Abstract
INTRODUCTION
It is believed that increased detection of earlier stage colorectal cancer can only be achieved by screening asymptomatic individuals. We describe a referral pathway for a symptomatic population which achieves a 30% Dukes' A detection rate.
PATIENTS AND METHODS
From October 1999, 4253 patients with distal colonic symptoms, referred by general practitioners, completed a patient consultation questionnaire (PCQ) linked to a computerised record. A weighted numerical score (WNS) was derived for each patient. Patients underwent flexible sigmoidoscopy, a diagnostic outcome was recorded and later Dukes' stage appended. Early and advanced colorectal cancers were separated and PCQ derived symptom profiles compared. Chi-square, Fisher exact, Student's t-test and logistic regression were used for statistical analysis.
RESULTS
A total of 183 patients had cancer, 55 (30%) were Dukes' A early colorectal cancers, 112 were advanced colorectal cancers (Dukes' B–D) and 16 could not be staged. Early colorectal cancers had significant symptoms and comparable profile to advanced colorectal cancers. The tendency in advanced colorectal cancers was towards greater symptom prevalence for only a few primary and systemic symptoms, as reflected by a higher WNS of 75 (P = 0.001)
CONCLUSIONS
Early colorectal cancers do have significant symptoms which can easily be captured by a PCQ and objective scoring tool in the secondary care setting. Detection of these cancers has the potential to improve survival.
Keywords: Early colorectal cancer, Symtomatology, Patient consultation questionnaire
Colorectal cancer is the second most common cancer in the UK, with 30,000 new cases and 19,000 deaths per annum.1–3 The 5-year survival rates are generally lower here than in any other European country.4 It is generally believed that diagnosis of colorectal cancer at an earlier stage improves outcome. However, most studies have failed to prove the temporal relationship between earlier diagnosis and an improvement in survival.5
Theoretical models suggest cancer passes through three phases, an invisible asymptomatic phase, followed by a visible asymptomatic phase and lastly the symptomatic phase,6 considered indicative of advanced disease.5 Contrary to this, we have shown that early colorectal cancers do in fact present with significant symptoms and with a profile comparable to advanced disease. Though, it is not individual symptoms which are suggestive of neoplastic pathology, as each alone has a low predictive value,1 but symptom clusters.
An individual symptom per se is often perceived to be a common experience and rarely synonymous with a particular disease.8 Consequently, separating the wheat from the chaff may be troublesome. Colorectal cancer, on the other hand, has been shown to present with primary symptoms,2,3 attributable to the tumour at its primary site.1 These symptoms include rectal bleeding, change in bowel habit and abdominal pain. They may also present with systemic symptoms – anorexia, significant weight loss, fatigue and symptoms of anaemia – features usually suggestive of advanced disease.5 The combination of rectal bleeding and a change in bowel habit, or rectal bleeding in the absence of peri-anal symptoms is believed to be a common mode of presentation,5 but this still has been shown to have limited discriminate value.1
As we have previously described an effective tool to help predict risk of colorectal cancer in a referred population,1 the aim of this study was to assess the symptomatic presentation of early cancers and look at symptom complexes which may differentiate early from advanced disease.
Patients and Methods
A prospective study was undertaken between October 1999 and June 2003. All patients with primary bowel symptoms were referred by general practitioners to a dedicated surgical colorectal assessment clinic. Prior to assessment, all patients completed a detailed questionnaire which accurately recorded a comprehensive history of bowel symptoms and related factors. This information was entered into a colorectal database and formed part of the patient record. On the basis of these symptoms, a Weighted Numerical Score (WNS) was automatically calculated by the computer program. The WNS is derived from the subjective weighting of symptoms and symptom complexes in relation to the likelihood of cancer outcome.1
All patients were seen in the assessment clinic and underwent a minimum investigation of flexible sigmoidoscopy. A diagnostic outcome was recorded or later appended on completion of further tests. Patients with high-risk polyps went on to have a completion colonoscopy; those with cardinal symptoms and negative flexible sigmoidoscopy or iron-deficiency anaemia had visualisation of the right side of the colon. Outcomes were validated against the hospital cancer records.
For the purpose of analysing symptomatology, the cancers were separated into two study groups – early colorectal cancers, Dukes' A; and advanced colorectal cancer, Dukes' B, C, and D.
Symptom profiles were compared between the two groups. Proportions were compared using either chi-squared or the Fisher exact test where appropriate, whereas difference in WNS was compared using the Student's t-test. Stepwise logistic regression was used to assess for the presence of symptom complexes that may differentiate between the two groups.
Results
In all, 4253 new referrals were seen in the colorectal assessment clinic during the time period; 183 of these had colorectal cancer (4.3%). Fifty-five patients had early colorectal cancers (30%), 46 Dukes' B (25%), 43 Dukes' C (23%), 23 Dukes' D (13%) and 16 ‘others’ were not staged (9%) as summarised in Table 1. The ‘others’ consisted of 12 patients unsuitable for resection (either because of significant co-morbidity or presumed advanced stage in the elderly unfit and, therefore, could not be staged histologically), 2 large bowel lymphomas, 1 squamous cell carcinoma in situ, and another proved unstageable due to the effects of adjuvant radiotherapy. Of the 55 patients with early colorectal cancers, 48 had T2 tumours, 7 had T1 tumours and 4 patients were down-staged histopathologically to T2 following short-course neo-adjuvant radiotherapy.
Table 1.
The study group
| Dukes' stage | n | Sex | Median age (years) | % |
|---|---|---|---|---|
| A | 55 | 34 M | 69 | 30 |
| 21 F | 70 | |||
| B | 46 | 31 M | 67 | 25 |
| 15 F | 69 | |||
| C | 43 | 28 M | 67 | 23 |
| 15 F | 64 | |||
| D | 23 | 11 M | 70 | 13 |
| 10 F | 69 | |||
| Other | 16 | 9 M | 72 | 9 |
| 7 F | 80 |
A greater number of men than women had colorectal cancer; 115 men (63%), median age 68 years (range, 25–88 years) and 68 women (37%), median 69 years (range, 33–93 years). There was no statistical difference between distribution amongst gender and age by Dukes' stage (P = 0.81).
Significant primary and systemic symptoms were present in both cancer groups (Table 2). In the early cancer group, 89% had rectal bleeding, 58% a change in bowel habit and 24% had abdominal pain, as compared to the advanced group, where abdominal pain (P = 0.001) and change in bowel habit (P < 0.001) were more common and reached significance. Systemic symptoms, decreased appetite and tiredness, were evenly distributed between both groups; though unexplained weight loss was not significant, there was a tendency towards this in the advanced colorectal cancers group (P = 0.17). A high proportion of patients in both the advanced and early groups had associated peri-anal symptoms, 40% and 45%, respectively, though only 2 patients with advanced colorectal cancers and 3 with early colorectal cancers had associated benign anorectal pathology.
Table 2.
Symptom profile for early and advanced colorectal cancers
| Early colorectal cancers Dukes' stage A (n = 55) | Advanced colorectal cancers Dukes' stages B–D (n = 112) | Significance (P) | |
|---|---|---|---|
| Symptom duration | < 4 weeks (33%) | < 4 weeks (19%) | |
| > 4 weeks (77%) | > 4 weeks (81%) | ||
| WNS (Weighted Numerical Score) | 612 | 75 | 0.001 |
| Blood PR | 49 (89%) | 91 (81%) | 0.2 |
| Change in bowel habits | 32 (58%) | 97 (87%) | < 0.001 |
| Abdominal pain | 13 (24%) | 53 (47%) | 0.001 |
| Peri-anal symptoms | 22 (45%) | 45 (40%) | 0.95 |
| Weight loss | 10 (18%) | 31 (28%) | 0.17 |
| Decreased appetite | 9 (16%) | 18 (16%) | 0.98 |
| Tiredness | 14 (25%) | 34 (30%) | 0.56 |
Of all cancers, 95% were distal and within reach of a flexible sigmoidoscope. The distribution of early and advanced carcinomas is summarised in Figure 1. Patients with proximal bowel cancers were usually advanced, had associated iron-deficiency anaemia and detected either following colonoscopy or barium enema. Of the 2 early cancers, one was sited in the distal transverse colon, the other in the ascending colon.
Figure 1.
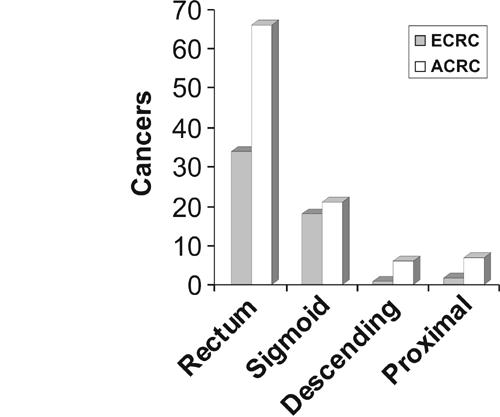
The distribution of early and advanced carcinomas.
Fresh/bright bleeding appeared to be the only individual symptom more likely associated with early colorectal cancers (78%) as opposed to advanced colorectal cancers (56%; P = 0.02. Although this was generally separate from the stool and low volume in over half of the patients, the associated symptom profiles were similar to the advanced group (Table 3).
Table 3.
Bleeding type with associated symptoms
| Type of bleeding | Dukes' A(e) | Dukes' B,C,D (a) | Bleeding description | No. with bleeding | Bleeding volume | Bleeding + change in bowel habit | Looseness | Increased frequency | Urgency | Mucus | Incomplete emptying | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | |||||||||||
| Fresh/bright | 38/49 (78%) | 51/91 (56%) | Separate | (e) 22 | (e) 19 | 3 | (e) 11 | (e) 6 | (e) 6 | (e) 5 | (e) 2 | (e) 4 |
| (a) 21 | (a) 20 | 1 | (a) 16 | (a) 11 | (a) 11 | (a) 5 | (a) 7 | (a) 6 | ||||
| P = 0.17 | P = 0.63 | P = 0.14 | P = 0.17 | P = 0.17 | P = 0.78 | P = 0.10 | P = 0.66 | |||||
| P = 0.02 | Mixed | (e) 7 | (e) 7 | 0 | (e) 4 | (e) 1 | (e) 1 | (e) 0 | (e) 0 | (e) 0 | ||
| (a) 12 | (a) 12 | 0 | (a) 9 | (a) 6 | (a) 6 | (a) 2 | (a) 4 | (a) 1 | ||||
| Separate + mixed | (e) 2 | (e) 2 | 0 | (e) 1 | (e) 0 | (e) 0 | (e) 0 | (e) 0 | (e) 0 | |||
| (a) 7 | (a) 6 | 1 | (a) 7 | (a) 6 | (a) 5 | (a) 4 | (a) 4 | (a) 5 | ||||
| Unspecififed | (e) 7 | (e) 3 | 4 | (e) 5 | (e) 5 | (e) 5 | (e) 2 | (e) 4 | (e) 0 | |||
| (a) 11 | (a) 11 | 0 | (a) 10 | (a) 7 | (a) 6 | (a) 4 | (a) 3 | (a) 4 | ||||
| Fresh + old | 9/49 (18%) | 23/91 (25%) | Separate | (e) 1 | (e) 1 | 0 | (e) 1 | (e) 1 | (e) 1 | (e) 1 | (e) 1 | (e) 1 |
| (a) 3 | (a) 3 | 0 | (a) 3 | (a) 2 | (a) 2 | (a) 2 | (a) 1 | (a) 1 | ||||
| P = 0.47 | Mixed | (e) 3 | (e) 3 | 0 | (e) 2 | (e) 1 | (e) 1 | (e) 1 | (e) 1 | (e) 1 | ||
| (a) 6 | (a) 6 | 0 | (a) 6 | (a) 4 | (a) 4 | (a) 4 | (a) 4 | (a) 3 | ||||
| Separate + mixed | (e) 4 | (e) 3 | 1 | (e) 3 | (e) 1 | (e) 1 | (e) 2 | (e) 3 | (e) 1 | |||
| (a) 11 | (a) 9 | 2 | (a) 10 | (a) 4 | (a) 3 | (a) 3 | (a) 3 | (a) 3 | ||||
| Unspecified | (e) 1 | (e) 1 | 0 | (e) 0 | (e) 0 | (e) 0 | (e) 0 | (e) 0 | (e) 0 | |||
| (a) 3 | (a) 2 | 1 | (a) 3 | (a) 3 | (a) 2 | (a) 1 | (a) 2 | (a) 1 | ||||
| Dark/altered | 2/49 (4%) | 8/91 (9%) | Separate | (e) 0 | (e) 0 | 0 | (e) 0 | (e) 0 | (e) 0 | (e) 0 | (e) 0 | (e) 0 |
| (a) 1 | (a) 1 | 0 | (a) 1 | (a) 0 | (a) 0 | (a) 0 | (a) 0 | (a) 0 | ||||
| P = 0.67 | Mixed | (e) 2 | (e) 2 | 0 | (e) 2 | (e) 1 | (e) 1 | (e) 0 | (e) 1 | (e) 0 | ||
| (a) 3 | (a) 2 | 1 | (a) 3 | (a) 3 | (a) 3 | (a) 1 | (a) 2 | (a) 3 | ||||
| Separate + mixed | (e) 0 | (e) 0 | 0 | (e) 0 | (e) 0 | (e) 0 | (e) 0 | (e) 0 | (e) 0 | |||
| (a) 0 | (a) 0 | 0 | (a) 0 | (a) 0 | (a) 0 | (a) 0 | (a) 0 | (a) 0 | ||||
| Unspecified | (e) 0 | (e) 0 | 0 | (e) 0 | (e) 0 | (e) 0 | (e) 0 | (e) 0 | (e) 0 | |||
| (a) 4 | (a) 4 | 0 | (a) 4 | (a) 4 | (a) 4 | (a) 2 | (a) 2 | (a) 4 | ||||
10 patients in the advanced group did not specify bleeding type.
When stepwise logistic regression was used to assess for a pattern of symptoms that may differentiate early from advanced cancer, the only factors found to be predictive of advanced disease were increased frequency of motion and abdominal pain (Table 4). However, overall, the regression model had a weak correlation coefficient (r2 = 0.17).
Table 4.
Forward stepwise logistic regression analysis
| Regression coefficient | Odds ratio | 95% Confidence limit | P-value | |
|---|---|---|---|---|
| Increased frequency | 1.42835 | 4.171808 | 2.0–8.6 | 0.000122 |
| Abdominal pain | 0.829582 | 2.292359 | 1.0–5.1 | 0.039574 |
| Bright blood Pr | −0.59131 | 0.553602 | 0.2–1.3 | 0.155779 |
| Blood separate | −0.72156 | 0.485993 | 0.2–1.1 | 0.0961 |
The WNS, an algorithm for symptoms and symptom interaction (symptom severity), clearly demonstrates that early colorectal cancers do have significant symptoms, with a high mean WNS (61), whilst advanced carcinomas had significantly higher WNS (75, P = 0.001). The WNS for benign disease, with the exception of colitis and moderate-to-severe diverticular disease was below 50 (Fig. 2).
Figure 2.
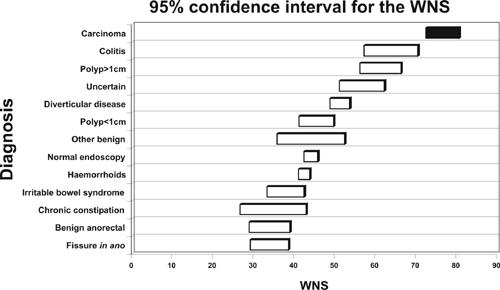
95% confidence intervals for the WNS.
The 12 patients excluded from the study, because they were unsuitable for surgical resection, also had significant symptomatology. In this group, there were 7 men and 5 women with a median age of 80 years and WNS of 91.
Discussion
The diagnosis of earlier stage disease has the potential to improve survival, with 5-year survival rates for Dukes' A being reported in excess of 90%. The challenge, therefore, must be to reduce the number of cancers presenting late and swing the pendulum in favour of early cancer detection. Screening per se is proclaimed the idealistic goal, as it aims to detect tumours at an early pre-invasive phase and thus prevent malignant transformation by their timely removal. This rationale is underpinned by Fearon and Volgestien's adenoma–carcinoma sequence,9 a multistep genetic model for which mutations in APC, K-ras and p53 are the cornerstones. Although it has been widely validated, only 7% of colorectal cancers harbour all three mutations, an observation which leads us to question its value. Certainly, there is a body of evidence to suggest alternative modalities of pathogenesis may be as important. Namely the emergence of de novo carcinogenesis and the flat adenoma–carcinoma sequence,10–13 which is now gaining recognition in the West. Alternative pathways also include micosatellite instability,14 predominant in proximal neoplasia and the serrated polyp theory, initiated by BRAF gene mutations.15 It is clear that we need to adopt a multipathway approach to understanding colorectal carcinogenesis, but whether this will impact on screening modalities remains to be seen. To date, one of the few studies to provide indirect evidence for the efficacy of colonoscopic polypectomy in reducing the incidence of colorectal cancer is the National Polyp Study.16 However, there are still no absolute criteria which can predict with certainty adenoma progression to cancer or recurrence.
Despite a wealth of knowledge in the field of genetics and molecular biology of colorectal cancer, there is a paucity of information with respect to symptomatology. With this in mind, a pragmatic approach maybe to try first identifying the prevalence of unreported symptoms within the primary care population, or indeed incorporate an accurate assessment of symptomatology into future screening modalities. After all, it is well documented that most colorectal cancers present with symptoms.2,3,19 In the UK, it is thought that up to 85% of cancers are diagnosed during the investigation of symptoms.20 The series of Gilbert et al.23 studying 449 relatives of patients with colorectal cancer who underwent colonoscopic screening, found 80% of those with cancer and 50% with adenomas greater than 5 mm already had bowel symptoms. Our results concur with this in the advanced and early cancer groups alike with respect to both primary and systemic symptoms. However, change in bowel habit towards loose motion and abdominal pain had a significant association with advanced disease (P < 0.001, P = 0.001, respectively). Conversely, systemic symptoms were distributed amongst both groups with similar proportions (Table 2) and thus appear to not necessarily reflect on advancing stage and tumour load. Likewise, symptom duration was similar in both early and advanced groups, with the majority of patients documenting symptoms lasting greater than 4 weeks’ duration. This underscores observations from previous studies in that symptom duration may not correlate with stage.4,17,18,21 Of particular interest, a large number of patients in both the early and advanced cancer groups had associated peri-anal symptoms, 45% and 40%, respectively – traditionally symptoms ‘protective’ of a diagnosis of colorectal cancer. It is important, therefore, to be aware that the presence of peri-anal symptoms does not obviate the need for a thorough examination beyond the anal canal and reach of a proctoscope.
At the primary care interface, managing symptoms is a task shrouded in difficulty. Not least helped by the heterogeneous nature in which present guidelines are applied, it is compounded by reports that a large number of patients in the community already have primary symptoms but benign disease.8,22 However, we should remember that these symptoms, in isolation, are neither sensitive nor specific, but together with other symptom combinations or clusters impact more on the likelihood of cancer risk.1 Symptom complexes or clusters are of paramount importance when assessing colorectal disease. Previous work by Majumber et al.19 suggested that certain symptoms share a common pathophysiology and, hence, occurred in clusters. Analysis of our own work supports this concept, as the WNS, an objective score and marker of symptom severity, is significantly higher in both early and advanced colorectal cancer and can help differentiate benign from malignant disease.
We have recognised that detailing and recording symptoms and symptom clusters is fundamental when assessing colorectal disease. Combining this with a mechanism to capture accurate information in conjunction with a dedicated referral pathway has enabled us to demonstrate significant symptomatology in early cancers and attain a detection rate for Dukes' A of 30%. A dedicated questionnaire completed by patients prior to assessment overcomes the problem of obtaining a detailed and comprehensive history and, when combined with a weighted scoring system, can provide an objective tool with high discriminatory power. However, it is clear that further studies are required and on a much larger scale to determine the overall efficacy of such a tool in its application to symptomatic patients in the community.
References
- 1.Selvachandran SN, Hodder R, Ballal M, Jones P, Cade D. Prediction of colorectal cancer by a patient consultation questionnaire and scoring system: a prospective study. Lancet. 2002;360:278–83. doi: 10.1016/s0140-6736(02)09549-1. [DOI] [PubMed] [Google Scholar]
- 2.Department of Health. Referral guidelines for suspected cancer. London: Department of Health, Health Service Circular; Nov 1999. pp. 1–19. HSC 1999/241. [Google Scholar]
- 3.The Royal College of Surgeons of England. Guidelines for the management of colorectal cancer. London: RCSE; 1996. [Google Scholar]
- 4.Kiran PR, Glass RE. Duration of symptoms and spread of colorectal cancer: a short history does not mean early disease. Ann R Coll Surg Engl. 2002;84:381–5. doi: 10.1308/003588402760978157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson M. Earlier symptomatic diagnosis of colorectal cancer. Colonews. 1999;8:3. [Google Scholar]
- 6.Sackett DL, Haynes RB, Guyatt GH, Tugwell P. Clinical Epidemiology – A basic science for clinical medicine. 2nd edn. Boston: Little Brown; 1991. pp. 119–39. [Google Scholar]
- 7.Smith D, Hodder R, Ballal M, Selvachandran SN, Cade D. Challenging accepted concepts in the early presentation of colorectal cancer. Colorectal Dis. 2003;4(Suppl 1):0–41. [Google Scholar]
- 8.Anon Symptoms of possible oncological significance: separating the wheat from the chaff. BMJ. 2002;325:1254–5. doi: 10.1136/bmj.325.7375.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 10.Stlote M, Bethke B. Colorectal mini-de novo carcinoma: a reality in Germany too. Endoscopy. 1995;27:286–90. doi: 10.1055/s-2007-1005694. [DOI] [PubMed] [Google Scholar]
- 11.Shimoda T, Ikegami M, Fujisaka J, Matsui T, Aizawa S, Ishikawa E. Early colorectal cancer with special reference to its development de-novo. Cancer. 1989;64:1138–46. doi: 10.1002/1097-0142(19890901)64:5<1138::aid-cncr2820640529>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 12.Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy. 1993;25:455–61. doi: 10.1055/s-2007-1010367. [DOI] [PubMed] [Google Scholar]
- 13.Kosaka T. Clinico-pathological study of the minute elevated lesion of the colorectal mucosa. J Jpn Soc Coloproctol. 1975;28:218–26. [Google Scholar]
- 14.Wheeler JMD, Bodmer WF, Mortensen NJMcC. DNA mismatch repair genes and colorectal cancer. Gut. 2000;47:148–53. doi: 10.1136/gut.47.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kambara T, Simms LA, Whitehall VLJ, et al. BRAF mutation and CpG island methylation: an alternative pathway to colorectal cancer. Gut. 2004;53:1137–44. doi: 10.1136/gut.2003.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977–81. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 17.Barillari, et al. Relationship of symptom duration and survival in patients with colorectal carcinoma. Eur J Surg Oncol. 1989;15:441–5. [PubMed] [Google Scholar]
- 18.Clinical Outcomes Group Guidance on Commissioning Cancer Services. Improving Outcomes in Colorectal Cancer, The Research Evidence. London: NHS Executive; 1997. p. 26. [Google Scholar]
- 19.Sumit R, Majumdar M, Fletcher R, Evans A. How does colorectal cancer present? Symptoms, duration, and clues to location. Am J Gastroenterol. 1999;94:3040–5. doi: 10.1111/j.1572-0241.1999.01454.x. [DOI] [PubMed] [Google Scholar]
- 20.Speights VO, et al. Colorectal cancer. Current trends in initial clinical manifestations. South Med J. 1991;84:575–8. [PubMed] [Google Scholar]
- 21.Stubbs RS, Long MG. Symptom duration and pathologic staging of colorectal cancer. Eur J Surg Oncol. 1986;12:127–30. [PubMed] [Google Scholar]
- 22.Thompson J, Pond C, Ellis B, Beach A, Thompson M. Rectal bleeding in general and hospital practice; ‘tip of the iceberg'. Colorectal Dis. 2000;2:288–93. doi: 10.1046/j.1463-1318.2000.00141.x. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert J, Vaizey C, Cassel P, Holden J. Feasibility study of colonoscopy as the primary screening investigation in relatives of patients with colorectal cancer. Ann R Coll Surg Engl. 2001;83:415–9. [PMC free article] [PubMed] [Google Scholar]


