Abstract
The sunburst diving beetle, Thermonectus marmoratus, ejects a milky fluid from its prothoracic defensive glands when disturbed. Two major volatile components of this secretion are steroids; cybisterone (structure 7) constitutes about 20% of the volatiles, and a new steroid, mirasorvone, about 50%. Mirasorvone is assigned an 18-oxygenated pregnane structure (structure 9) on the basis of extensive spectroscopic data. Although no 18-oxygenated steroid has been described previously from an insect source, a closely related hormone with mineralocorticoid activity, 18-hydroxydeoxycorticosterone (structure 13), has been isolated from the adrenal glands of rats.
Keywords: steroids, hemiketal, Dytiscidae
In the mind of the general public, steroids loom large. Consider cholesterol (1). Millions of Americans are aware of their blood cholesterol levels, and many take cholesterol biosynthesis inhibitors regularly. Steroidal oral contraceptives have been responsible for a quiet social revolution, and closely related steroids are widely used for the relief of postmenopausal symptoms. Many steroids find application as antiinflammatory agents. The abuse of anabolic steroids by both amateur and professional athletes has become a much-publicized problem in the world of sports. But the importance of this almost ubiquitous family of tetracyclic isoprenoids is hardly limited to humans.
Among insects, for example, there are not only steroidal hormones but also steroidal pheromones and defensive agents. α-Ecdysone (2) controls the molting process and plays an important role in pupation (1). Blattellastanosides A and B (3 and 4) have been characterized as active arrestants in the aggregation pheromone of the German cockroach, Blattella germanica (2).
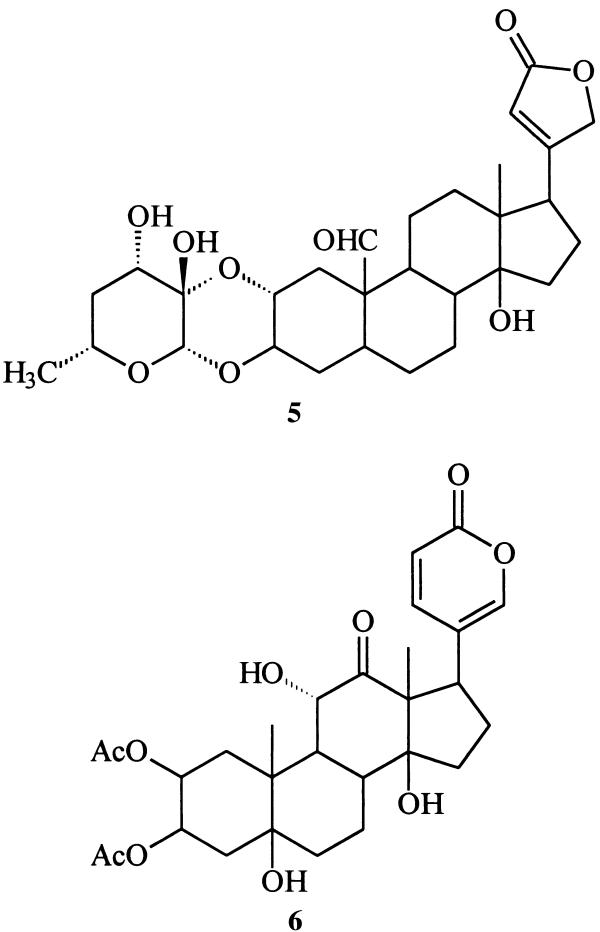
The demonstration that Monarch butterflies defend themselves by sequestering cardiotonic glycosides such as calotropin (5) from milkweeds has become one of the classic examples of defensive strategies in chemical ecology (3), and the more recently elucidated defensive role of lucibufagins (of as yet unknown origin), for example 6, among fireflies involves interactions of comparable interest (4).
Whereas these defensive compounds are present systemically within the insects, others are deployed as constituents of glandular fluids emitted in response to attack. The silphid beetle Silpha americana, for example, discharges a defensive mixture of steroids (chiefly pregnanes) when disturbed (5). Pregnanes are also ejected by diving beetles (Dytiscidae) (6) and a species of water bug (Belostomatidae) (7). The steroids, in the case of these aquatic insects, have been shown to be distasteful and toxic to both fish and amphibians (6, 8–11). We have studied the defensive chemistry of Thermonectus marmoratus, an aposematic dytiscid beetle recently named the “sunburst diving beetle” (12) (Fig. 1), and report the chemical characterization of two steroida1 components from the secretion of its prothoracic defensive glands.
Figure 1.

(Left) T. marmoratus, swimming. (Right) Same, held in fingers, discharging secretion; the milky fluid, emitted from glands opening just behind the head, is spreading over the pronotum.
MATERIALS AND METHODS
The Beetle.
T. marmoratus is a moderately sized dytiscid (body length ≈ 12–14 mm). It occurs in the southwestern United States and extends southward into Mexico (13). It lives in rocky creeks and streams, is at times gregarious, and is exceptional among dytiscids in bearing bright markings (13). Larvae and adults are carnivorous, and the beetles can be readily reared on insect prey in aquaria (13). Our work was done with beetles from a colony maintained at the Cincinnati Zoo, derived from individuals originally collected in southern Arizona.
The prothoracic defensive glands of dytiscids (14) consist of a pair of elongate sacs positioned beneath the pronotum and opening by single pores at the anterolateral angles of the prothorax, just behind the head. Such glands are typically filled with a dense suspension of white crystalline material, as we found also to be the case in T. marmoratus. Dytiscids discharge their prothoracic glands in response to direct physical disturbance. This proved true for T. marmoratus, which usually emitted its secretion promptly when seized by hand (Fig. 1). To collect secretion for analysis, we simply grasped beetles in the fingers and wiped up the fluid that oozed from their neck with small pieces of filter paper. Samples analyzed consisted invariably of the collective output of several beetles of both sexes.
Chemistry.
Mass spectra were obtained with a Hewlett–Packard (HP) 5890 gas chromatograph, with a 30 m × 0.2 mm fused-silica capillary column coated with a 0.25 μm film of DB-5, coupled to an HP-5970 Mass Selective Detector. Oven temperature was kept at 60°C for 4 min, increased to 270°C at 8°C/min, and maintained at 270°C for 50 min. Introduction of samples (in ethyl acetate solution) into the chromatograph was by splitless injection. The major peak in the gas chromatogram obtained from the GC-MS analysis of the defensive secretion of T. marmoratus gave the following spectrum [m/z (%)]: 310 (100), 295 (2), 267 (3),175 (87), 157 (12), 136 (72), 131 (46), 129 (36), 91 (42), 77 (22), and 43 (26). High-resolution GC-MS data were obtained on a VG 70-VSE instrument (resolution 5,000), and chemical ionization data were collected with methane as the reagent gas.
Ultraviolet spectra were obtained with a diode-array detector linked to a HP 1090 HPLC instrument. A 25 cm × 4.6 mm ID Supelcosil LC-Si (5 μm) column was eluted with a solvent gradient of 0–7.5% CH3OH/CH2Cl2 over a period of 15 min at a rate of 1 ml/min. Mirasorvone eluted at 7.5 min and showed an ultraviolet absorption maximum at 282 nm.
Gas phase infrared spectra were recorded with a HP 5890 gas chromatograph (HP 5 column, 25 m × 0.32 mm ID, 0.52 μm film thickness) linked to a HP 5965A infrared detector. The dehydration product of mirasorvone showed absorption maxima at 3036, 2954, 2867, 1689, 1622, 1452, 1261, 1052, 877, and 811 cm−1.
Mirasorvone was isolated on a preparative scale by TLC. Secretion-impregnated filter paper from many “milkings” was extracted with ethyl acetate. After most of the solvent was removed by a stream of nitrogen, the residue was loaded onto Baker Si254F plates (20 × 20 cm, 250 μm thickness) and developed in CH2Cl2/EtOAc (1:1). A total of six bands were visualized on the plate under ultraviolet irradiation. The third band from the origin was collected and eluted with ethyl acetate. This extract was evaporated and resubjected to preparative TLC with diethyl ether as the mobile phase. Mirasorvone was obtained by eluting the second band from the origin with ethyl acetate. Gas chromatographic analysis of a sample of prothoracic glandular secretion collected from a large number of beetles, using progesterone as a standard and assuming that mirasorvone is converted completely to its dehydration product during analysis, indicated the presence of about 3 μg of mirasorvone per beetle.
NMR studies were carried out with a Varian Unity 500 spectrometer. A solution of mirasorvone dissolved in 350 μl of CDCl3 was transferred to a Shigemi NMR tube and used for 1H, gHMQC, gHMBC, dqCOSY, and NOESY experiments. Additonal dqCOSY and NOESY experiments were performed with C6D6 as the solvent. 13C and DEPT spectra were obtained using a Varian XL 400 instrument.
The high-temperature hydrogenolysis of mirasorvone was performed by a reaction gas chromatographic procedure with a precolumn of palladium catalyst prepared according to Beroza and Sarmiento (15, 16). In an attempted hydrolysis experiment, mirasorvone was treated with 0.5 M p-toluenesulfonic acid in CH3CN/H2O (4:1) at 50°C. The progress of the reaction was monitored by GC-MS analysis. After 26 hr, mirasorvone was recovered unchanged, and no hydrolysis product was detected.
RESULTS
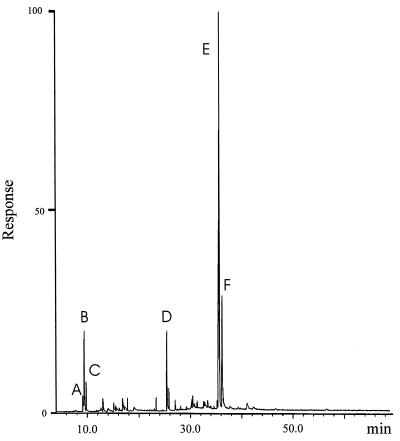
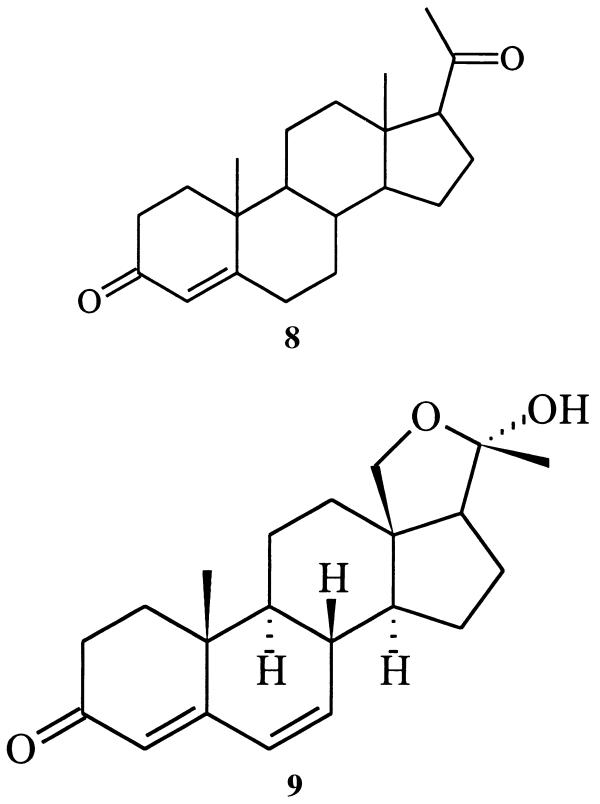
GC analysis of an ethyl acetate extract of a secretion sample revealed the presence of two major components (designated as peaks E and F in Fig. 2), along with several more volatile compounds present in lesser amounts. The mass spectrum corresponding to the major gas chromatographic peak (E, ca. 50% of the volatiles) appeared to be that of a previously unknown compound (17). A second component (F, ca. 20%), with a slightly longer GC retention time, was readily characterized on the basis of spectroscopic data as cybisterone (7), well known as a defensive steroid from the prothoracic glands of other dytiscid species (18).
Figure 2.
A gas chromatogram obtained by GC-MS analysis of an ethyl acetate extract of T. marmoratus secretion: A–D, unknowns; E, dehydration product of mirasorvone (9); F, cybisterone (7).
The accurate mass of the molecular ion corresponding to peak E (Fig. 2), obtained by high-resolution electron ionization MS, suggested a molecular formula of C21H26O2 (obs 310.1929; calculated for C21H26O2 310.1932). In addition, the spectrum showed two prominent fragment ions with the compositions C9H12O (136.0884; calculated for C9H12O 136.0888) and C12H15O (175.1125; calculated for C12H15O 175.1123, M-135). As part of an extensive study of the mass spectra of steroids, Brown and Djerassi (19) found that steroidal 3-keto-4,6-dienones cleave consistently and characteristically in just this way, yielding important fragment ions with m/z 136 and M-135. The suggested mechanisms for the formation of these ions, however, are of byzantine complexity. We could not have deduced that we were dealing with a steroidal 3-keto-4,6-dienone on the basis of MS data had we not found these earlier studies in the literature. The observation of a strong absorption maximum at 282 nm in the ultraviolet spectrum of this compound confirmed the presence of this conjugated dienone moiety. The gas-phase infrared absorption spectrum (obtained by coupled GC-FT infrared spectrometry) showed as expected, a strong peak at 1689 cm−1 for the conjugated carbonyl group in this chromophore.
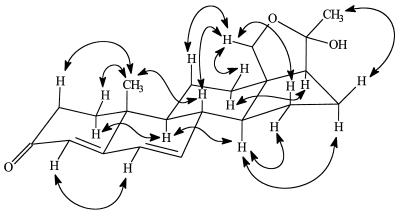
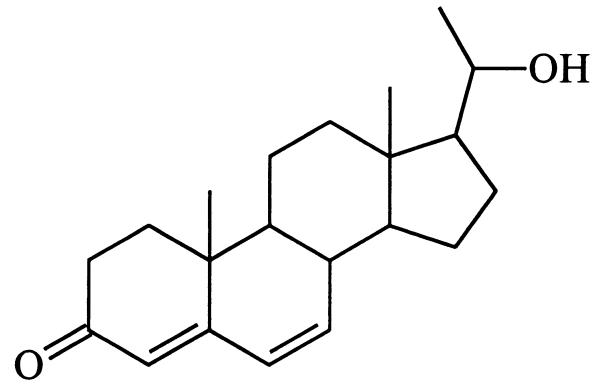
A high-temperature hydrogenolysis experiment performed with mirasorvone, which gave the same pattern of hydrocarbon products (GC-MS analysis) as that of an authentic sample of progesterone (8), established the presence of a pregnane skeleton (15, 16). Because all natural pregnanes are oxygenated (or aminated) at C-3 and C-20, we assumed that mirasorvone must also be oxygenated at these sites. The 1H NMR spectrum of mirasorvone (see Table 1) shows the presence of only three olefinic protons (δ 5.69, 6.12, and 6.17, corresponding to the protons at C-4, C-6, and C-7, respectively), only two methyl groups (both as singlets; δ 1.07 and 1.51), and two protons (δ 3.77 and 3.79) on an oxygen-bearing carbon. The totality of these data led us initially to consider a number of enol ether structures, but mirasorvone proved remarkably resistant to acid-catalyzed hydrolysis, making these structures unlikely. In fact, it finally became apparent that C21H26O2 is not the molecular formula of mirasorvone. As soon as we were able to obtain a sufficient quantity of mirasorvone for 13C NMR analysis and a series of 2D NMR experiments (see Table 1), we obtained unequivocal evidence in favor of the 18-oxygenated hemiketal structure 9. The high-resolution chemical-ionization mass spectrum of mirasorvone [observed for (M+1)+ 329.2117; calculated for C21H29O3 329.2117] confirmed that its molecular formula is in actuality C21H28O3 (m/z 328). It is now clear that mirasorvone dehydrates when subjected to GC analysis and that the peak E in the gas chromatogram (Fig. 2) represents the dehydration product. Some key 1H–1H correlations observed in the nuclear Overhauser effect spectroscopy spectrum of mirasorvone serve to confirm both the structure and complete stereochemistry of this steroid (see Fig. 3).
Table 1.
1H (500 MHz) and 13C (100 MHz) data for mirasorvone in CDC3
|
13C data
|
1H data
|
gHMBC correlations, carbon no. | |||
|---|---|---|---|---|---|
| Carbon no. | δ, ppm | Proton no. | δ, ppm | J, Hz | |
| C-1 | 33.9 | 1-Hα | 1.71 | J1α,1β = 12.9, J1a,2β = 12.8, J1α,2α = 5.4 | C-2, 9, 10, 19 |
| 1-Hβ | 2.00 | J1β,2β = 4.8, J1β,2α = 2.4 | C-2, 3, 5, 10, 19 | ||
| C-2 | 33.9 | 2-Hα | 2.42 | J2α,2β = 17.8 | C-1, 3, 10 |
| 2-Hβ | 2.60 | C-1, 3 | |||
| C-3 | 199.5 | ||||
| C-4 | 123.8 | 4-H | 5.69 | C-1(2), 6, 10 | |
| C-5 | 163.3 | ||||
| C-6 | 128.1 | 6-H | 6.12 | J6,7 = 9.7, J6,8 = 1.7 | |
| C-7 | 140.3 | 7-H | 6.17 | J7,8 = 2.7 | C-5, 8, 9 |
| C-8 | 39.8 | 8-H | 1.96 | J8,9 = 9.6, J8,14 = 11.3 | C-6, 7, 14 |
| C-9 | 49.9 | 9-H | 1.22 | J9,11β = 12.5, J9,11α = 2.9 | C-11, 19 |
| C-10 | 36.0 | ||||
| C-11 | 22.5 | 11-Hβ | 1.12 | J11β,11α = J11β,12α = 12.5, J11β,12β = 3.9 | C-9 |
| 11-Hα | 1.72 | J11α,12α = 3.9, J11α,12β = 2.8 | C-9 | ||
| C-12 | 37.8 | 12-Hα | 1.55 | J12α,12β = 12.6 | C-18 |
| 12-Hβ | 2.40 | C-9, 14 | |||
| C-13 | 56.7 | ||||
| C-14 | 52.7 | 14-H | 1.46 | J14,15β = 12.9, J14,15α = 5.0 | C-15, 18 |
| C-15 | 25.7 | 15-Hβ | 1.35 | *J15β,15α = *J15β,16α = 10.0, *J15β,16β = 7.6 | C-14 |
| 15-Hα | 1.83 | *J15α,16α = 7.7, *J15α,16β < 1 | C-13, 14 | ||
| C-16 | 27.2 | 16-Hα | 1.68 | *J16α,16β = 11.0, *J16α,17 = 10.3 | |
| 16-Hβ | 1.68 | *J16β,17 = 3.3 | |||
| C-17 | 56.0 | 17-H | 2.14 | ||
| C-18 | 72.9 | 18-Ha | 3.77 | J18α,18β = 9.5 | |
| 18-Hb | 3.79 | C-14, 20 | |||
| C-19 | 16.3 | 19-H | 1.07 | C-1, 5, 9, 10 | |
| C-20 | 107.6 | ||||
| C-21 | 24.8 | 21-H | 1.51 | C-17, 20 | |
(1H,1H)-Coupling constants marked with asterisks were determined by dqCOSY experiments with C6D6 as the solvent, which afforded better separation of these proton signals.
Figure 3.
Some key 1H–1H correlations observed in the nuclear Overhauser effect spectroscopy spectrum of mirasorvone.
DISCUSSION
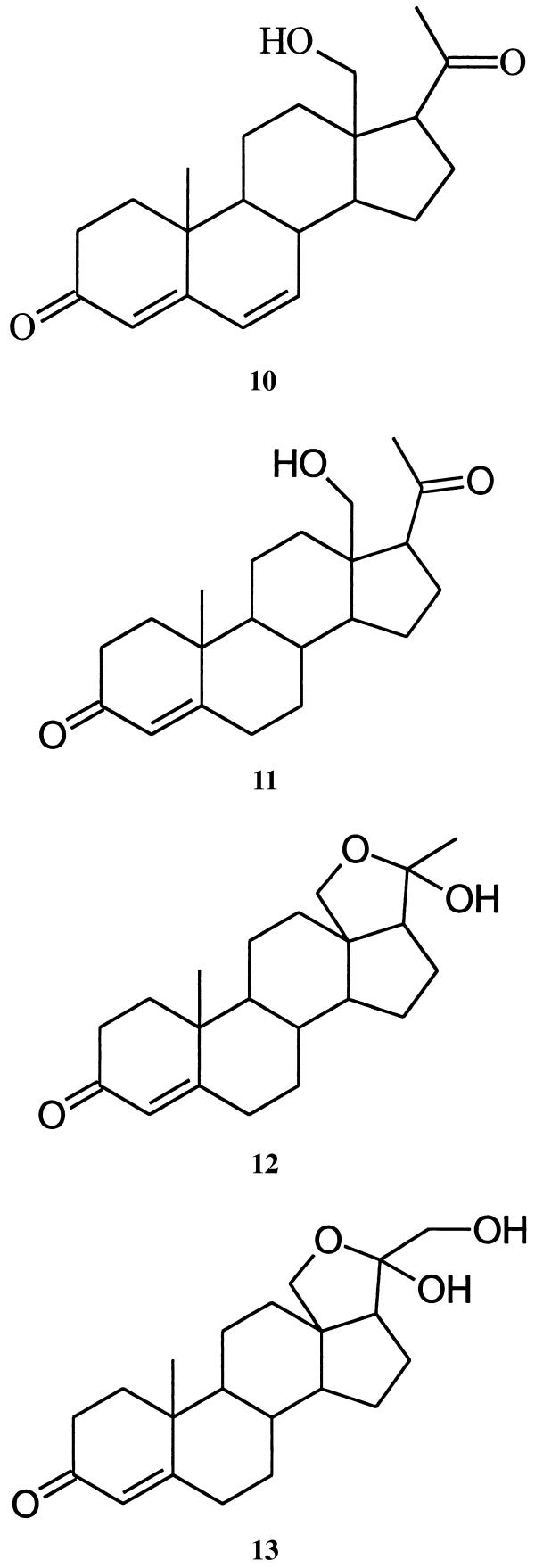
Is there anything interesting about this new beetle-derived compound? Structure 9 represents a cyclic hemiketal corresponding to the 18-hydroxy-20-ketopregnane 10 . Although no 18-oxygenated steroids appear to have been isolated from insect sources, a search of the literature reveals that 18-hydroxyprogesterone (11) (20) and several close relatives are well known. Like mirasorvone, these compounds all exist not as the hydroxy ketones but as the corresponding cyclic hemiketals. For example, 11 actually exists as the pentacyclic tautomer 12, of undefined stereochemistry at C-20. Of particular biological interest is 18-hydroxydeoxycorticosterone (again, as its cyclic tautomer, 13), first isolated from the adrenal cortex of rats (21). This hormone has been shown to have mineralocorticoid activity both in vivo and in vitro, and may be of significance in human essential hypertension.
We suppose that mirasorvone functions as a fish deterrent, as do the previously characterized dytiscid steroids. However, a determination of its behavioral and physiological effects on vertebrate predators or, for that matter of its possible pharmacological properties, must await its isolation or synthesis in sufficient quantity to permit more extensive experimentation.
Figure .
1
Figure .
7
Acknowledgments
This paper is no. 152 in the series Defense Mechanisms of Arthropods; paper no. 151 is ref. 22. We have named compound 9 affectionately in honor of Mira Sorvino, who, as Dr. Susan Tyler in the motion picture Mimic, successfully confronted the ultimate insect challenge. The study was supported by Grants AI02908 and GM53830 from the National Institutes of Health. Support (to J.M.) from the Bert L. and N. Kuggie Vallee Foundation, Inc. during the preparation of this report is gratefully acknowledged.
ABBREVIATIONS
- gHMQC
gradient-echo heteronuclear multiple quantum correlation
- gHMBC
gradient-echo heteronuclear multiple bond correlation
- dqCOSY
double quantum filtered correlated spectroscopy
- NOESY
nuclear Overhauser effect spectroscopy
- DEPT
distortionless enhancement by polarization transfer
References
- 1.Karlson P, Hoffmeister H, Hummel H, Hocks P, Spiteller G. Chem Ber. 1965;98:2394–2402. doi: 10.1002/cber.19650980743. [DOI] [PubMed] [Google Scholar]
- 2.Sakuyama M, Fukami H. Tetrahedron Lett. 1993;38:6059–6062. [Google Scholar]
- 3.Brower L P. In: The Biology of Butterflies. Vane-Wright R I, Ackery P R, editors. New York: Academic; 1984. pp. 109–134. [Google Scholar]
- 4.Eisner T, Goetz M A, Hill D E, Smedley S R, Meinwald J. Proc Natl Acad Sci USA. 1997;94:9723–9728. doi: 10.1073/pnas.94.18.9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meinwald J, Roach B, Hicks K, Alsop D, Eisner T. Experientia. 1987;41:516–519. doi: 10.1007/BF01966178. [DOI] [PubMed] [Google Scholar]
- 6.Scrimshaw S, Kerfoot W C. In: Predation: Direct and Indirect Impacts on Aquatic Communities. Kerfoot W C, Sih A, editors. London: University Press of New England; 1987. pp. 240–262. [Google Scholar]
- 7.Lokensgard J, Smith R L, Eisner T, Meinwald J. Experientia. 1993;49:175–176. doi: 10.1007/BF01989425. [DOI] [PubMed] [Google Scholar]
- 8.Blunck H. Z Wiss Zool. 1917;117:205–256. [Google Scholar]
- 9.Miller R J, Mumma R O. J Chem Ecol. 1976;2:115–130. [Google Scholar]
- 10.Miller R J, Mumma R O. J Chem Ecol. 1976;2:131–146. [Google Scholar]
- 11.Gerhart D J, Bondura M E, Commito J A. J Chem Ecol. 1991;17:1363–1370. doi: 10.1007/BF00983769. [DOI] [PubMed] [Google Scholar]
- 12.Morgan R C. Backyard BUGwatching. 1992;14:4–8. [Google Scholar]
- 13.Morgan R C. Invertebrates in Captivity Conference Proceedings. Tucson, AZ: Sonoran Arthropod Studies Institute; 1995. pp. 50–57. [Google Scholar]
- 14.Forsyth D C. Trans R Entomol Soc London. 1968;120:159–182. [Google Scholar]
- 15.Beroza M, Sarmiento R. Anal Chem. 1963;35:1353–1357. [Google Scholar]
- 16.Adhikary B A, Harkness R A. Anal Chem. 1969;41:470–476. [Google Scholar]
- 17.McLafferty F W, Stauffer D B. The Wiley/NBS Registry of Mass Spectral Data. New York: Wiley; 1989. [Google Scholar]
- 18.Schildknecht H, Siewerdt R, Maschwitz U. Liebigs Ann Chem. 1967;703:182–189. doi: 10.1002/jlac.19677030122. [DOI] [PubMed] [Google Scholar]
- 19.Brown F J, Djerassi C. J Org Chem. 1981;46:954–963. [Google Scholar]
- 20.Buzzetti F, Wicki W, Kalvoda J, Jeger O. Helv Chim Acta. 1959;42:388–390. [Google Scholar]
- 21.Birmingham M K, Ward P J. J Biol Chem. 1961;236:1661–1667. [Google Scholar]
- 22.Eisner T, Eisner M, Attygalle A B, Deyrup M, Meinwald J. Proc Natl Acad Sci USA. 1998;95:1108–1113. doi: 10.1073/pnas.95.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]