Abstract
INTRODUCTION
Angiogenesis is the process of new blood vessel formation from pre-existing vessels, and is a key feature of malignant tumours. Surgeons involved in the management of patients with malignant disease need to be aware of angiogenic mechanisms and their surgical implications.
PATIENTS AND METHODS
A literature search was used to review recent developments in our understanding of the factors and processes involved in tumour angiogenesis, and how these will impact on the care of patients with malignant disease encountered by surgeons.
RESULTS
Angiogenesis is fundamental to all stages of the malignant process, and involves a complex interaction between mediators secreted by tumour cells and host cells. Intense investigation continues into therapies targeting components of the angiogenic cascade. Imaging modalities capable of measuring the angiogenic activity of a tumour are also being studied in order to predict prognosis and select suitable patients for anti-angiogenic therapy.
CONCLUSIONS
As the use of these anti-angiogenic therapies becomes more wide-spread, they may have implications on the healing rates of cutaneous wounds and intracorporeal anastomoses.
Keywords: Neovascularisation, Angiogenesis inhibitors, Surgery
Angiogenesis, the process of new blood vessel formation from pre-existing vessels, and vasculogenesis, the process of vessel development from progenitor cells, are both important in a range of physiological and pathological conditions, including cancer formation.1,2 An understanding of the mechanisms involved in angiogenesis is vital to surgeons involved in the management of patients with malignant disease.
Literature search
A comprehensive Medline search was performed using the terms ‘angiogenesis’ and ‘neovascularisation’. Further searches were performed by combining these terms with several other key terms, for example ‘tumour’, ‘anti-angiogenic therapy’, and ‘surgery’. Article reference lists were also reviewed. This article represents the authors' collation and interpretation of data from recently published studies.
Angiogenic mediators and mechanisms
Angiogenesis may be a physiological phenomenon associated with wound healing, inflammation and menstruation, or it may be a pathological process (when it is often termed neovascularisation), in conditions such as diabetic retinopathy, rheumatoid arthritis, and cancer.3 Physiological angiogenesis is tightly controlled; however, in the pathological setting, it escapes regulation and excessive neovascularisation ensues.4 The angiogenic cascade involves a complex interaction between tumour cells and host immune cells and stromal cells, namely endothelia and modified smooth muscle cells called pericytes. Throughout the angiogenic process, the cellular and molecular mediators are regulated by autocrine and paracrine mechanisms, resulting in a coherent interplay between pro- and anti-angiogenic factors (Table 1).
Table 1.
| Angiostimulators | Angio-inhibitors |
|---|---|
| Vascular endothelial growth factor (VEGF) | Thrombospondins-1, -2 |
| Basic and acidic fibroblast growth factors (bFGF, aFGF) | Endostatin |
| Platelet-derived endothelial cell growth factor | Angiostatin |
| Matrix metalloproteinases (MMPs) | Interferons α and β |
| Insulin-like growth factor (IGF) | Interleukin-12 (IL-12) |
| Epidermal growth factor (EGF) | Tamoxifen |
| IL-1, IL-4, IL-6, IL-8, IL-15 | Thalidomide |
| Angiogenin | Captopril |
| Integrins αv1β3 and αv1β5 | Dexamethasone |
| Endotoxin | Indomethacin |
| Endothelin-1 | Diclofenac |
| Angiopoietin-1 (Ang-1) | Angiopoietin-2 (Ang-2) |
| Tumour necrosis factor (TNF)-α (in vivo) | TNF-α (in vitro) |
One of the most extensively studied angiogenic factors is vascular endothelial growth factor (VEGF), which is thought to have a role in many of the steps of the angiogenic cascade. It is a protein secreted by nearly all cells2 and occurs as several isoforms.5 Its release is regulated by cytokines, oncogenes, and tumour suppressor genes,6 and its action is to activate endothelia and increase the vascular permeability, allowing escape of proteins into the extravascular space to provide the lattice needed for endothelial migration.7 The conversion of immature to mature vessels requires the migration of pericytes and other accessory cells under the influence of angiopoietin-1 and platelet-derived growth factors (PDGFs).8 Many other angiogenic mediators are involved in endothelial activation and migration, as well as extracellular matrix invasion and capillary tube formation (Table 1). The important thing to consider is that these processes are not the result of a single mediator, but result from the interaction of multiple factors.
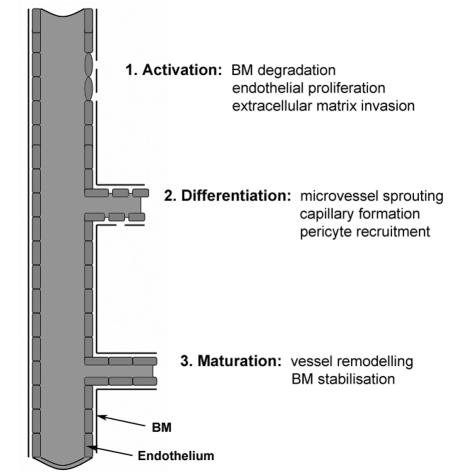
Angiogenic mediators produced by tumour and host cells diffuse to nearby existing vessels and bind to endothelial receptors, resulting in endothelial activation and anti-apoptotic molecule expression, an effect mediated primarily by VEGF and bFGF.9,10 The proliferation of activated endothelial cells characteristically leads to sprouting of microvessels (Fig. 1), involving initial basement membrane degradation (secondary to proteases such as MMPs and plasminogen activator11), destabilisation of the endothelial lining (under the influence of VEGF and angiopoietin-2), and finally migration of activated endothelia along a fibrin skeleton of extracellular matrix (facilitated by the release of adhesion molecules such as αv1β3 and αv1β5 integrins12). The new vessels formed are leaky as they have an incomplete basement membrane, and so are stabilised by recruitment of smooth muscle cells and pericytes via cytokines such as PDGF.13
Figure 1.
The stages of neovascularisation (BM, basement membrane).
Tumour angiogenesis
Angiogenesis is fundamental to tumour growth, invasion and metastasis. For a cancer to grow more than 2–3 mm3, it requires its own blood supply to meet the demands of tumour cell metabolism.14 The rich vascular network typical of solid tumours allows entry of tumour cells into the circulation, thereby facilitating the metastatic process.2 Similarly, micrometastases need to acquire a circulation at a site distant to the primary tumour in order to survive. Angiogenesis is, therefore, associated with all stages of the angiogenic cascade,15 and involves a gradual loss of cell cycle regulation, with an imbalance of pro- and anti-angiogenic factors. For most tumours, the switch to an angiogenic phenotype is a discrete step in the malignant process,16 and may be brought about by changes in the local tumour environment, such as inflammation, hypoxia, and acidosis, as well as altered gene expression by tumour and host cells, such as the loss of the tumour suppressor gene p53.17 Once the angiogenic switch has occurred, tumours grow exponentially due, in part, to the symbiotic relationship that exists between tumour and endothelial cells, in that angiogenic factors secreted by tumour cells result in an increase in the number of local blood vessels, which in turn secrete paracrines that increase tumour growth.18
Pro- and anti-angiogenic therapies
There has been considerable recent interest in anti-angiogenic therapies, with a wide range of agents being devised that inhibit one or more steps of the angiogenic process. Direct anti-angiogenic agents, such as thalidomide and endostatin, specifically inhibit endothelial cell proliferation, whereas indirect agents, such as the VEGF monoclonal antibody bevacizumab, inhibit the endothelial-tumour cell communication which co-ordinates the angiogenic response.19,20 Anti-angiogenic therapies can also be classified as true angiogenic inhibitors, which halt vessel sprouting but have no effect on established vessels, vascular targeting agents, that target pre-existing vessels and result in tumour necrosis, and non-selective anti-angiogenic agents, which exert a range of effects on a number of cell types, not just tumour vessel endothelia.3 Optimal anti-angiogenic agent selection as well as dosing schedule and timing of administration have yet to be determined. Combinations of agents, each with a different mechanism of action, may be synergistic;21 similarly, combining anti-angiogenic agents with standard chemotherapy22 and radiotherapy23 regimens may be beneficial. Further clinical studies are needed to optimise patient selection for anti-angiogenic therapies, and determine their contribution alongside standard treatment modalities, as inhibition of a single agent is unlikely to affect the complex interaction of the multiple factors characterising the angiogenic process.
Promotion of angiogenesis may actually be therapeutically desirable in ischaemic conditions such as coronary and peripheral artery disease. There has been recent interest in gene therapy techniques aimed at increasing the concentration of angiogenic mediators such as VEGF and FGF in ischaemic tissues, in order to augment new vessel formation in response to ischaemia.24 In contrast, the inhibition of deleterious genes has been investigated in vasoproliferative disorders, such as those occurring after vascular bypass procedures leading to restenosis and graft failure.25 Pro-angiogenic therapies involve administering a viral or plasmid vector containing the target gene to the region of interest, either by intravascular infusion or intramuscular injection, and the vector DNA encoding the gene becomes incorporated into the host tissue DNA. An alternative approach is to deliver recombinant protein directly to the ischaemic tissue, but this requires prolonged administration of high concentration protein to ensure adequate tissue uptake, thereby increasing the likelihood of adverse effects.24 Safety concerns regarding pro-angiogenic therapy have been raised,26 such as non-specific vascular stimulation resulting in conditions such as angiomas and diabetic retinopathy.27 Infection resulting from administration of an adenoviral vector has also been reported,28 and local effects of VEGF administration have been documented, such as increased vascular permeability leading to oedema following intramuscular injection.26 Controlled clinical trials are being conducted into the efficacy of gene therapy techniques for ischaemic heart and brain diseases as well as peripheral vascular disease.
The effect of surgery on the angiogenic cascade
Surgical wounds are perfect environments for tumour growth and metastases.29 A colonic anastomosis or laparotomy wound is a thousand times more likely to develop a metastatic deposit from circulating tumour cells than normal tissue.4 This is because the physiological angiogenesis evident in surgical wounds creates suitable conditions for implantation and subsequent proliferation of circulating cancer cells, thereby increasing the likelihood of local tumour recurrence.30 In addition, primary tumours may inhibit the growth of metastases through the actions of the anti-angiogenic mediators angiostatin and endostatin,31 which act to keep a metastatic cell cluster in a state of dormancy. Subsequent resection of the primary tumour may, therefore, favour metastatic growth by creating a pro-angiogenic environment. This has been demonstrated in renal cell and breast carcinomas,32 as well as in the residual liver following resection of colorectal metastases.33
For patients undergoing major surgery for malignant disease, there appears to be a theoretical balance between the physiological angiogenesis needed for wound healing, and the concurrent facilitation of tumour progression secondary to angiogenic promotion. This latter concern might suggest the need for intra- or peri-operative anti-angiogenic therapy, but at the expense of impaired wound healing. Animal studies suggest anti-angiogenic agents do not significantly impair cutaneous wound healing,32 but are deleterious on intestinal anastomotic healing;34 however, the paucity of human data in this area prevents firm conclusions being drawn.
Measuring angiogenesis
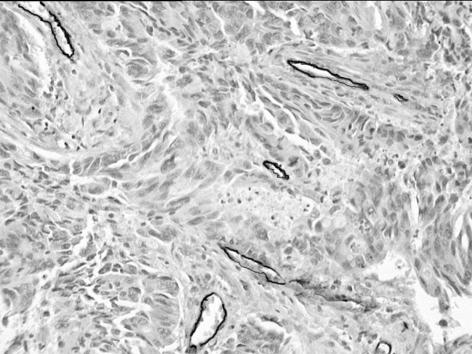
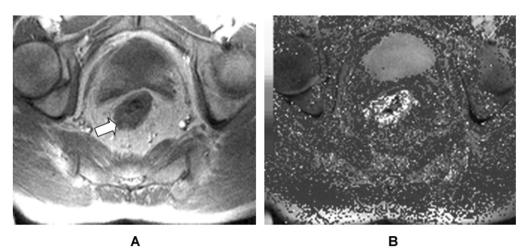
The angiogenic potential of a tumour may be measured in order to predict prognosis, monitor the tumour burden, and assess the response to anti-angiogenic therapy. Direct methods assess angiogenic mediators in actual tumour samples, whereas indirect methods involve radiological imaging of the tumour vasculature or measurement of circulating angiogenic mediators.35 Direct methods, such as immunohistochemical staining of tumour sections (Fig. 2), have been considered the gold standard, but are limited by the need for repeated invasive sampling procedures and the lack of data on the functional status of the tumour vasculature.36 Imaging modalities, such as CT and MRI, have the advantage of being non-invasive and can be repeated on multiple occasions in order to monitor disease progression and the response to therapy. Conventional angiography is too insensitive for accurate visualisation of tumour microvessels,3 but ultrasound has been used to measure tumour blood flow and volume, as well as deriving a vascularity index.37 CT and MRI techniques involve observation of changes in tissue enhancement following intravascular contrast administration, with quantification of tumour vascular estimates by mathematical modelling of the contrast enhancement patterns (Fig. 3). Both have excellent spatial resolution, but MRI is non-ionising, thereby allowing repeated imaging without biological sequelae. Nuclear medicine techniques, such as positron emission tomography (PET) and single photon emission computed tomography (SPECT), have also been investigated as angiogenic imaging modalities.38 PET imaging in particular appears promising for the measurement of tumour perfusion and hypoxia.39,40
Figure 2.
Histological rectal carcinoma section showing blood vessels stained by the endothelial marker CD31 (diaminobenzidine labelled, ×100).
Figure 3.
(A) T1-weighted magnetic resonance image of a rectal carcinoma (white arrow). (B) Corresponding map of transfer constant (Ktrans), a marker of capillary permeability (white areas represent high capillary permeability).
Conclusions
Angiogenesis is an important aspect of tumour biology, and surgeons need to be aware of the mechanisms involved in order to understand the pathophysiology of the disease processes encountered in surgical practice.
References
- 1.Noden DM. Embryonic origins and assembly of blood vessels. Am Rev Respir Dis. 1989;140:1097–103. doi: 10.1164/ajrccm/140.4.1097. [DOI] [PubMed] [Google Scholar]
- 2.Ellis LM, Liu W, Ahmad SA, Fan F, Jung YD, Shaheen RM, et al. Overview of angiogenesis: biologic implications for antiangiogenic therapy. Semin Oncol. 2001;28(Suppl 16):94–104. doi: 10.1016/s0093-7754(01)90287-8. [DOI] [PubMed] [Google Scholar]
- 3.Li WW. Tumor angiogenesis: molecular pathology, therapeutic targeting, and imaging. Acad Radiol. 2000;7:800–11. doi: 10.1016/s1076-6332(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 4.McNamara DA, Harmey JH, Walsh TN, Redmond HP, Bouchier-Hayes DJ. Significance of angiogenesis in cancer therapy. Br J Surg. 1998;85:1044–55. doi: 10.1046/j.1365-2168.1998.00816.x. [DOI] [PubMed] [Google Scholar]
- 5.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, et al. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–54. [PubMed] [Google Scholar]
- 6.Boedefeld WM, 2nd, Bland KI, Heslin MJ. Recent insights into angiogenesis, apoptosis, invasion, and metastasis in colorectal carcinoma. Ann Surg Oncol. 2003;10:839–51. doi: 10.1245/aso.2003.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36:127–37. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- 8.Bikfalvi A, Bicknell R. Recent advances in angiogenesis, anti-angiogenesis and vascular targeting. Trends Pharmacol Sci. 2002;23:576–82. doi: 10.1016/s0165-6147(02)02109-0. [DOI] [PubMed] [Google Scholar]
- 9.Nor JE, Christensen J, Mooney DJ, Polverini PJ. Vascular endothelial growth factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am J Pathol. 1999;154:375–84. doi: 10.1016/S0002-9440(10)65284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Connor DS, Schechner JS, Adida C, Mesri M, Rothermel AL, Li F, et al. Control of apoptosis during angiogenesis by survivin expression in endothelial cells. Am J Pathol. 2000;156:393–8. doi: 10.1016/S0002-9440(10)64742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pepper MS, Ferrara N, Orci L, Montesano R. Vascular endothelial growth factor (VEGF) induces plasminogen activators and plasminogen activator inhibitor-1 in microvascular endothelial cells. Biochem Biophys Res Commun. 1991;181:902–6. doi: 10.1016/0006-291x(91)91276-i. [DOI] [PubMed] [Google Scholar]
- 12.Zetter BR. Migration of capillary endothelial cells is stimulated by tumour-derived factors. Nature. 1980;285:41–3. doi: 10.1038/285041a0. [DOI] [PubMed] [Google Scholar]
- 13.Darland DC, D'Amore PA. Blood vessel maturation: vascular development comes of age. J Clin Invest. 1999;103:157–8. doi: 10.1172/JCI6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 15.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 16.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 17.Poon RT, Fan ST, Wong J. Clinical significance of angiogenesis in gastrointestinal cancers: a target for novel prognostic and therapeutic approaches. Ann Surg. 2003;238:9–28. doi: 10.1097/01.sla.0000075047.47175.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rak J, Filmus J, Kerbel RS. Reciprocal paracrine interactions between tumour cells and endothelial cells: the ‘angiogenesis progression’ hypothesis. Eur J Cancer. 1996;32A:2438–50. doi: 10.1016/s0959-8049(96)00396-6. [DOI] [PubMed] [Google Scholar]
- 19.Scappaticci FA. Mechanisms and future directions for angiogenesis-based cancer therapies. J Clin Oncol. 2002;20:3906–27. doi: 10.1200/JCO.2002.01.033. [DOI] [PubMed] [Google Scholar]
- 20.Abdollahi A, Lipson KE, Sckell A, Zieher H, Klenke F, Poerschke D, et al. Combined therapy with direct and indirect angiogenesis inhibition results in enhanced antiangiogenic and antitumor effects. Cancer Res. 2003;63:8890–8. [PubMed] [Google Scholar]
- 21.Scappaticci FA, Smith R, Pathak A, Schloss D, Lum B, Cao Y, et al. Combination angiostatin and endostatin gene transfer induces synergistic antiangiogenic activity in vitro and antitumor efficacy in leukemia and solid tumors in mice. Mol Ther. 2001;3:186–96. doi: 10.1006/mthe.2000.0243. [DOI] [PubMed] [Google Scholar]
- 22.Sweeney CJ, Miller KD, Sissons SE, Nozaki S, Heilman DK, Shen J, et al. The antiangiogenic property of docetaxel is synergistic with a recombinant humanized monoclonal antibody against vascular endothelial growth factor or 2-methoxyestradiol but antagonized by endothelial growth factors. Cancer Res. 2001;61:3369–72. [PubMed] [Google Scholar]
- 23.Mauceri HJ, Hanna NN, Beckett MA, Gorski DH, Staba MJ, Stellato KA, et al. Combined effects of angiostatin and ionizing radiation in antitumour therapy. Nature. 1998;394:287–91. doi: 10.1038/28412. [DOI] [PubMed] [Google Scholar]
- 24.Freedman SB, Isner JM. Therapeutic angiogenesis for coronary artery disease. Ann Intern Med. 2002;136:54–71. doi: 10.7326/0003-4819-136-1-200201010-00011. [DOI] [PubMed] [Google Scholar]
- 25.Mann MJ, Whittemore AD, Donaldson MC, Belkin M, Conte MS, Polak JF, et al. Ex-vivo gene therapy of human vascular bypass grafts with E2F decoy: the PREVENT single-centre, randomised, controlled trial. Lancet. 1999;354:1493–8. doi: 10.1016/S0140-6736(99)09405-2. [DOI] [PubMed] [Google Scholar]
- 26.Epstein SE, Kornowski R, Fuchs S, Dvorak HF. Angiogenesis therapy: amidst the hype, the neglected potential for serious side effects. Circulation. 2001;104:115–9. doi: 10.1161/01.cir.104.1.115. [DOI] [PubMed] [Google Scholar]
- 27.Freedman SB. Clinical trials of gene therapy for atherosclerotic cardiovascular disease. Curr Opin Lipidol. 2002;13:653–61. doi: 10.1097/00041433-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Ferber D. Gene therapy. Safer and virus-free? Science. 2001;294:1638–42. doi: 10.1126/science.294.5547.1638. [DOI] [PubMed] [Google Scholar]
- 29.Murthy MS, Scanlon EF, Silverman RH, Goodheart CR, Goldschmidt RA, Jelachich ML. The role of fibronectin in tumor implantation at surgical sites. Clin Exp Metastasis. 1993;11:159–73. doi: 10.1007/BF00114974. [DOI] [PubMed] [Google Scholar]
- 30.Hofer SO, Molema G, Hermens RA, Wanebo HJ, Reichner JS, Hoekstra HJ. The effect of surgical wounding on tumour development. Eur J Surg Oncol. 1999;25:231–43. doi: 10.1053/ejso.1998.0634. [DOI] [PubMed] [Google Scholar]
- 31.O'Reilly MS, Holmgren L, Chen C, Folkman J. Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med. 1996;2:689–92. doi: 10.1038/nm0696-689. [DOI] [PubMed] [Google Scholar]
- 32.van der Bilt JD, Borel Rinkes IH. Surgery and angiogenesis. Biochim Biophys Acta. 2004;1654:95–104. doi: 10.1016/j.bbcan.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Picardo A, Karpoff HM, Ng B, Lee J, Brennan MF, Fong Y. Partial hepatectomy accelerates local tumor growth: potential roles of local cytokine activation. Surgery. 1998;124:57–64. [PubMed] [Google Scholar]
- 34.Hendriks JM, Hubens G, Wuyts FL, Vermeulen P, Hubens A, Eyskens E. Experimental study of intraperitoneal suramin on the healing of colonic anastomoses. Br J Surg. 1999;86:1171–5. doi: 10.1046/j.1365-2168.1999.01223.x. [DOI] [PubMed] [Google Scholar]
- 35.George ML, Dzik-Jurasz AS, Padhani AR, Brown G, Tait DM, Eccles SA, et al. Non-invasive methods of assessing angiogenesis and their value in predicting response to treatment in colorectal cancer. Br J Surg. 2001;88:1628–36. doi: 10.1046/j.0007-1323.2001.01947.x. [DOI] [PubMed] [Google Scholar]
- 36.Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer Res. 2000;60:1388–93. [PubMed] [Google Scholar]
- 37.Cheng WF, Lee CN, Chu JS, Chen CA, Chen TM, Shau WY, et al. Vascularity index as a novel parameter for the in vivo assessment of angiogenesis in patients with cervical carcinoma. Cancer. 1999;85:651–7. doi: 10.1002/(sici)1097-0142(19990201)85:3<651::aid-cncr15>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 38.Blankenberg FG, Eckelman WC, Strauss HW, Welch MJ, Alavi A, Anderson C, et al. Role of radionuclide imaging in trials of antiangiogenic therapy. Acad Radiol. 2000;7:851–67. doi: 10.1016/s1076-6332(00)80633-9. [DOI] [PubMed] [Google Scholar]
- 39.Lehtio K, Eskola O, Viljanen T, Oikonen V, Gronroos T, Sillanmaki L, et al. Imaging perfusion and hypoxia with PET to predict radiotherapy response in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;59:971–82. doi: 10.1016/j.ijrobp.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Barthel H, Wilson H, Collingridge DR, Brown G, Osman S, Luthra SK, et al. In vivo evaluation of [18F]fluoroetanidazole as a new marker for imaging tumour hypoxia with positron emission tomography. Br J Cancer. 2004;90:2232–42. doi: 10.1038/sj.bjc.6601862. [DOI] [PMC free article] [PubMed] [Google Scholar]