Abstract
INTRODUCTION
The aim of this study was to investigate the effect of Saccharomyces boulardii treatment on preventing bacterial translocation in an obstructive jaundice animal model.
MATERIALS AND METHODS
Sixty adult rats were divided into five groups: group 1 – the sham-operated group; group 2 – the common bile duct ligation group; group 3 – the S. boulardii group; group 4 – the ampicillin-sulbaktam group; and group 5 – the S. boulardii plus ampicillin-sulbaktam group. The saline, antibiotics and S. boulardii were given, respectively, for a 7-day period as a single dose per day via temporary orogastric intubation. Seven days following the obstructive jaundice, the animal had laparatomy under sterile conditions. Segments of ileum were removed for histopathological examination. Blood, liver, spleen and mesenteric lymph nodes were taken for microbiological culture.
RESULTS
Bacterial translocation rates were 0% in the sham-operated group, 83% in group 2, 42% in group 3, 42% in group 4 and 33% in group 5. Bacterial translocation significantly increased in group 2 compared to groups 3, 4 and 5 (P = 0.001). The bacterial counts (CFU/g) of group 2 were significantly higher than those of groups 3, 4 and 5 (P = 0.001). Histopathological examination of ileum specimens revealed a significant decrease in the heights of villi in groups 2–5 compared to the sham-operated group (P = 0.001). The mean villus height in groups 3 and 5 was significantly higher than that of group 4 (P = 0.001).
CONCLUSIONS
S. boulardii was found to be effective in the successful control of translocation and improvement of intestinal barrier function.
Keywords: Saccharomyces boulardii, Bile, Obstructive, Jaundice, Translocation
Bacterial translocation is defined as the passage of viable indigenous bacteria from the gastrointestinal tract to extra-intestinal sites, such as the mesenteric-lymph-node complex, liver, spleen and bloodstream. Three major mechanisms promote bacterial translocation: intestinal bacterial overgrowth, deficiencies in host immune defences and increased permeability or damage to the intestinal mucosal barrier.1 Most transmigrating organisms are subsequently subject to phagocytosis by macrophages, but some are found to be free in blood and lymph vessels.2 Increased intestinal permeability and bacterial translocation were demonstrated following both experimental biliary obstruction and in jaundiced patients.3,4
Saccharomyces boulardii is non-pathogenic yeast that is used for the prevention and treatment of antibiotic-associated diarrhoea, acute and chronic enterocolopathies, trophic intestinal effects and for the treatment of pseudomembranous colitis.5–7 Clinical trials and experimental studies have demonstrated that oral treatment with a lyophilised preparation of S. boulardii has beneficial effects in preventing the occurrence of complications linked to changes in the normal gut flora and significantly increased the intestinal secretion of secretory component of immunoglobulins and secretory IgA concentration in the gut.8–12
According to our review of the literature, this is the first in vivo study of the relationship between S. boulardii and the development of bacterial translocation following obstructive jaundice in rats. The aim of this study was to investigate the effect of S. boulardii treatment on preventing bacterial translocation in an obstructive jaundice animal model.
Materials and Methods
Animals
This study was approved by the Animal Ethics Committee of the Dicle University Hospital and performed following standard guidelines for the care and use of laboratory animals. A total of 60 adult Sprague-Dawley male rats (250–300 g weight) were housed under constant temperature (22°C) and humidity, with 12-h dark/light cycles and allowed tap water and rat pellets ad libitum before and after the operation.
Surgery
Rats were randomised into 5 groups containing 12 rats each. All rats were initially anaesthetised by intramuscular injection of ketamine (25 mg/kg) and xylasine (5 mg/kg). All procedures were performed under sterile conditions. After midline abdominal incision, the common bile duct was identified and mobilised. It was then doubly ligated using 5-0 silk and divided. Sham-operated group rats had a similar incision followed by mobilisation of the common bile duct, without ligation or division. At the end of the experiment, rats were killed by an overdose of intravenous pentobarbital.
Treatment
The rats were divided in to five groups: group 1 – the sham-operated group; group 2 – the common bile duct ligation group; group 3 – the S. boulardii group (Reflor sase®, Sanofi, Istanbul, Turkey; 100 mg/day); group 4 – the ampicillin-sulbaktam group (Alfasid suspension®, Fako AS, Istanbul, Turkey; 50 mg/kg/day); group 5 – the S. boulardii plus ampicillin-sulbaktam group (Reflor sase®; 100 mg/day plus Alfasid suspension®; 50 mg/kg/day). The saline, antibiotics and S. boulardii were given respectively for a 7-day period as a single dose per day via temporary orogastric intubation. There was no death in any group after 7 days. Seven days following the obstructive jaundice, the animal had laparatomy under sterile conditions. Samples of systemic blood, liver, spleen and mesenteric lymph nodes (MLNs) were taken for microbiological culture under sterile conditions. Segments of ileum were removed for histopathological examination. Liver, spleen and MLNs were taken for microbiological culture.
Collection of tissues and bacterial translocation
Blood samples were obtained from the portal vein and cultured aerobically and anaerobically using the BacTec™ Peds battles (Becton-Dickinson Diagnostic Inc., Sparks, MD, USA). Blood cultures were continuously monitored for 7 days. Positive cultures were plated out on blood agar, chocolate agar, eosin methylene blue (EMB) agar or Sabouraud-dextrose agar. Identification was performed by the Sceptor microdilution method. At the same time, MLNs, spleen and the right lobe of the liver were removed and placed in sterile glass bottles containing sterile brain-heart infusion media. The bottles were re-weighed and tissue homogenates were prepared in 2 ml brain-heart infusion using a sterile mortar and pestle. A portion (0.1 ml) of each of homogenates was cultured on blood agar, chocolate agar, EMB agar and Sabouraud-dextrose agar . All the plates were examined after 24 h and 48 h of incubation at 37°C. Individual colonies were identified and quantified as colony-forming units (CFUs) per gram tissue. The results of all CFUs were averaged and expressed as mean Log10.
Assessment of ileal villus heights
Following retrieval of the solid organs at laparotomy, a 2-cm segment of terminal ileum was removed. The bowel was stripped from its mesentery, each of the segments was opened along its length, and rinsed in a cold solution. The specimens of terminal ileum were fixed in 10% formalin in 0.15 M phosphate buffer (pH 7.2), embedded in paraffin and then 5-μm sections were cut. The specimens were stained with hematoxylin and eosin and examined under the light microscope (Olympus BH-2, Tokyo, Japan). An independent pathologist who had no knowledge of the experimental groups from which the specimens were derived performed histological evaluation. Morphometric analysis was fulfilled using an eyepiece micrometer (Olympus). For each rat, 10 randomly chosen mucosal regions were traced and villus height (μm) was measured and the mean was calculated.
Statistical analysis
Statistical analyses were made using analysis of variance (one-way ANOVA) with Tukey HSD honestly significant test for post hoc multiple comparisons on an IBM-compatible personal computer using SPSS v10.0 software. A P value of < 0.05 was considered to be statistically significant.
Results
The predominant bacteria obtained from blood, liver, spleen and MLNs samples were Escherichia coli (29%) Klebsiella spp. (21%), Enterobacter cloaca (13%), Proteus mirabilis (6%), Enterococcus faecalis (3%) and mixed cultures (28%). The effects of the treatments on bacterial translocation are presented in Figure 1. Bacterial translocation rates were 0% in the sham-operated group, 83% in group 2, 42% in group 3, 42% in group 4 and 33% in group 5. Bacterial translocation significantly increased in group 2 compared to groups 3, 4 and 5 (P = 0.001). There was no significant difference among groups 3, 4 and 5 for bacterial translocation rates (P > 0.05). The bacterial counts (CFU/g) of group 2 were significantly higher than those of groups 3, 4 and 5 (P = 0.001; Table 1).
Figure 1.
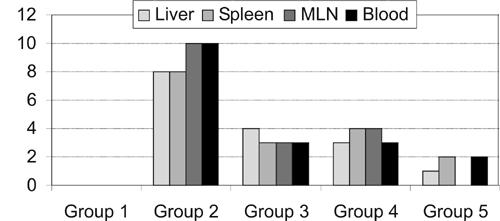
The number of rats with bacterial translocation for each group.
Table 1.
Log colony forming unit (CFU) ratios in the second group and treated groups
| Group 2 LogCFU | Group 3 LogCFU | Group 4 LogCFU | Group 5 LogCFU | |
|---|---|---|---|---|
| 1 | 5.9 | 5.3 | 5.1 | 3.7 |
| 2 | 5.8 | 3.8 | 4.7 | 3.8 |
| 3 | 6.1 | 3.3 | 2.6 | 3.0 |
| 4 | 4.3 | 5.0 | 2.9 | 3.2 |
| 5 | 5.0 | 4.6 | 3.5 | 0 |
| 6 | 5.5 | 0 | 0 | 0 |
| 7 | 5.8 | 0 | 0 | 0 |
| 8 | 5.6 | 0 | 0 | 0 |
| 9 | 5.7 | 0 | 0 | 0 |
| 10 | 5.8 | 0 | 0 | 0 |
| 11 | 0 | 0 | 0 | 0 |
| 12 | 0 | 0 | 0 | 0 |
| Mean | 4.6 | 1.8 | 1.6 | 1.1 |
| (± SD) | (± 2.2) | (± 2.3) | (± 2.1) | (± 1.7) |
Serum bilirubin levels, alkaline phosphatase (ALP), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) activities were significantly higher in groups 2, 3, 4 and 5 compared to group 1 (P < 0.001); however, there was no significant difference among groups 2, 3, 4 and 5 (P > 0.05; Table 2). The mean ileal villus heights in the sham-operated group, and groups 2, 3, 4 and 5 were 484.5 ± 32.1 μm, 328.1 ± 22.7 μm, 442.8 ± 29.7 μm, 344.2 ± 34.5 μm and 462.7 ± 29.6 μm, respectively. Histopathological examination of ileum specimens revealed a significant decrease in the heights of villi in groups 2–5 compared to the sham-operated group (P = 0.001). The mean villus height in groups 3 and 5 was significantly higher than that of group 4 (P = 0.001). However, there was no statistically significant difference between group 3 and group 5 for mean villus height (P> 0.05).
Table 2.
The average of serum bilirubin, ALT, AST and ALP values of the groups
| Bilirubin (mg/dl) | ALT (IU/l) | AST (IU/l) | ALP (IU/l) | |
|---|---|---|---|---|
| Group 1 | 0.4 ± 0.3* | 69.7 ± 14.9* | 65.9 ± 12.2* | 99.6 ± 13.4* |
| Group 2 | 8.8 ± 0.9 | 360 ± 15.6 | 402.4 ± 48.4 | 384.3 ± 20.1 |
| Group 3 | 8.7 ± 0.8 | 358.8 ± 15.1 | 421.9 ± 17.5 | 379.9 ± 21.2 |
| Group 4 | 8.5 ± 0.6 | 353.5 ± 16.4 | 407.4 ± 27.2 | 378.0 ± 22.8 |
| Group 5 | 8.9 ± 0.7 | 371.5 ± 16.3 | 414.1 ± 32.6 | 384.5 ± 20.5 |
P = 0.000 group 1 compared with groups 2–5.
Discussion
Infectious complications such as biliary sepsis, wound infections, intra-abdominal abscess formation, and renal failure frequently occur during obstructive jaundice.2,3,13,14 Bacterial translocation from the intestinal mucosal barrier implicated in the pathophysiology of complications has been associated with obstructive jaundice.3,4 Several factors have been proposed as promoters of bacterial translocation. These include alterations in gastrointestinal microbiota, impairment of gut barrier function, and deficiencies in the host immunity.15,16 Experimental obstructive jaundice induces regional loss of occluding expression in the intestinal epithelium, which may be a key factor contributing to the disruption of the mucosal barrier.17 Treatment with growth hormone and insulin-like growth factor I in rats with experimental obstructive jaundice has been shown to reduce endotoxin and to improve liver histopathology.13 Intestinal absorption of endotoxin is increased in patients with obstructive jaundice that causes endotoxaemia in the portal blood and depression of reticulo-endothelial functions.18
Many authors have studied the effects of different drugs on preventing bacterial translocation in animal models of obstructive jaundice. Berg et al.19 demonstrated that a combination of antibiotics and immunosuppressive drugs promotes the systemic spread of bacterial translocation, resulting in lethal sepsis. According to Reid et al.,20 bacterial colonisation or infection of the intestine by bacteria such as Escherichia, Clostridium, Klebsiella, Salmonella, Shigella, Campylobacter, Pseudomonas, Streptococcus, Enterococcus, Staphylococcus aureus, and coagulase-negative staphylococci increases the risk of necrotising enterocolitis. Bile salts are known to inhibit the growth of intestinal bacteria and may contribute to the regulation of the indigenous gut microbiota. Absence of intraluminal bile salts and their anti-endotoxic effects may result in overgrowth of bacteria.2,3 In the present study, we also showed significant increase in bacterial translocation in the liver, spleen, MLNs and blood as well as significant reduction in mean villus height in jaundiced rats. Bacterial translocation after obstructive jaundice may be due to the inhibition of bile salts, reduction of villus height or disruption of the ecological balance of the normal indigenous microbiota.
S. boulardii is a yeast presently used as a lyophilised powder in the prevention and treatment of diarrhoea associated with antibiotic use.21 S. boulardii has been used in the treatment of intestinal disorders in recent studies.22–25 Berg et al.19 reported that bacterial translocation is caused by oral antibiotics due to the disruption of the gastrointestinal ecological equilibrium, leading to intestinal overgrowth. However, they also reported that S. boulardii is widely used as a probiotic which decreases the incidence of Candida albicans translocation to the MLNs, liver, and kidneys. The mucosal damage in the gut may increase the invasion of bacteria through the disrupted mucosal barrier; it is possible that the suppression of bacterial translocation is simply caused by a decrease in the severity of intestinal lesions induced by S. boulardii.
Kakkos et al.26 reported that administration of non-absorbable antibiotics had a positive effect on bacterial and endotoxin translocation after extended hepatectomy, and related this to reduction of colonic bacterial load as an intraluminal effect of antibiotics. Our study has demonstrated that ampicillin-sulbaktam administration reduces the level of bacterial translocation. In addition, S. boulardii also has shown a significant suppression against the increase of bacterial translocation as well as ampicillin-sulbaktam and additionally S. boulardii preserved intestinal mucosal integrity. It should be noted from the present study that both S. boulardii and ampicillin-sulbaktam exhibited a significant suppression of bacterial translocation.
Morphometric evidence of ileal mucosal injury with reduction in villus height and total thickness in jaundiced rats have also been reported.10,27 The effect of S. boulardii on intestinal trophic architecture in pigs was indicated by increased villus length in the small intestine.28 In this study, mean villus height in groups 1, 3 and 5 was higher than that of group 2. Obstructive jaundice may contribute to the breakdown of gastrointestinal barrier functions, thus promoting bacterial translocation. Additionally, bacterial overgrowth can promote bacterial translocation. This study showed that S. boulardii preserved mucosal integrity. On the other hand, the effect of S. boulardii may inhibit overgrowth of pathogenic organisms.
Conclusions
The administration of S. boulardii to rats suffering from obstructive jaundice is effective in the successful control of translocation and improvement of intestinal barrier function. S. boulardii is a non-toxic preparation and has been found to be experimentally effective in decreasing bacterial translocation. Further studies are needed for use in humans.
Acknowledgments
No financial support or benefits were received by any author.
References
- 1.Berg RD. Bacterial translocation from the gastrointestinal tract. Trends Microbiol. 1995;3:149–54. doi: 10.1016/s0966-842x(00)88906-4. [DOI] [PubMed] [Google Scholar]
- 2.Deitch EA. Bacterial translocation of the gut flora. J Trauma. 1990;30:184–9. doi: 10.1097/00005373-199012001-00037. [DOI] [PubMed] [Google Scholar]
- 3.Wells CL, Maddaus MA, Simmons RL. Proposed mechanisms for the translocation of intestinal bacteria. Rev Infect Dis. 1988;10:958–79. doi: 10.1093/clinids/10.5.958. [DOI] [PubMed] [Google Scholar]
- 4.Wang XD, Parsson H, Andersson R, Soltesz V, Johansson K, Bengmark S. Bacterial translocation intestinal ultrastructure and cell membrane permeability early after major liver resection in the rat. Br J Surg. 1994;81:579–84. doi: 10.1002/bjs.1800810434. [DOI] [PubMed] [Google Scholar]
- 5.Buts JP, De Keyser N, Stilmant C, Sokal E, Marandi S. Saccharomyces boulardii enhances N-terminal peptide hydrolysis in suckling rat small intestine by endoluminal release of a zinc-binding metalloprotease. Pediatr Res. 2002;51:528–34. doi: 10.1203/00006450-200204000-00021. [DOI] [PubMed] [Google Scholar]
- 6.McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Moyer KA, Melcher SA, et al. Prevention of beta-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am J Gastroenterol. 1995;90:439–48. [PubMed] [Google Scholar]
- 7.Brandao RL, Castro IM, Bambirra EA, Amaral SC, Fietto LG, Tropia MJ, et al. Intracellular signal triggered by cholera toxin in Saccharomyces boulardii and Saccharomyces cerevisiae. Appl Environ Microbiol. 1998;64:564–8. doi: 10.1128/aem.64.2.564-568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buts JP, Bernasconi P, Vaerman JP, Dive C. Stimulation of secretory IgA and secretory component of immunoglobulins in small intestine of rats treated with Saccharomyces boulardii. Dig Dis Sci. 1990;35:251–6. doi: 10.1007/BF01536771. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues AC, Cara DC, Fretez SH, Cunha FQ, Vieira EC, Nicoli JR, et al. Saccharomyces boulardii stimulates sIgA production and the phagocytic system of gnotobiotic mice. J Appl Microbiol. 2000;89:404–14. doi: 10.1046/j.1365-2672.2000.01128.x. [DOI] [PubMed] [Google Scholar]
- 10.Aldemir M, Kokoglu OF, Geyik MF, Buyukbayram H. Effects of octreotide acetate and Saccharomyces boulardii on bacterial translocation in an experimental intestinal loop obstruction model of rats. Tohoku J Exp Med. 2002;198:1–9. doi: 10.1620/tjem.198.1. [DOI] [PubMed] [Google Scholar]
- 11.Buts JP, Bernasconi P, Van Craynest MP, Maldague P, De Meyer R. Response of human and rat small intestinal mucosa to oral administration of Saccharomyces boulardii. Pediatr Res. 1986;20:192–6. doi: 10.1203/00006450-198602000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Peret Filho LA, Penna FJ, Bambirra EA, Nicoli JR. Dose effect of oral Saccharomyces boulardii treatments on morbidity and mortality in immunosuppressed mice. J Med Microbiol. 1998;47:111–6. doi: 10.1099/00222615-47-2-111. [DOI] [PubMed] [Google Scholar]
- 13.Scopa CD, Koureleas S, Tsamandas AC, Spiliopoulou I, Alexandrides T, Filos KS, et al. Beneficial effects of growth hormone and insulin-like growth factor I on intestinal bacterial translocation, endotoxemia, and apoptosis in experimentally jaundiced rats. J Am Coll Surg. 2000;190:423–31. doi: 10.1016/s1072-7515(99)00285-9. [DOI] [PubMed] [Google Scholar]
- 14.Sileri P, Morini S, Sica GS, Schena S, Rastellini C, Gaspari AL, et al. Bacterial translocation and intestinal morphological findings in jaundiced rats. Dig Dis Sci. 2002;47:929–34. doi: 10.1023/a:1014733226337. [DOI] [PubMed] [Google Scholar]
- 15.Parks RW, Stuart Cameron CH, Gannon CD, Pope C, Diamond T, Rowlands BJ. Changes in gastrointestinal morphology associated with obstructive jaundice. J Pathol. 2000;192:526–32. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH787>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 16.Parks RW, Clements WD, Smye MG, Pope C, Rowlands BJ, Diamond T. Intestinal barrier dysfunction in clinical and experimental obstructive jaundice and its reversal by internal biliary drainage. Br J Surg. 1996;83:1345–9. doi: 10.1002/bjs.1800831007. [DOI] [PubMed] [Google Scholar]
- 17.Assimakopoulos SF, Scopa CD, Charonis A, Spiliopoulou I, Georgiou C, Nikolopoulou V, et al. Experimental obstructive jaundice disrupts intestinal mucosal barrier by altering occludin expression: beneficial effect of bombesin and neurotensin. J Am Coll Surg. 2004;198:748–57. doi: 10.1016/j.jamcollsurg.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Unal AE, Cevikel MH, Ozgun H, Tunger A. Effect of granulocyte-macrophage colony stimulating factor on bacterial translocation after experimental obstructive jaundice. Eur J Surg. 2001;167:366–70. doi: 10.1080/110241501750215267. [DOI] [PubMed] [Google Scholar]
- 19.Berg RD, Wommack E, Deitch EA. Immunosuppression and intestinal bacterial overgrowth synergistically promote bacterial translocation. Arch Surg. 1988;123:1359–64. doi: 10.1001/archsurg.1988.01400350073011. [DOI] [PubMed] [Google Scholar]
- 20.Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev. 2003;16:658–72. doi: 10.1128/CMR.16.4.658-672.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McFarland LV, Bernasconi P. Saccharomyces boulardii A review of an innovative biotherapeutic agent. Microb Ecol Health Dis. 1993;6:157–71. [Google Scholar]
- 22.Herek O, Kara IG, Kaleli I. Effects of antibiotics and Saccharomyces boulardii on bacterial translocation in burn injury. Surg Today. 2004;34:256–60. doi: 10.1007/s00595-003-2677-1. [DOI] [PubMed] [Google Scholar]
- 23.Johnson S, Gerding DN. Clostridium difficile-associated diarrhea. Clin Infect Dis. 1998;26:1027–34. doi: 10.1086/520276. [DOI] [PubMed] [Google Scholar]
- 24.Zaouche A, Loukil C, Lagauise PD, Peuchmaur M, Macry J, Fitoussi F, et al. Effects of oral Saccharomyces boulardii on bacterial overgrowth, translocation, and intestinal adaptation after small-bowel resection in rats. Scand J Gastroenterol. 2000;2:160–5. doi: 10.1080/003655200750024326. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigues AC, Nardi RM, Bambirra EA, Vieira EC, Nicoli JR. Effect of Saccharomyces boulardii against experimental oral infection with Salmonella typhimurium and Shigella flexneri in conventional and gnotobiotic mice. J Appl Bacteriol. 1996;81:251–6. doi: 10.1111/j.1365-2672.1996.tb04325.x. [DOI] [PubMed] [Google Scholar]
- 26.Kakkos SK, Kirkilesis J, Scopa CD, Arvaniti A, Alexandrides T, Vagianos CE. Nonabsorbable antibiotics reduce bacterial and endotoxin translocation in hepatectomised rats. HPM Surg. 1997;10:283–9. doi: 10.1155/1997/49681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aldemir M, Geyik MF, Kokoglu OF, Buyukbayram H, Hosoglu S, Yagmur Y. Effects of ursodeoxycholic acid, glutamine and polyclonal immunoglobulins on bacterial translocation in common bile duct ligated rats. Aust NZ J Surg. 2003;73:722–6. doi: 10.1046/j.1445-2197.2003.02749.x. [DOI] [PubMed] [Google Scholar]
- 28.Kamm K, Hoppe S, Breves G, Schroder B, Schemann M. Effects of the probiotic yeast Saccharomyces boulardii on the neurochemistry of mysenteric neurones in pig jejunum. Neurogastroenterol Motil. 2004;16:53–60. doi: 10.1046/j.1365-2982.2003.00458.x. [DOI] [PubMed] [Google Scholar]


