Abnormal semen quality or sexual dysfunction are contributing factors in about half of subfertile couples. As natural pregnancy is substantially reduced in these cases, the man should be assessed by an appropriately trained gynaecologist in a reproductive medicine clinic, or by a clinical andrologist. Subfertile men often defer consultations because they perceive subfertility as a threat to their masculinity. Consultations should help them to distinguish between fertility and virility, which may ease their anxiety. However, achievement of a wanted pregnancy is more likely to restore manly feelings.
Table 1.
Semen analysis terminology
| • Normozoospermia—All semen parameters normal |
|---|
| • Oligozoospermia—Reduced sperm numbers Mild to moderate: 5-20 million/ml of semen Severe: <5 million/ml of semen |
| • Asthenozoospermia—Reduced sperm motility |
| • Teratozoospermia—Increased abnormal forms of sperm |
| • Oligoasthenoteratozoospermia—Sperm variables all subnormal |
| • Azoospermia—No sperm in semen |
| • Aspermia (anejaculation)—No ejaculate (ejaculation failure) |
| • Leucocytospermia—Increased white cells in semen |
| • Necrozoospermia—All sperm are non-viable or non-motile |
Causes of male subfertility
Subfertility affects one in 20 men. Idiopathic oligoasthenoteratozoospermia is the commonest cause of male subfertility. Although sexual function is normal, there is a reduced count of mainly dysfunctional spermatozoa. Reduced fertilising capacity is related to raised concentrations of reactive oxygen species in semen, which may damage the cell membrane. Abnormal sperm morphology—an indicator of deranged sperm production or maturation—is also associated with reduced fertilising capacity. Less common types of male subfertility are caused by testicular or genital tract infection, disease, or abnormalities. Systemic disease, external factors (such as drugs, lifestyle, etc), or combinations of these also result in male subfertility. Male subfertility is rarely caused by endocrine deficiency.
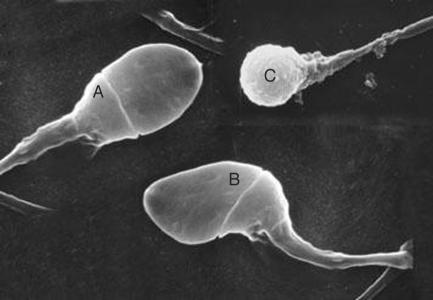
Figure 1.
Sperm morphology is related to the fertilising capacity by in vitro fertilisation. (A=normal sperm head; B=abnormal head; C=globozoospermia—a rare syndrome in which all sperm heads lack acrosome caps and cannot fertilise)
Falling sperm counts have not affected global fertility, although the effect of increased oestrogenic compounds in drinking water is of concern because the incidence of cryptorchidism and testicular cancer is increasing.
Table 2.
Normal seminal fluid analysis (World Health Organization, 2002)
| • Volume >2 ml |
|---|
| • Sperm concentration >20 million/ml |
| • Sperm motility >50% progressive or >25% rapidly progressive |
| • Morphology (strict criteria) >15% normal forms |
| • White blood cells <1 million/ml |
| • Immunobead or mixed antiglobulin reaction test* <10% coated |
Tests for the presence of antibodies coating the sperm
Clinical assessment
History taking should include frequency of coitus, erectile function, ejaculation, scrotal disorders or surgery, urinary symptoms, past illnesses, lifestyle factors, and any drugs taken. Physical examination should seek signs of hypogonadism (small testes), hypoandrogenism (lack of facial or body hair), systemic disease, and abnormalities of the penis or testicles. Testicular sizes are assessed by length (cm) or volume (ml) and are measured with an orchidometer. Because of the risk of cancer, a urological opinion is essential if there is an intratesticular lump or the testes are undescended or absent. Scrotal ultrasonography is helpful in confirming a varicocele or testicular tumour. Transrectal ultrasonography of the prostate may identify the cause of a low volume ejaculate. Serum follicle stimulating hormone is a useful index of impaired spermatogenesis. Genetic screening (karyotype, or DNA analysis for Y chromosome microdeletions or cystic fibrosis) is indicated for men with severe oligozoospermia and most men with azoospermia.
Figure 2.
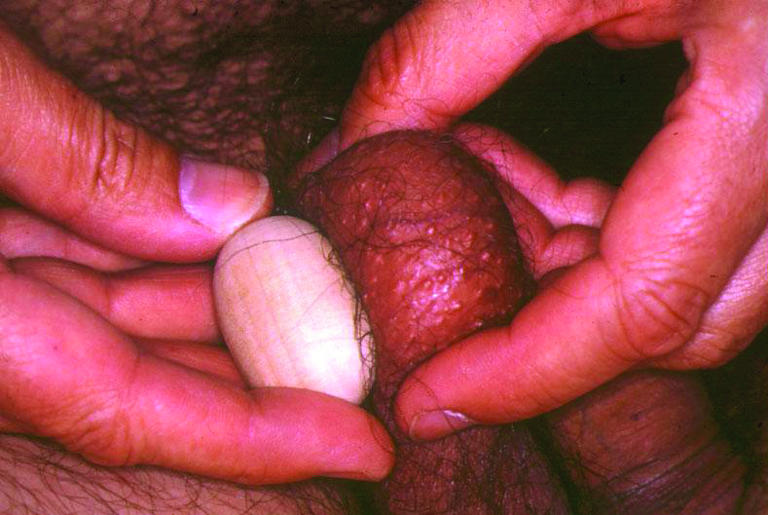
A simple orchidometer is a 4 cm long (20 ml) ovoid used to assess testis size in subfertile men. Normal testes are more than 4 cm and firm in consistency. Abnormal testes are soft and smaller. Both testes should be carefully examined to exclude a tumour
Semen analysis is the cornerstone of male fertility assessment and is often the trigger to refer patients for a specialist opinion. Semen samples are best sent to laboratories linked with infertility services. Ideally two samples around six weeks apart (unless the first is unequivocally normal) are required. The samples should be produced by masturbation after three days' abstinence. Men may use non-spermicidal condoms if they have difficulty with, or religious objections to, masturbation
Treatment options for subfertile men
As all couples hope and prefer to conceive naturally, a specific diagnosis should be sought and corrected where appropriate. However, a couple with subfertility of longer than three years, or with a non-reversible form of subfertility is unlikely to conceive spontaneously and should join an assisted conception programme without delay.
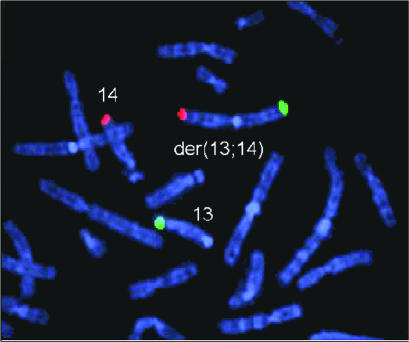
Figure 3.
Autosomal Robertsonian reciprocal translocation may be associated with poor sperm quality and subfertility
Stopping adverse drugs and drug misuse
Several drugs impair spermatogenesis or sexual function. Most common are sulfasalazine and anabolic steroids when misused by athletes. These effects are reversible, allowing fertility to return to normal in six to 12 months if the drugs are withdrawn. Chemotherapy and radiotherapy damage spermatogenesis, hence sperm banking should be offered to male patients with cancer irrespective of sperm quality.
Table 3.
What doesn't work
| • Abstaining from coitus until ovulation does not improve the semen or likelihood of conception. Increasing coital frequency (alternate days) supplies more viable spermatozoa that normally remain motile in the female tract for two to three days |
|---|
| • Treatment with gonadotrophin injections, androgens (mesterolone) or antioestrogens (clomifene or tamoxifen) is not indicated because although they may improve the sperm count, fertility rates are not improved as the spermatozoa remain dysfunctional |
Timing and lifestyle changes
Most cases of mild to moderate oligozoospermia are idiopathic, but transient oligozoospermia can follow influenza or a major illness and improves within three to six months. The incidence of spontaneous conception each month is 1-2% and justifies conservative or empirical treatment for younger couples. The incidence also explains the powerful placebo effect of some treatments. Advice can be given on lifestyle changes and on the avoidance of fertility impairing drugs.
Table 4.
Genetics and male infertility
| Clinical diagnosis | Genetic tests | Most common defects | Incidence (%) |
|---|---|---|---|
| Congenital bilateral absence of vas deferens (CBAVD) | Cystic fibrosis (CFTR gene) | ΔF508, R117H | 66 |
| Non-obstructive azoospermia | Karyotype | 47, XXY | 15-30 |
| AZFa, AZFb*, AZFc | 10-15 | ||
| Severe (<5M/ml) oligozoospermia | Karyotype | 47, XXY | 1-2 |
| Translocation | 0.2-0.4 | ||
| Y chromosome microdeletions | Partial AZFb, AZFc | 7-10 |
CFTR = cystic fibrosis transmembrane conductance regulator;
AZF = azoospermia factor; *AZFb the most severe (DAZ gene—deleted in azoospermia) causes the most severe defects of spermatogenesis; AZFc causes the mildest defects of spermatogenesis
Treating accessory gland infection
With the increased prevalence of chlamydia, accessory gland infection may cause partial obstruction, focal epididymitis, or subclinical prostatitis. Semen cultures are rarely useful but antibiotic treatment (for example, doxycycline, erythromycin, ciprofloxacin) is often given empirically. Antioxidants (for example, vitamins C and E) absorb reactive oxygen species and are purported to improve sperm motility, although no convincing evidence exists that pregnancy rates are improved.
Table 5.
that can impair male fertility
| • Impaired spermatogenesis—Sulfasalazine, methotrexate, nitrofurantoin, colchicine, chemotherapy |
|---|
| • Pituitary suppression—Testosterone injections, gonadotrophin releasing hormone analogues |
| • Antiandrogenic effects—Cimetidine, spironolactone |
| • Ejaculation failure—α blockers, antidepressants, phenothiazines |
| • Erectile dysfunction—β blockers, thiazide diuretics, metoclopramide |
| • Drugs of misuse—Anabolic steroids, cannabis, heroin, cocaine |
Assisted conception
Assisted conception gives most infertile men the chance of biological fatherhood, and it is most successful if the woman is under 35 years. The method indicated depends on the quantity and quality of sperm isolated from the semen after “washing” or density gradient techniques. The resulting sperm preparations have improved counts of morphologically normal progressively motile spermatozoa.
Table 6.
Conservative measures for men with suboptimal semen analyses
| • Stop smoking—Nicotine reduces seminal plasma antioxidants |
|---|
| • Have frequent intercourse—Increases output of non-senile spermatozoa |
| • Reduce alcohol intake—Alcohol can suppress spermatogenesis |
| • Wear boxer shorts and avoid hot baths—Heat suppresses spermatogenesis |
| • Avoid pesticides, herbicides, heat, and radiation at work—All impair spermatogenesis |
Intrauterine insemination
In mild or moderate oligozoospermia some spermatozoa are functionally normal. Intrauterine insemination is feasible with preparations of three to five million progressively motile sperm. The woman must have at least one normal patent fallopian tube for successful interuterine insemination. Combined with ovarian stimulation, three to four cycles of intrauterine insemination result in conception in 15-30% of couples.
In vitro fertilisation and intracytoplasmic sperm injection
A prepared sample containing around one to two million motile sperm is required for adequate oocyte fertilisation with in vitro fertilisation. Not surprisingly, fertilisation is lowest when it is the man who is infertile. However, intracytoplasmic sperm injection needs only one viable sperm for microinjection into each egg. The technique is indicated if the semen preparation yields too few normal motile sperm for in vitro fertilisation, as occurs in severe oligozoospermia. Intracytoplasmic sperm injection is appropriate after unexpectedly, poor, or absent fertilisation in vitro. It is also an effective technique for men with azoospermia by using spermatozoa that have been surgically retrieved from the epididymis or testis.
Severe oligoasthenoteratozoospermia
Most cases of severe oligoasthenoteratozoospermia are caused by idiopathic testicular abnormality or disorder. Genetic tests are abnormal in 7-10% of men with sperm counts less than 5 million/ml. Severely impaired sperm motility may be caused by antibodies, chronic prostatitis, or rare recessive intrinsic defects of the sperm tail linked to sinopulmonary disease (for example, Kartagener's syndrome or Young's syndrome). Treatment of severe oligoathenoteratoazoospermia rarely improves the semen quality, but intracytoplasmic sperm injection is often successful even if only few weakly motile spermatozoa can be isolated from the ejaculate.
Table 7.
Varicocele controversy
| Varicoceles occur in 15% of men in general and in 30% of subfertile men. Varicoceles probably impair spermatogenesis by increasing the temperature in the scrotum. Achievement of pregnancies is often attributed to varicocele surgery because semen quality may improve after surgery and because conceptions occur in 15% of infertile men with or without surgery. However, clinical trials are equivocal. Varicoceles can be ligated or embolised, but as it may take one to two years for the couple to achieve a pregnancy, clinicians may recommend assisted conception if the woman is older than 35 years |
Azoospermia
Azoospermia (absence of sperm from the semen) can be caused by hypothalamic-pituitary failure, testicular failure, or testicular obstruction. Testicular size and concentration of follicle stimulating hormone determine the clinical diagnosis. Although azoospermia is uncommon, 75% of men who have the condition now have the opportunity of biological fatherhood through assisted conception techniques.
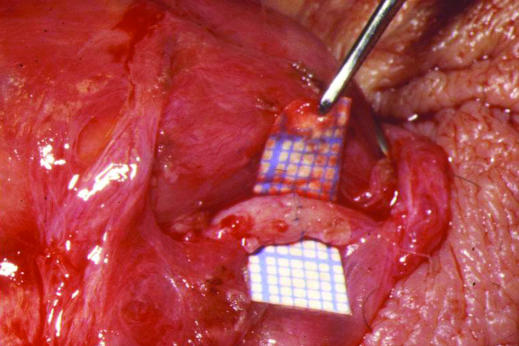
Figure 4.
Microsurgical vasovasectomy (vasectomy reversal). Using an operating microscope, the cut and often fibrosed ends of the vas deferens are dissected free from surrounding tissue and anastomosed using fine nylon sutures to re-establish patency. The small squares on the graph paper are 1 mm wide
Hypogonadotrophic hypogonadism
Hypogonadotrophic hypogonadism is rare but can be treated with gonadotrophin injections or by administering gonadotrophin releasing hormone by infusion pump. Natural conceptions often occur within a year of treatment because any spermatozoa secreted will be functionally normal.
Obstructive azoospermia
Men with obstructive azoospermia have normal spermatogenesis and hence normal size testes, normal concentrations of serum follicle stimulating hormone, and they are normally virilised. If neither vas is palpable, congenital bilateral absence of the vas deferens is diagnosed, which cannot be corrected surgically. As two thirds of men with palpable congenital bilateral absence of the vas deferens carry cystic fibrosis mutations, both partners require screening.
Other cases of obstructive azoospermia occur after vasectomy or they are caused by epididymal obstruction after chlamydia or gonorrhoea. Vasectomy reversal will return sperm to the ejaculate in 80-90% of men, and pregnancies occur in 40-50% of couples in one to two years. Testicular exploration may be indicated for other obstructions because reconstructive surgery results in sperm positive semen in 30-50% of cases, and pregnancies in 20-25% of couples. If necessary, sperm retrieved from the epididymis during these reconstructive procedures can be frozen for future intracytoplasmic sperm injection cycles.
Testing for sperm antibodies is controversial. False positive results occur and antibodies do not necessarily impair sperm function. Antibodies may be found in genital infections and obstructions, but specific treatment is of limited value. Corticosteroids have been used successfully for high titre sperm antibodies. However, severe side effects may occur, including bilateral necrosis of the hip and gastric ulceration. In vitro fertilisation is a more effective and safer way to achieve pregnancy
Live birth rates of 20-30% per in vitro fertilisation cycle are achievable, even in severe oligozoospermia or azoospermia, if the woman is under 35 years
Non-obstructive azoospermia
Non-obstructive azoospermia may be caused by cryptorchidism, Klinefelter's syndrome (47,XXY), or Y chromosome deletions after chemotherapy or radiotherapy—for example, for lymphoma or testicular cancer. However, many cases of non-obstructive azoospermia are idiopathic. Multiple testicular biopsy may show scattered foci of spermatogenesis in about half the cases with potential for surgical sperm retrieval and intracytoplasmic sperm injection. Men with non-obstructive azoospermia should have genetic testing as 15-30% of them have sex chromosome aneuploidy or Y chromosome deletions.
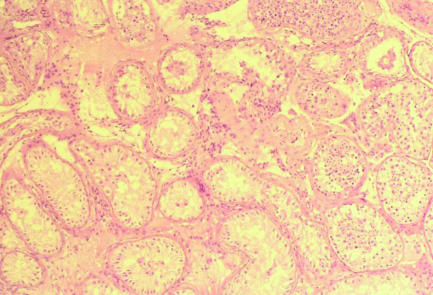
Figure 5.
Testicular biopsy of non-obstructive azoospermia showing Sertoli cell only syndrome with a focus of spermatogenesis. All biopsies obtained for testicular sperm extraction in non-obstructive azoospermia are assessed histologically because of the increased prevalence (0.4-1.1%) of carcinoma in situ in subfertile men
Surgical sperm retrieval
Surgical sperm retrieval for intracytoplasmic sperm injection is indicated in obstructive azoospermi where spermatogenesis is usually normal, or non-obstructive azoospermia where spermatogenesis is present on biopsy in 30-50% of cases. Results of intracytoplasmic sperm injection using surgically retrieved sperm are similar to cycles where ejaculated sperm is used. Percutaneous epididymal or testicular sperm aspiration or extraction are usually feasible under local anaesthetic and sedation.
Sexual dysfunction
Most men with erectile or ejaculation failure have normal sperm function and are managed according to whether ejaculation can be stimulated. Men unable to produce the sample for in vitro fertilisation usually respond to sildenafil.
In retrograde ejaculation, where the emission enters the bladder because of non-surgical sphincter failure (for example, in diabetes), oral sympathomimetics (for example, pseudoephedrine) may close the incompetent bladder neck and produce antegrade ejaculation. Retrograde ejaculation caused by anatomical sphincter defects (for example, after prostatectomy or other bladder neck incision) is managed by intrauterine insemination. The sperm that are used are isolated from post-ejaculation urine, which is suitably alkalinised by oral sodium bicarbonate and adjusted for osmolarity.
In men whose spinal cord is injured, semen is usually obtained with a vibrator when vaginal insemination at home may be successful. If this fails, and in cases of aspermia caused by pelvic injury or multiple sclerosis, rectal electrostimulation usually provides semen suitable for assisted conception. If ejaculation is not induced, sperm can be retrieved by vas deferens aspiration or by testicular aspiration for intracytoplasmic sperm injection.
Donor insemination
Donor insemination is the principal choice for the 1 in 200 infertile men (and their partners) who have no sperm because of traumatic or congenital anorchia or total germ cell aplasia. As donor semen is selected for high quality, the live birth rate of 7-15% per cycle is largely dependent on the fertility of the woman. Adoption is the only other option at present.
The scanning electron micrographs of sperm morphology are published with permission of Alpha from Scientists in Reproductive Medicine newsletters 1996 and 2000. The testicular biopsy showing non-obstructive azoospermia is at a magnification x 400 and is reprinted with permission from Dr K Shah, London.
The ABC of subfertility is edited by Peter Braude, professor and head of department of women's health, Guy's, King's, and St Thomas's School of Medicine, London, and Alison Taylor, consultant in reproductive medicine and director of the Guy's and St Thomas's assisted conception unit. The series will be published as a book in the winter.
Competing interests: None declared.
Further reading and resources
- • Hargreave TB, Mills JA. Investigating and managing infertility in general practice. BMJ 1998;316: 1438-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- • Hirsh AV. Investigation and therapeutic options for infertile men presenting in assisted conception units. In: Brinsden PR, ed. In-vitro fertilisation and assisted reproduction. 2nd ed. London: Parthenon, 1999
- • Hull MGR, Glazener CMA, Kelly NJ, Conway DI, Foster PA, Hinton RA, et al. Population study of causes, treatment, and outcome of infertility. BMJ 1985;291: 1693-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- • Royal College of Obstetricians and Gynaecologists. Evidence-based guidelines: initial investigation and management of the infertile couple. London: RCOG, 1998
- • Royal College of Obstetricians and Gynaecologists. Evidence-based guidelines: management of infertility in secondary care. London: RCOG, 1998 [PubMed]
- • Royal College of Obstetricians and Gynaecologists. Evidence-based guidelines: management of infertility in tertiary care. London: RCOG, 2000
- • Rowe PJ, Comhaire FH, Hargreave TB, Mahmoud AMA. WHO manual for the standard investigation, diagnosis and management of the infertile male. Cambridge: Cambridge University Press, 2000
- • Skakkebaek NE, Giwercman A, de Kretser D. Pathogenesis and management of male infertility. Lancet 1994;343: 1473-9 [DOI] [PubMed] [Google Scholar]
- • Vale J, Hirsh AV. Male sexual dysfunction. Oxford: Blackwell Science, 2001