [Google Scholar]
- 12.Theocharopoulos N, Perisinakis K, Damilakis J. Occupational exposure from common fluoroscopic projections used in orthopaedic surgery. J Bone Joint Surg Am. 2003;85:1698–703. doi: 10.2106/00004623-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Madan S, Blakeway C. Radiation exposure to surgeon and patient in intramedullary nailing of the lower limb. Injury. 2002;33:723–27. doi: 10.1016/s0020-1383(02)00042-6. [DOI] [PubMed] [Google Scholar]
- 14.Giannoudis PV, McGuigan J, Shaw DL. Ionising radiation during internal fixation of extracapsular neck of femur fractures. Injury. 1998;29:469–72. doi: 10.1016/s0020-1383(98)00090-4. [DOI] [PubMed] [Google Scholar]
- 15.Levin PE, Schoen RW, Browner BD. Radiation exposure to the surgeon during closed interlocking intramedullary nailing. J Bone Joint Surg Am. 1987;69:761–6. [PubMed] [Google Scholar]
- 16.Miller ME, Davis ML, MacClean CR, Davis JG, Smith BL, Humphries JR. Radiation exposure and associated risks to operating-room personnel during use of fluoroscopic guidance for selected orthopaedic surgical procedures. J Bone Joint Surg Am. 1983;65:1–4. [PubMed] [Google Scholar]
- 17.Mehlman CT, DiPasquale TG. Radiation exposure to the orthopaedic surgical team during fluoroscopy: ‘How far away is far enough?’. J Orthop Trauma. 1997;11:392–8. doi: 10.1097/00005131-199708000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Alonso JA, Shaw DL, Maxwell A, Hart GP. Scattered radiation during fixation of hip fractures. Is distance alone enough protection? J Bone Joint Surg Br. 2001;83:815–8. doi: 10.1302/0301-620x.83b6.11065. [DOI] [PubMed] [Google Scholar]
- 19.Muller LP, Suffner J, Wenda K, Mohr W, Rommens PM. Radiation exposure to the hands and the thyroid of the surgeon during intramedullary nailing. Injury. 1998;29:461–8. doi: 10.1016/s0020-1383(98)00088-6. [DOI] [PubMed] [Google Scholar]
- 20.Dewey P, Incoll I. Evaluation of thyroid shields for reduction of radiation exposure to orthopaedic surgeons. Aust NZ J Surg. 1998;68:635–6. doi: 10.1111/j.1445-2197.1998.tb04832.x. [DOI] [PubMed] [Google Scholar]
- 21.Noordeen MHH, Shergill N, Twyman RS, Cobb JP, Briggs T. Hazard of ionizing radiation to trauma surgeons: reducing the risk. Injury. 1993;24:562–4. doi: 10.1016/0020-1383(93)90039-9. [DOI] [PubMed] [Google Scholar]
- 22.Maruthainar N, Bentley G, Williams A, Danin JC. Availability of thyroid protective lead shields and their use by trainee orthopaedic surgeons. Occup Environ Med. 2003;60:381. doi: 10.1136/oem.60.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barry TP. Radiation exposure to an orthopaedic surgeon. Clin Orthop. 1984;182:160–2. [PubMed] [Google Scholar]
- 24.Rampersaud YR, Foley KT, Shen AC, Williams S, Solomito M. Radiation exposure to the spine surgeon during fluroscopically assisted pedicle screw insertion. Spine. 2000;25:2637–45. doi: 10.1097/00007632-200010150-00016. [DOI] [PubMed] [Google Scholar]
- 25.The Ionising Radiation Regulations. 1999 < http://www.legislation.hmso.gov.uk> (Accessed 7 September 2003)