Abstract
The ability of Giardia lamblia to undergo two distinct differentiations in response to physiologic stimuli is central to its pathogenesis. The giardial cytoskeleton changes drastically during encystation and excystation. However, the signal transduction pathways mediating these transformations are poorly understood. We tested the hypothesis that PP2A, a highly conserved serine/threonine protein phosphatase, might be important in giardial differentiation. We found that in vegetatively growing trophozoites, gPP2A‐C protein localizes to basal bodies/centrosomes, and to cytoskeletal structures unique to Giardia: the ventral disk, and the dense rods of the anterior, posterior‐lateral, and caudal flagella. During encystation, gPP2A‐C protein disappears from only the anterior flagellar dense rods. During excystation, gPP2A‐C localizes to the cyst wall in excysting cysts but is not found in the wall of cysts with emerging excyzoites. Transcriptome and immunoblot analyses indicated that gPP2A‐C mRNA and protein are upregulated in mature cysts and during the early stage of excystation that models passage through the host stomach. Stable expression of gPP2A‐C antisense RNA did not affect vegetative growth, but strongly inhibited the formation of encystation secretory vesicles (ESV) and water‐resistant cysts. Moreover, the few cysts that formed were highly defective in excystation.
Thus, gPP2A‐C localizes to universal cytoskeletal structures and to structures unique to Giardia. It is also important for encystation and excystation, crucial giardial transformations that entail entry into and exit from dormancy.
Keywords: Giardia, encystation, excystation, PP2A, cytoskeleton, differentiation
1. Introduction
Differentiation from one life cycle stage into another is an elegant adaptation by which many parasites ensure their transmission and survival. The protozoan Giardia lamblia is a major cause of water‐borne diarrheal disease [1]. The pathogenesis of G. lamblia depends on its ability to undergo two very different differentiations [2]. Upon ingestion, the oval cysts pass through the stomach where the cyst wall protects the parasite from the gastric acid. In the small intestine each cyst quickly releases an excyzoite, which divides into two trophozoites. Cysts are non‐adherent and rapid excystation allows the parasite to attach to the intestinal epithelium to avoid being swept downstream in the intestine. Trophozoites are half‐pear shaped and are characterized by 2 nuclei, a ventral disk, and 8 flagella: 2 anterior, 2 posterior‐lateral, 2 ventral and 2 caudal. Each of the flagella is anchored to a basal body and traverses the cell body to emerge at distinct loci. Moreover, the intracellular portions of the flagella are accompanied by paraflagellar dense rods (PDR), whose functions are enigmatic [3]. Trophozoites that are carried downstream in the small intestine differentiate into the infectious cyst stage in order to survive outside the host. Encysting trophozoites undergo cytoskeletal remodeling leading to reduced adhesion, motility and cytokinesis, and are characterized by the presence of numerous encystation secretory vesicles (ESV) which transport proteins to the nascent cyst wall [4–6].
The ability of the complex giardial cytoskeleton to undergo dramatic remodeling throughout Giardia’s life cycle is central to its success as a parasite and makes it an excellent model for study of regulation of growth versus differentiation, as well as motility versus attachment across a broad spectrum of organisms. The external stimuli of encystation and excystation are known, and we can complete Giardia’s life cycle in vitro [2]. Nonetheless, the signal transduction pathways leading to the cytoskeletal changes during differentiation are largely unknown. Previously, it was shown that protein kinase A (PKA) and calmodulin both localize to the Giardia cytoskeleton and regulate different steps in the resumption of motility and cytokinesis during the cellular awakening of excystation [7], while PKA and ERK kinases have been implicated in encystation [8, 9]. These data suggest that specific cell signaling mechanisms may regulate cytoskeletal functions during differentiation.
In the current study, we tested the hypothesis that protein phosphatase 2A (PP2A) may be important in G. lamblia differentiation. Heterodimeric PP2A core enzyme is comprised of a catalytic C subunit (PP2A‐C) and a scaffolding A subunit (PP2A‐A). The heterotrimeric PP2A holoenzyme is formed by binding of various regulatory B subunits (B, B’, B”, B’”) to the core enzyme. The different B subunits are structurally unrelated and convey functional specificity to the core enzyme and target it to different cellular domains [10, 11]. The activity of PP2A‐C is further regulated by phosphorylation of Y‐307 and by C‐terminal carboxymethylation of L‐309 [12, 13]. PP2A‐C is one of the most highly conserved enzymes known and its expression is tightly regulated, underlining its essential roles. PP2A localizes to and regulates microtubule dynamics in a variety of cell types and its localization changes upon various stimuli [14–18]. Moreover, PP2A is involved in differentiation of Trypanosoma cruzi and Plasmodium falciparum [19–21]. Therefore, we asked whether PP2A‐C might localize to unique elements of the giardial cytoskeleton and be an important mediator of its differentiation.
Materials and Methods
2.1 Chemicals
All chemicals were purchased from Sigma‐Aldrich unless otherwise noted.
2.2. Cell culture and differentiation
Giardia trophozoites (strain WB, clone C6, ATCC 50803) were cultured in modified TYI‐S‐33 medium with bovine bile [22, 23]. Encystation and excystation were induced essentially as described [2]. Briefly, trophozoites were cultured 72 h in medium without bile at pH 7.2 and subsequently transferred to encystations medium containing 0.25 mg bovine bile/ml and 5 mM lactic acid (hemi‐calcium salt) at pH 7.8. At 21 h encystation, the numbers of ESV were counted microscopically in live trophozoites using differential interference contrast optics. Cysts were harvested after 48 h encystation from the bottom of the culture flasks and residual trophozoites were lysed by incubation in double‐distilled water for 20 min at 4 °C. Water resistant cysts were washed 3 times, counted and stored overnight in double distilled water at 4 °C. In stage 1 of excystation, cysts were exposed to an acidic‐reducing solution (57 mM L‐cysteine HCl, 32.5 mM reduced glutathione, 0.1 M NaHCO3, in Hank’s balanced salt solution, pH 2.0) for 30 min at 37 °C. In stage 2, acid‐treated cysts were washed and treated with trypsin in pH 8.0 bicarbonate‐buffered Tyrode’s solution for 1 h at 37 °C. Subsequently, excysting cysts were transferred to regular growth medium for 60 min at 37 °C. To test the role of gPP2A‐C in excystation, 10 µM okadaic acid (ammonium salt, Alexis Biochemicals) was added to all stages (Stage 1, Stage 2, growth medium) or to each stage separately and the number of excyzoites was compared to untreated excystations (in the presence of equal amounts of DMSO in the respective stages).
The human colon adenocarcinoma cell line HT‐29 (ATCC HTB 38) was grown in Dulbecco’s modified Eagle’s medium (Invitrogen) with 10% fetal calf serum and 1% penicillin‐streptomycin‐amphotericin B. Confluent cell layers were washed three times with PBS and lysed in PBS + 1% NP‐40. Cells were scraped from the bottom of the flask and sonicated for 15 s. Lysates were centrifuged at 13000 rpm for 10 min and supernatants were processed for Western blot analysis.
2.3. Immunofluorescence microscopy
Trophozoites were harvested by chilling and allowed to adhere to coverslips at 37°C for 10 min. Cytoskeletons were extracted in situ for 10 min with 0.5% Triton X‐100 in 10 mM Tris (pH 8.3), 2 mM EDTA, 2 mM MgCl2, 150 mM KCl, and 2 mM dithiotreitol [24]. Whole trophozoites were fixed in methanol and permeabilized for 10 min with 0.5% Triton X‐100 in PBS [7]. Water‐treated and excysting cysts (in Stages 1 and 2 and 30 and 60 min excystation) were dried on the coverslips. Subsequently, cytoskeletons, permeabilized trophozoites and cysts were blocked for 1 h in blocking buffer (5% goat serum, 1% glycerol, 0.1% bovine serum albumin, 0.1% fish gelatin and 0.04% sodium azide) and incubated for 1 h with the (non‐methylation sensitive) rabbit anti‐PP2A‐C polyclonal antibody directed against a synthetic peptide (KVTRRTPDYFL) corresponding to the C terminus of the human PP2A‐C subunit [25] or the mouse monoclonal antibody against CWP‐1 [26]. As a control for specificity, the Giardia C‐terminal PP2A‐C peptide ‘HISRRVPDYFL’ (GenScript Corporation) was pre‐incubated for 30 min with the PP2A‐C antibody. Subsequently coverslips were washed 4 times with PBS and trophozoites were incubated with secondary goat anti‐rabbit‐Alexa 568 or goat anti‐mouse‐Alexa 488 (Molecular Probes) and cysts with goat anti‐rabbit‐Texas‐Red (Zymed) conjugates. Coverslips were washed 4 times with PBS, postfixed with 4% paraformaldehyde, for 5 min, rinsed with PBS and mounted with Prolong Gold + 4′,6‐Diamino‐2‐phenylindole (Molecular Probes). Localization was monitored on a Nikon Eclipse E800 microscope with an X‐Cite™ 120 fluorescence lamp (Nikon Instruments Inc, NY).
2.4. mRNA quantification by SAGE
The RNA expression profiles of gPP2A‐C in the different stages of the Giardia life cycle, were examined by Serial Analysis of Gene Expression (SAGE) [27] as described by Palm et al. [5] and on the Giardia genome project website (www.mbl.edu/Giardia).
2.5. Western blot analysis
Trophozoites and cysts in the different stages of the life cycle were counted in a haemocytometer, washed with PBS and collected by centrifugation. Total cell protein was precipitated with 6% TCA [28], and 20 µg/lane (=5×105 cells) were separated by SDS‐polyacrylamide gel electrophoresis on 4–20% Tris‐glycine gels (Invitrogen) and transferred to PVDF (Amersham). For detection with the methylation sensitive antibody, filters were treated for 10 min with 0.2 M KOH prior to blocking of the membrane; control membranes were untreated. Filters were incubated with either the methylation sensitive (Ab299/309) [29] or the non‐methylation sensitive anti‐PP2A‐C polyclonal antibody [25], the rabbit polyclonal antibody against PDI‐2 [30] as a protein loading control or the mouse monoclonal antibody against CWP‐1. As a control for specificity, the KVTRRTPDYFL peptide used for immunization [25] or the Giardia C‐terminal PP2A‐C peptide HISRRVPDYFL (GenScript Corporation) was pre‐incubated with the non‐methylation sensitive PP2A‐C antibody for 30 min.
Subsequently, filters were washed 4 times with PBS and probed with donkey anti‐rabbit‐HRP or goat anti‐mouse‐HRP (Zymed). Filters were developed with ECL® or ECL‐Plus® (Amersham Life Science) and chemiluminescent signals exposed to X‐ray film. Intensity of reactive bands was quantified by Quantity One program (Bio‐Rad) and the ratio (gPP2A‐C / PDI‐2) was normalized so that C6 = 1.
2.6. Expression of gPP2A‐C Antisense
A new vector, called pOCT‐PP2Aas was generated to drive the overexpression of gPP2A‐C antisense RNA (Fig. 4A). Briefly, a standard AU1 epitope tagging vector [31] was modified to contain the 5’ UTR (−502 to −1) of the most highly expressed trophozoite transcript, ornithine carbamoyltransferase (OCT) [32], followed by an in frame ATG start codon and XhoI ‐ EcoRV restriction sites at the EcoRV juncture of the original AU1 vector. The 3′ UTR was replaced with 73 nucleotides of the 3’ UTR of a gene encoding a highly conserved eukaryotic protein (HCEP) (Genbank Accession no. XP_770639). The coding sequence of gPP2A‐C was amplified from G. lamblia strain WB clone C6 genomic DNA with primers 5’‐ATAGGGCCCATGGGCATCAAGTCTTGCCTT‐3’ and 5’‐ATACTCGAGCTAAAGGAAGTAATCCGGAAC‐3’. The gPP2A‐C fragment (1035bp) and the OCT‐AU1 vector were digested with XhoI and ApaI restriction enzymes, gel extracted using a QIAquick Gel Extraction Kit (Qiagen), and ligated at 14°C overnight. Plasmids were transformed into E. coli JM109 (Promega). Bacteria were grown overnight in Luria broth and plasmid DNA was purified using a Maxiprep kit (Qiagen) and sequenced (Etonbio). Trophozoites were electroporated with 50 µg plasmid DNA and transfectants were maintained through puromycin selection [30].
Fig. 4.
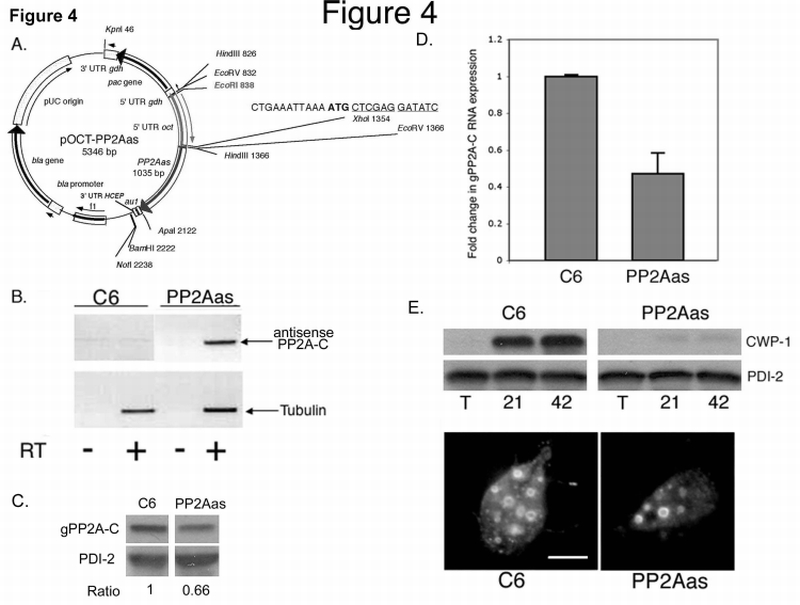
PP2Aas expression inhibits Giardia encystation.
A. Diagram of the pOCT‐PP2Aas vector used to overexpress Giardia PP2Aas (see Experimental Procedures).
B. Expression of gPP2A‐C antisense RNA in transfected (PP2Aas) and control cells (C6). RT‐PCR with (+) or without (−) reverse transcriptase are shown. PP2A‐C antisense transcripts were only apparent in cells transfected with the gPP2A‐C antisense vector (+RT/antisense PP2A‐C). The lower panel is a control for tubulin expression in both strains.
C. Western blot analysis revealed a reduction in gPP2A‐C protein expression in PP2Aas compared to C6 control trophozoites. PDI‐2 was used as a protein loading control. Band intensities were quantified and the ratio (PP2A‐C / PDI‐2) revealed a 34% reduction in total gPP2A‐C protein in the PP2Aas strain.
D. Expression of PP2Aas decreases the level of gPP2A‐C sense RNA as shown by quantitative real time RT‐PCR analyses. Bars show the average fold change ± s.d. (n=3) in gPP2A‐C cDNA in the PP2Aas strain as compared to the wild type C6 strain and relative to tubulin cDNA [33]. (p‐value<0.05).
E. CWP‐1 protein levels and the number of ESV are decreased in PP2Aas encysting trophozoites. Upper panel: Total proteins from vegetative (T), 21 h and 42 h encysting C6 and PP2Aas cells were separated by SDS‐PAGE, transferred to PDVF, and probed with the antibody to CWP‐1 or PDI‐2 (loading control).
Lower panel: Immunolocalization of CWP‐1 in a 21 h encysting C6 (left) and PP2Aas (right) cell. Bar = 5µm
2.7. Reverse transcriptase polymerase chain reaction (RT‐PCR) and real time PCR
cDNA from wild type WB clone 6 and antisense expressing trophozoites was synthesized using Superscript III Reverse Transcriptase (Invitrogen) according to the manufacturer’s instructions. The cDNA was used as a template for PCR with a constitutive alpha‐tubulin control (Genbank Accession no. XM_764294) (5’‐ TTTCTTCATGGGCGATTATGTCGATCGCGG‐3’ and 5’‐TTTCTCCAT GGTAAGCTGGTGGGCACGCGC‐3’) and primers for detection of gPP2A‐C antisense (5’‐ATACTCGAGCTAAAGGAAGTAATCCGGAAC‐3’ and 5’‐CTAGATGTAGCGGTACGTGTC‐3’). The PCR products were separated on a 1% agarose gel containing ethidium bromide and visualized with UV light. Oligonucleotides for real time PCR (gPP2A‐C: 5’‐AGCAGTGCGTGACCATCTTCT‐3’ and 5’‐TTGCCCCGCGGTTTC‐3’; alpha‐tubulin: 5’‐CCGTCGACCTTTGCATCAT‐3’ and 5’‐GAGGCCCAGGAACAAGCA‐3’) were designed by Primer Express 2.0 (Applied Biosystems) and synthesized (Proligo). Oligonucleotide specificity was tested by sequence similarity searches (BLAST, NCBI) of the Giardia genome and confirmed by a dissociation curve analysis. PCR reactions were performed with the SYBR Green method in an ABI 7900HT sequence detection system (Applied Biosystems, USA) following manufacturer's guidelines. The Ct value was set constant at 0.1 relative fluorescence units throughout the study. Each condition was analyzed in triplicate in the same PCR session and standard deviations were calculated. The results were analyzed according to the 2−ΔΔCT method with ΔΔCT= (CTPP2A − CTTubulin)PP2Aas − (CTPP2A − CTTubulin)wild type and 2−ΔΔCT = fold change [33]. Results were analyzed with the One sample T‐test (Graphpad Instat 3) and p‐value < 0.05 was considered significant.
3. Results
3.1. PP2A‐C gene is present in Giardia
Similarity sequence searches using human PP2A‐Cα (GenBank accession no. P62714) to query the Giardia genome database (http://gmod.mbl.edu/perl/site/giardia?page=intro) revealed an ORF (GenBank Accession no. XP_767901) with an e‐value of 1e−124. The amino acid sequence of this ORF (gPP2A‐C) shares 63% identity and 82% similarity with its human homologue, 58% and 78%, with yeast (GenBank Accession no. P23594), 48% and 66% with Trypanosoma cruzi (GenBank Accession no. AAO17777.1) and 54% and 68% with Plasmodium falciparum (GenBank Accession no. AAC47800.1) respectively (Fig. 1A). The highly conserved phosphoesterase domains I, II, and III, as well as the histidine and aspartate residues that act as an ion‐pair in catalysis, are all present in Giardia PP2A‐C (gPP2A‐C). The apparent molecular weight of the gPP2A‐C (41.2 kDa) is similar to the hPP2A‐C (Fig. 1B) and slightly larger than its theoretical molecular weight (39.7 kDa). hPP2A‐C phosphatase activity is regulated by alkali‐sensitive methyl‐esterification of the invariant C‐terminal Leu 309 [12, 34]. gPP2A‐C also appears to be methylated, since treatment of protein with 0.2M KOH ‐ to remove the methyl group ‐ was necessary to reveal the gPP2A‐C epitope on membranes probed with a methylation sensitive polyclonal antibody (Fig. 1B). Controls with the non‐methylation‐sensitive anti‐PP2A‐C antibody showed that the KOH treatment did not remove the protein (data not shown).
Fig. 1.
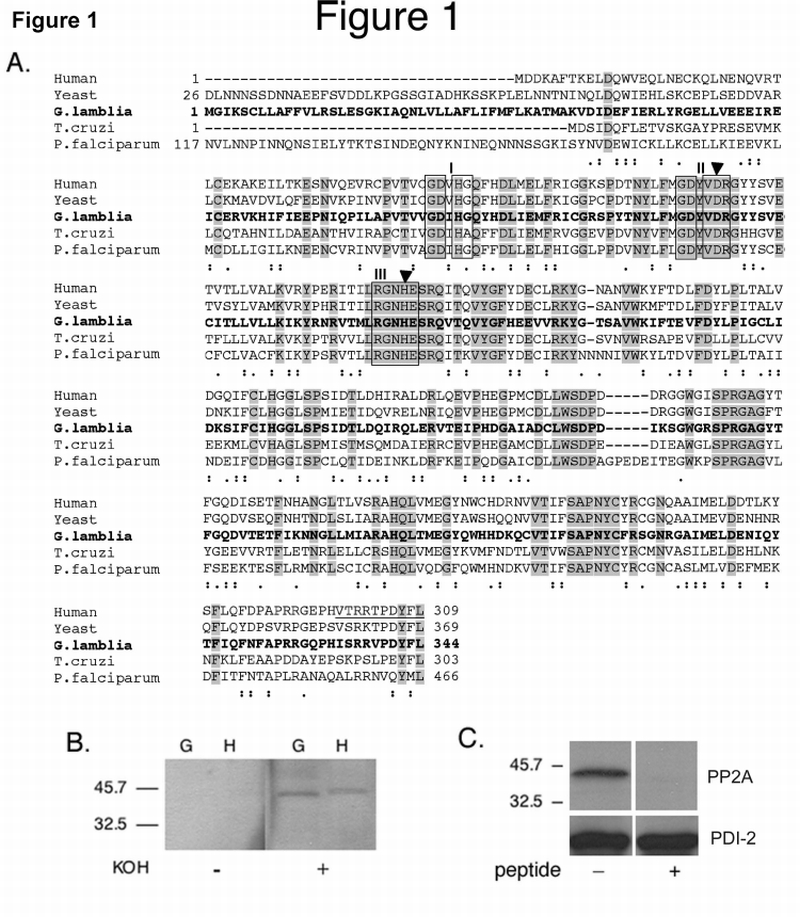
Amino acid sequence homology comparison between Giardia and other PP2A‐C subunits, evidence for L‐methylation of Giardia PP2A‐C and control for anti‐PP2A‐C antibody specificity.
A. Amino acid sequence alignment of human PP2A‐C (GenBank Accession no. P62714), yeast, (Saccharomyces cerevisiae, GenBank Accession no. P23594), Giardia (GenBank Accession no. XP_767901), Trypanosoma cruzi (GenBank Accession no. AAO17777.1) and Plasmodium falciparum (GenBank Accession no. AAC47800.1) PP2A‐C subunits. Identical and semi‐conserved amino acids are shaded shown with dots, respectively. The highly conserved phosphoesterase domains I, II, and III are boxed. The filled triangles show the conserved histidine and aspartate residues, which act as an ion‐pair in catalysis. The underlined peptide indicates the sequence used to generate the polyclonal Ab299/309.
B. Methylation of gPP2A‐C: Western blot of total lysates of vegetative Giardia trophozoites (G) and HT‐29 cells (H). Prior to reacting with the methylation sensitive polyclonal Ab299/309, the membrane was treated with 0.2M KOH (+) to remove methyl groups or with PBS (−). Demethylation of both giardial and human PP2A‐C proteins is necessary for antibody reactivity.
C. Antibody specificity: Western blot of total cell lysates of vegetative Giardia trophozoites in the presence (+) or absence (−) of the Giardia specific peptide. Competition with the gPP2A‐C C‐terminal peptide ‘HISRRVPDYFL’ inhibited all binding of the anti‐PP2A‐C antibody to gPP2A‐C.
Antibody specificity for gPP2A‐C was shown by successful competition with both the KL immunizing peptide antigen (data not shown) and the gPP2A‐C C‐terminal peptide HISRRVPDYFL (Fig. 1C).
Leucine methylation of gPP2AC is also supported by the presence of a single putative homologue for human leucine carboxymethyltransferase 2 in the Giardia genome (1e−23) (GenBank Accession no. XP_767048.1). In addition, we searched the Giardia genome for other gPP2A subunits (Table 1). ORF 7439 and ORF 16567 have both been annotated as PP2A scaffolding A subunits and have 33% and 19% identity and share 52% and 39% similarity with the hPP2A regulatory chain A subunit / PR 65 (GenBank Accession no. P30153), respectively. The major difference is that the gPP2A A subunits have divergent amino acid inserts (50AA between AA 283 and 341 in ORF 7439 and several small inserts in ORF 16567 among a 19AA one between AA 275 and AA 293), which are common amongst conserved giardial genes [7, 35]. There are several PP2A regulatory B subunits and blast searches identified 8 putative B subunits in the Giardia genome (Table 1).
Table 1.
Candidate PP2A scaffolding (A) and regulatory (B) subunits in Giardia lamblia
| PP2A subunit gene family | ORF IDa | GenBank Accession no. | Swiss‐Prot search result | E‐value |
|---|---|---|---|---|
| A | 7439 | EAA37044 | Mouse, PP2A subunit A | 7e−96 |
| PR65‐alpha isoform | ||||
| 16567 | EAA40784 | S. pombe, PP2A subunit A | 1e−13 | |
| PR65 isoform | ||||
| B | 4079 | EAA40324 | Mouse, PP2A subunit B | 4e−82 |
| PR55‐delta isoform | ||||
| 32398 | EAA42029 | Mouse, PP2A subunit B | 3e−24 | |
| PR55‐alpha isoform | ||||
| B’ | 16443 | EAA36693 | Human, PP2A subunit B | 7e−62 |
| PR61 epsilon isoform | ||||
| B” | 9894 | EAA40706 | Human, PP2A subunit B | 3e−52 |
| PR72/PR130 isoforms | ||||
| 17538 | EAA40850 | Human, PP2A subunit B | 2e−41 | |
| PR72/PR130 isoforms | ||||
| 2261 | EAA38169 | Human, PP2A subunit B | 2e−15 | |
| PR72/PR130 isoforms | ||||
| 17157 | EAA46474 | Human, PP2A subunit B | 5e−10 | |
| PR48 isoform | ||||
| B’” | 17518 | EAA37984 | Rat, Striatin | 3e−7 |
ORF ID numbers can be used to view the data on GiardiaDB (www.mbl.edu/Giardia).
3.2. Localization of gPP2A‐C changes during differentiation
We used immunofluorescence techniques to determine the cellular localization of gPP2A‐C in isolated cytoskeletons with a heterologous antibody made against the C‐terminus of hPP2A‐C (Fig. 1A). The antibody recognizes the basal bodies, the ventral disk, and the anterior, posterior‐lateral, and caudal PDR (Fig. 2A). Thus, gPP2A‐C localizes to specific PDR, which accompany only the intracellular portions of the corresponding axonemes, rather than to the flagellar axonemes themselves. Similar results were observed with permeabilized intact cells, regardless of the method of fixation and also with the methylation sensitive antibody against PP2A‐C (Ab299/309) [29] (data not shown). We did not detect gPP2A‐C in the nuclei or in the cytosol. The antibody detects a single major band in immunoblots of total giardial lysates (Fig. 2B). Universal signaling proteins often attain specificity by localization to target cellular structures [36]. We found that gPP2A‐C localization also changes in response to external signals. Specifically, during encystation, gPP2A‐C is no longer associated with the anterior PDR, while the localization to the basal bodies, posterior‐lateral and caudal PDR is maintained (Fig. 2C). In addition, gPP2A‐C localization to the ventral disk increased after 21 hours of encystation (Fig. 2C). In cysts, gPP2A‐C localizes to the basal bodies, PDR and occasionally to the cyst wall. During Stage 1 of excystation, we observed a punctate gPP2A‐C staining pattern within the cyst wall. In Stage 2 of excystation, gPP2A‐C was evenly distributed in the walls of some cysts, while in other cysts, gPP2A‐C was only seen in the cell body. Binding of anti‐PP2A‐C to the cyst wall was specific, since the Giardia‐specific PP2A‐C peptide eliminated antibody binding (data not shown). There may be some localization to the cytoplasm in cysts and during excystation. gPP2A‐C was no longer associated with the cyst wall during emergence of excyzoites, but was observed in the cytoplasm of excyzoites (Fig. 2C).
Fig. 2.
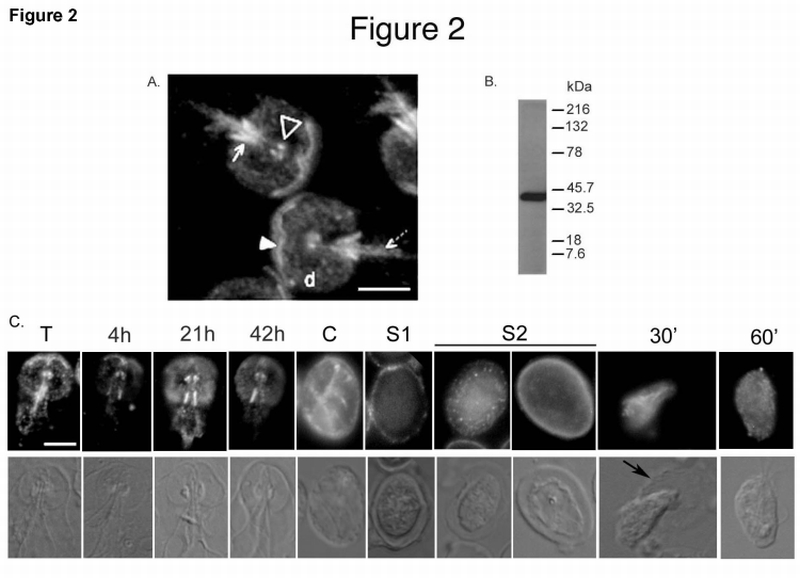
A. Immunolocalization of gPP2A‐C to the Giardia cytoskeleton. Cytoskeletons of vegetative trophozoites were probed with the anti‐PP2A‐C antibody. The filled arrowhead shows the anterior PDR, the open arrowhead shows the basal bodies. The solid arrow points to posterior‐lateral PDR and the dashed arrow to the caudal PDR. “d” is the ventral disk. Bar = 5 µm
B. Antibody specificity: Western blot of a total Giardia lysate reacted with the anti‐PP2A‐C antibody used for immunolocalization.
C. Immunolocalization of gPP2A‐C changes during Giardia differentiation. Cytoskeletons of vegetative (T), 4 h, 21 h and 42 h encysting trophozoites, whole water‐treated cyst (C), excysting cysts in Stages 1 (S1) and 2 (S2) and 30 (30’) and 60 (60’) min emerging cells were probed with the same anti‐PP2A‐C antibody. Note: Intensities of the signals in the panels should not be compared as the exposure times were optimized individually. The black arrow points to the degraded cyst wall surrounding an emerging excyzoite. Bar = 5 µm
3.3. gPP2A‐C mRNA is highly expressed in cysts and excystation stage 1
SAGE transcriptome analyses of steady state mRNA throughout the Giardia life cycle indicated that gPP2A‐C is expressed at increased levels in cysts (0.05% of total tags) and during Stage 1 of excystation (0.11%) as compared with vegetative (0.03%) and encysting trophozoites (0.01–0.02%) (Fig. 3, upper panel). Western blot analyses showed reproducibly that the protein level of gPP2A‐C resembles mRNA expression and is upregulated in cysts and highest in Stage 1 excysting cells. A representative Western blot is shown in Fig. 3 (lower panel). The protein loading control, protein disulfide isomerase 2 (PDI‐2), appeared constant until excystation Stage 1. We observed a general reduction in both the loading control and gPP2A‐C protein levels after treatment of cysts in the trypsin‐containing Stage 2 solution. SAGE patterns for the scaffolding A and regulatory B subunits did not reveal upregulation of respective mRNAs in cyst or excystation samples (data not shown).
Fig. 3.
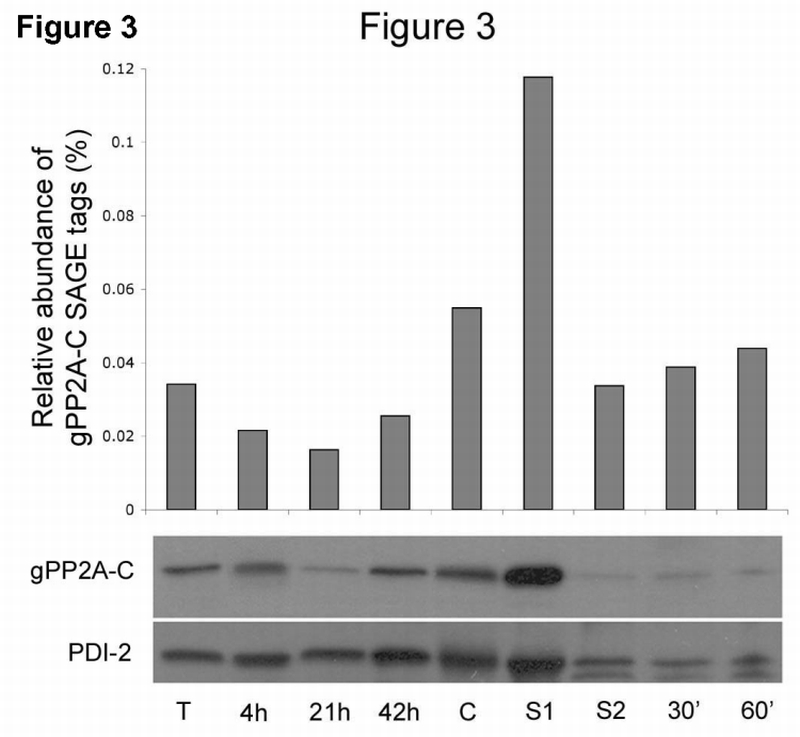
gPP2A‐C mRNA and protein are upregulated in cysts and during excystation stage 1.
Upper panel: SAGE analyses show steady state mRNA levels in vegetative trophozoites (T), total cultures encysting for 4, 21 or 42 h, cysts (C), stage 1 (S1) and stage 2 (S2) excysting cells and 30 and 60 min after excystation.
Lower panel: Western blot analysis of total cell protein from the same stages in Giardia differentiation. The highest amount of gPP2A‐C protein was detected in cysts and Stage 1 of excystation relative to the PDI‐2 protein loading control shown at the bottom of the figure (PDI‐2).
3.4. Knockdown of gPP2A‐C RNA results in decreased differentiation
Due to its high expression in cysts and during excystation Stage 1, we asked whether gPP2A‐C is important for encystation and excystation. Therefore, we stably transfected trophozoites with a plasmid encoding antisense RNA against gPP2A‐C, driven by a strong constitutive promoter (PP2Aas; Fig. 4A). The expression of the antisense RNA in PP2Aas‐transfected Giardia trophozoites was confirmed by RT‐PCR (Fig. 4B). Western analyses showed that the gPP2A‐C protein levels in the PP2Aas expressing trophozoites was reduced by ∼34% (Fig. 4C) and real‐time RT‐PCR confirmed that the RNA expression level of native gPP2A‐C sense in the PP2Aas trophozoites was reduced to 47 ± 11% of the control wild type C6 trophozoites (Fig. 4D). Expression of PP2Aas did not affect vegetative growth of trophozoites (data not shown). Cyst wall protein 1 (CWP‐1), a specific marker of encystation, was greatly reduced in PP2Aas encysting trophozoites compared to the wild type C6 (Fig. 4E upper panel). After 21 h of encystation the encystation secretory vesicles (ESV) were reduced by 64.1 ± 20.5% in PP2Aas trophozoites (Table 2, Fig. 4E lower panel). We also observed a significant reduction in the number of water‐resistant cysts (Table 2). Control cells transfected with the same plasmid containing irrelevant gene sequences had ESV and cyst counts equivalent to wild type C6 (Table 2).
Table 2.
Expression of PP2Aas inhibits encystation and excystation compared to the wild type and irrelevant transfected C6 controls.
| C6 | transfected C6b | PP2Aasc | |
|---|---|---|---|
| ESV/ 50 trophozoites | 104.0 ± 33.8 | 160.3 ± 53.5 | 19.3 ± 8.3 |
| ESV/positivea trophozoite | 3.0 ± 0.3 | 5.1 ± 2.2 | 1.1 ± 0.8 |
| % positivea trophozoites | 55.7 ± 12.0 | 56.6 ± 1.5 | 26.3 ± 7.5 |
| Number of cysts/ml | 25.2 ± 5.7 × 104 | 22.4 ± 9.3 × 104 | 6.1 ± 3.3 × 104 |
| Excyzoites/ml | 966.6 ± 208.2 | 755.7 ± 72.6 | 66.6 ± 115.5 |
Having one or more ESV
data are an average of three independent transformed C6 cell lines (orf ID: 4018, 3582, 4689; ORF ID numbers can be used to view the data on GiardiaDB(www.mbl.edu/Giardia),
significantly different from the C6 controls as tested with the T‐test, with a p‐value <0.05.
We questioned whether the smaller number of water‐resistant PP2Aas cysts that formed were able to excyst. We found that PP2Aas cysts were >90% reduced in excystation compared with wild type (Table 2). Since it is unclear whether the strong inhibition of excystation is secondary to reduced gPP2A‐C activity during formation of the PP2Aas cysts, or is due to the requirement for gPP2A‐C activity during the actual process of excystation, we excysted C6 cysts in the presence or absence of okadaic acid, a potent inhibitor of serine/threonine phosphatases, including PP1 and PP2A. SAGE analysis demonstrated that gPP2A‐C is expressed at a 6.5‐fold higher level than PP1 during excystation Stage 1. Excystation of wild type cysts in the presence of 10µM okadaic acid was inhibited by 82.5 ± 4.4%, showing that gPP2A‐C and/or PP1 are needed during excystation. To determine when in excystation serine/threonine phosphatases are needed, we incubated cysts in each stage of excystation separately with okadaic acid or equal amounts of DMSO as a control. Excystation was inhibited when cysts were incubated with okadaic acid during Stage 1 (39.0 ± 22.6%), but not Stage 2. Emergence was inhibited when okadaic acid was added to the growth media following Stage 2 (65.5 ± 20.5%).
4. Discussion
Encystation and excystation are critical differentiations that enable G. lamblia to persist in the environment and to infect a new host. Although these differentiations involve dramatic changes in cell division, shape, and motility, there is little understanding of the roles of the cytoskeleton in mediating these key transformations. PP2A is a ubiquitously expressed serine/threonine phosphatase involved in the regulation of numerous cellular processes in eukaryotic cells, including growth and differentiation [11, 37]. The present study shows that the Giardia PP2A C subunit localizes to cytoskeletal structures and to the cyst wall and regulates encystation and excystation.
In Giardia trophozoites, gPP2A‐C localizes to the eight basal bodies, as previously reported for giardial PKAc/r, the MAP kinase ERK1, centrin, and calmodulin [7–9, 24, 38, 39]. gPP2A‐C also localizes to the ventral disk (similar to ERK 1 [9]), to the anterior and caudal PDR (similar to PKAc/r [7, 8]) and to the posterior‐lateral PDR (similar to centrin [24]). Activity of cell signaling proteins is often regulated by phosphorylation and dephosphorylation, leading to complex signaling networks. Involvement of PP2A in such networks has been extensively reviewed [11, 37, 40, 41]. Interestingly, PP2A has been reported to regulate the PKA, the calmodulin and the ERK1/2 pathways in mammalian cells [42–44]. We speculate that the proximity of these diverse signaling proteins in the PDR and the basal bodies may regulate the signaling underlying the distinct flagellar activities [3]. Cellular responses to external stimuli may be mediated by changes in localization of signal transducing proteins. Exposure of Giardia to the physiologic signals that induce encystation led to loss of gPP2A‐C from the anterior PDR and an increased localization to the disk, while localization to the posterior‐lateral PDR and basal bodies remained unchanged. Similarly, localization of PKAc to both anterior and caudal PDR, but not the basal bodies, was lost when Giardia was deprived of growth factors, leading to decreased cAMP levels [7]. Ellis et al. showed recently that localization of ERK 2 to the nuclei and anterior PDR was lost during encystation [9]. Thus, the localization of gPP2A‐C, PKAc, ERK1, centrin and calmodulin to the basal bodies is constitutive, while their localization to (para)flagellar structures is responsive to environmental signals.
Knockdown of gPP2A‐C RNA by expression of antisense RNA under a strong constitutive promoter greatly inhibited both encystation and excystation. During encystation, giardial trophozoites gradually round up and lose their ability to attach and to swim. We speculate that decreased mobility and cytokinesis may be coordinated by the basal bodies, while changes in individual flagella may be mediated by signaling proteins localized to the specific PDR. For example, the loss of gPP2A‐C from the anterior PDR during encystation suggests that complex changes in phosphorylation of proteins associated with this pair of flagella may be important in the decreased motility during encystation.
Although gPP2A‐C localizes to the cytoskeleton and not to the endoplasmatic reticulum or ESV pathway in encysting cells, our results show that gPP2A‐C is needed for the trophozoite’s ability to encyst. In PP2Aas cells CWP expression, the earliest step in encystation, is inhibited. Therefore, all subsequent steps including formation of the ESV and the cyst wall are also greatly decreased [4, 45, 46]. Since we have never seen gPP2A‐C in the nuclei, we do not know whether it is directly needed for CWP synthesis or affects a transcription factor or other upstream protein or event.
Although cysts can remain dormant in cold water for months [47], upon ingestion they respond rapidly to host signals. Excystation entails re‐activation of motility, cell polarity, cytokinesis, and attachment. The increased expression of gPP2A‐C in cysts and during the first stage of excystation strongly suggest that gPP2A‐C plays a role in giardial differentiation. Interestingly, we observed that gPP2A‐C localization to the cyst wall is not homogenous and changes throughout excystation. This suggests that there may be a maturation‐dependent expression/translocation of gPP2A‐C via a novel non‐ESV pathway in the non‐synchronous encysting population. It is possible that excyzoites only emerged from cysts that had no gPP2A‐C in their wall. Previous studies reported that dephosphorylation of cyst wall proteins by an acid phosphatase is essential for Giardia excystation [48, 4949]. We speculate that gPP2A‐C, in addition to the acid phosphatases, may be involved in the dephosphorylation of cyst wall proteins during excystation. These findings also suggest that there is a novel ESV independent pathway by which proteins can be translocated to the giardial cyst wall in response to physiological stimuli inducing excystation.
We found that the small number of normal‐appearing, water‐resistant cysts produced by the PP2Aas strain excysted very poorly (∼10% of wild type). Because the antisense data did not reveal whether gPP2A‐C was required for a specific stage during excystation, we used okadaic acid. Okadaic acid inhibited excystation when it was present in Stage 1, and during emergence following Stage 2. Since okadaic acid inhibits multiple serine/threonine phosphatases, we concluded that gPP2A‐C and possibly other serine/threonine phosphatases are needed for Stage 1 and for the resumption of motility during emergence of excyzoites. Based on our gPP2A‐C localization and functional data, we hypothesize that gPP2A‐C regulates the pathways that lead to loss of motility, adhesion, and cell division during encystation and may regulate the gain of these functions during excystation, along with dephosphorylation of cyst wall proteins. These opposing roles could be explained by the changes in localization and/or association with different gPP2A regulatory B subunits leading to proximity to different target proteins. In Chlamydomonas, kinases and phosphatases, including PKA and PP2A‐C, localize to the flagellar axonemes [15, 50]. These enzymes have opposing effects on dynein motor phosphorylation that may regulate microtubule sliding and flagellar beating. Since PKA and gPP2A‐C co‐localize to paraflagellar structures, rather than to the axonemes, Giardia may employ novel regulatory pathways.
Protein phosphatases have long been considered to be subordinate to protein kinases and have only recently been identified as dynamic regulators of important cellular processes [37, 51]. Dysregulation of PP2A by viruses, parasites or carcinogenesis leads to human disease. Our data demonstrate the importance of gPP2A‐C as a regulator of crucial stages in the life cycle of G. lamblia. Moreover, gPP2A‐C is another differentiation regulating signaling molecule which ‐ together with PKA, calmodulin and ERK1 ‐ localizes to the basal bodies, supporting the idea that giardial basal bodies are centers of cell signaling that regulate the parasite’s switches between active growth and dormancy. Our studies show that generalized signaling proteins can serve highly specialized roles that are requisite for disease in the unique biology of an early diverging parasite.
Acknowledgements
We thank Dr. G. Walter, Dr. R. Ruediger and Dr. M. Gentry (UCSD, CA, US) for critically reading the manuscript. We thank Dr. G. Walter and Dr. R. Ruediger for the non‐methylation sensitive anti‐PP2A‐C antibody and the KL peptide, Dr. J. Goris (KUL, Belgium) for the methylation sensitive anti‐PP2A‐C antibody (Ab299/309) and Dr. H. Stibbs (NIAID, MD, US) for the CWP‐1 antibody. This work was funded by NIH grants GM61896, AI51687, AI42488, and DK35108. Dr. A.G. McArthur was supported by NIH grant AI51089 and the Marine Biological Laboratory’s Program in Global Infectious Diseases, funded by the Ellison Medical Foundation.
Abbreviations
- CWP
cyst wall protein
- ERK
extracellular signal regulated kinase
- ESV
encystation secretory vesicles
- PBS
phosphate‐buffered saline
- PDI
protein disulfide isomerase
- PDR
paraflagellar dense rods
- PKA
protein kinase A
- PP1
protein phosphatase 1
- PP2A‐A/‐B/‐C
protein phosphatase 2A subunit A, B, or C
- PP2Aas
PP2A‐C antisense
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kramer MH, Herwaldt BL, Craun GF, et al. Surveillance for waterborne‐disease outbreaks‐‐United States, 1993–1994. MMWR CDC Surveill Summ. 1996;45:1–33. [PubMed] [Google Scholar]
- [2].Boucher SE, Gillin FD. Excystation of in vitro‐derived Giardia lamblia cysts. Infect Immun. 1990;58:3516–3522. doi: 10.1128/iai.58.11.3516-3522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Elmendorf HG, Dawson SC, McCaffery JM. The cytoskeleton of Giardia lamblia. Int J Parasitol. 2003;33:3–28. doi: 10.1016/s0020-7519(02)00228-x. [DOI] [PubMed] [Google Scholar]
- [4].Reiner DS, McCaffery M, Gillin FD. Sorting of cyst wall proteins to a regulated secretory pathway during differentiation of the primitive eukaryote, Giardia lamblia. Eur J Cell Biol. 1990;53:142–153. [PubMed] [Google Scholar]
- [5].Palm D, Weiland M, McArthur AG, et al. Developmental changes in the adhesive disk during Giardia differentiation. Mol Biochem Parasitol. 2005;141:199–207. doi: 10.1016/j.molbiopara.2005.03.005. [DOI] [PubMed] [Google Scholar]
- [6].Marti M, Regos A, Li Y, et al. An ancestral secretory apparatus in the protozoan parasite Giardia intestinalis. J Biol Chem. 2003;278:24837–24848. doi: 10.1074/jbc.M302082200. [DOI] [PubMed] [Google Scholar]
- [7].Abel ES, Davids BJ, Robles LD, et al. Possible roles of protein kinase A in cell motility and excystation of the early diverging eukaryote Giardia lamblia. J Biol Chem. 2001;276:10320–10329. doi: 10.1074/jbc.M006589200. [DOI] [PubMed] [Google Scholar]
- [8].Gibson C, Schanen B, Chakrabarti D, Chakrabarti R. Functional characterisation of the regulatory subunit of cyclic AMP‐dependent protein kinase A homologue of Giardia lamblia: Differential expression of the regulatory and catalytic subunits during encystation. Int J Parasitol. 2006 doi: 10.1016/j.ijpara.2005.11.008. [DOI] [PubMed] [Google Scholar]
- [9].Ellis JGt, Davila M, Chakrabarti R. Potential involvement of extracellular signal‐regulated kinase 1 and 2 in encystation of a primitive eukaryote, Giardia lamblia. Stage‐specific activation and intracellular localization. J Biol Chem. 2003;278:1936–1945. doi: 10.1074/jbc.M209274200. [DOI] [PubMed] [Google Scholar]
- [10].Gentry MS, Hallberg RL. Localization of Saccharomyces cerevisiae protein phosphatase 2A subunits throughout mitotic cell cycle. Mol Biol Cell. 2002;13:3477–3492. doi: 10.1091/mbc.02-05-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. [Google Scholar]
- [12].Turowski P, Fernandez A, Favre B, et al. Differential methylation and altered conformation of cytoplasmic and nuclear forms of protein phosphatase 2A during cell cycle progression. J Cell Biol. 1995;129:397–410. doi: 10.1083/jcb.129.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Chen J, Martin BL, Brautigan DL. Regulation of protein serine‐threonine phosphatase type‐2A by tyrosine phosphorylation. Science. 1992;257:1261–1264. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- [14].Hiraga A, Tamura S. Protein phosphatase 2A is associated in an inactive state with microtubules through 2A1‐specific interaction with tubulin. Biochem J. 2000;346(Pt 2):433–439. [PMC free article] [PubMed] [Google Scholar]
- [15].Yang P, Fox L, Colbran RJ, Sale WS. Protein phosphatases PP1 and PP2A are located in distinct positions in the Chlamydomonas flagellar axoneme. J Cell Sci. 2000;113(Pt 1):91–102. doi: 10.1242/jcs.113.1.91. [DOI] [PubMed] [Google Scholar]
- [16].Awotunde OS, Lechward K, Krajewska K, et al. Interaction of maize (Zeamays) protein phosphatase 2A with tubulin. Acta Biochim Pol. 2003;50:131–138. [PubMed] [Google Scholar]
- [17].Sontag E, Nunbhakdi‐Craig V, Bloom GS, Mumby MC. A novel pool of protein phosphatase 2A is associated with microtubules and is regulated during the cell cycle. J Cell Biol. 1995;128:1131–1144. doi: 10.1083/jcb.128.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lu Q, Dunn RL, Angeles R, Smith GD. Regulation of spindle formation by active mitogen‐activated protein kinase and protein phosphatase 2A during mouse oocyte meiosis. Biol Reprod. 2002;66:29–37. doi: 10.1095/biolreprod66.1.29. [DOI] [PubMed] [Google Scholar]
- [19].Gonzalez J, Cornejo A, Santos MR, et al. A novel protein phosphatase 2A (PP2A) is involved in the transformation of human protozoan parasite Trypanosoma cruzi. Biochem J. 2003;374:647–656. doi: 10.1042/BJ20030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li JL, Baker DA. Protein phosphatase beta, a putative type‐2A protein phosphatase from the human malaria parasite Plasmodium falciparum. Eur J Biochem. 1997;249:98–106. doi: 10.1111/j.1432-1033.1997.t01-2-00098.x. [DOI] [PubMed] [Google Scholar]
- [21].Erondu NE, Donelson JE. Characterization of trypanosome protein phosphatase 1 and 2A catalytic subunits. Mol Biochem Parasitol. 1991;49:303–314. doi: 10.1016/0166-6851(91)90074-g. [DOI] [PubMed] [Google Scholar]
- [22].Diamond LS, Harlow DR, Cunnick CC. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72:431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- [23].Keister DB. Axenic culture of Giardia lamblia in TYI‐S‐33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77:487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- [24].Meng TC, Aley SB, Svard SG, et al. Immunolocalization and sequence of caltractin/centrin from the early branching eukaryote Giardia lamblia. Mol Biochem Parasitol. 1996;79:103–108. doi: 10.1016/0166-6851(96)02636-9. [DOI] [PubMed] [Google Scholar]
- [25].Ruediger R, Roeckel D, Fait J, et al. Identification of binding sites on the regulatory A subunit of protein phosphatase 2A for the catalytic C subunit and for tumor antigens of simian virus 40 and polyomavirus. Mol Cell Biol. 1992;12:4872–4882. doi: 10.1128/mcb.12.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stibbs HH. Monoclonal antibody‐based enzyme immunoassay for Giardia lamblia antigen in human stool. J Clin Microbiol. 1989;27:2582–2588. doi: 10.1128/jcm.27.11.2582-2588.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Velculescu VE, Zhang L, Vogelstein B, Kinzler KW. Serial analysis of gene expression. Science. 1995;270:484–487. doi: 10.1126/science.270.5235.484. [DOI] [PubMed] [Google Scholar]
- [28].Hetsko ML, McCaffery JM, Svard SG, et al. Cellular and transcriptional changes during excystation of Giardia lamblia in vitro. Exp Parasitol. 1998;88:172–183. doi: 10.1006/expr.1998.4246. [DOI] [PubMed] [Google Scholar]
- [29].De Baere I, Derua R, Janssens V, et al. Purification of porcine brain protein phosphatase 2A leucine carboxyl methyltransferase and cloning of the human homologue. Biochemistry. 1999;38:16539–16547. doi: 10.1021/bi991646a. [DOI] [PubMed] [Google Scholar]
- [30].Knodler LA, Noiva R, Mehta K, et al. Novel protein‐disulfide isomerases from the early‐diverging protist Giardia lamblia. J Biol Chem. 1999;274:29805–29811. doi: 10.1074/jbc.274.42.29805. [DOI] [PubMed] [Google Scholar]
- [31].Weiland ME, Palm JE, Griffiths WJ, et al. Characterisation of alpha‐1 giardin: an immunodominant Giardia lamblia annexin with glycosaminoglycan‐binding activity. Int J Parasitol. 2003;33:1341–1351. doi: 10.1016/s0020-7519(03)00201-7. [DOI] [PubMed] [Google Scholar]
- [32].Palm JE, Weiland ME, Griffiths WJ, et al. Identification of immunoreactive proteins during acute human giardiasis. J Infect Dis. 2003;187:1849–1859. doi: 10.1086/375356. [DOI] [PubMed] [Google Scholar]
- [33].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [34].Floer M, Stock J. Carboxyl methylation of protein phosphatase 2A from Xenopus eggs is stimulated by cAMP and inhibited by okadaic acid. Biochem Biophys Res Commun. 1994;198:372–379. doi: 10.1006/bbrc.1994.1052. [DOI] [PubMed] [Google Scholar]
- [35].Langford TD, Silberman JD, Weiland ME, et al. Giardia lamblia: identification and characterization of Rab and GDI proteins in a genome survey of the ER to Golgi endomembrane system. Exp Parasitol. 2002;101:13–24. doi: 10.1016/s0014-4894(02)00037-1. [DOI] [PubMed] [Google Scholar]
- [36].Hancock JF. Ras proteins: different signals from different locations. Nat Rev Mol Cell Biol. 2003;4:373–384. doi: 10.1038/nrm1105. [DOI] [PubMed] [Google Scholar]
- [37].Sontag E. Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell Signal. 2001;13:7–16. doi: 10.1016/s0898-6568(00)00123-6. [DOI] [PubMed] [Google Scholar]
- [38].Reiner DS, Hetsko ML, Meszaros JG, et al. Calcium signaling in excystation of the early diverging eukaryote, Giardia lamblia. J Biol Chem. 2003;278:2533–2540. doi: 10.1074/jbc.M208033200. [DOI] [PubMed] [Google Scholar]
- [39].Correa G, Morgado‐Diaz JA, Benchimol M. Centrin in Giardia lamblia‐ultrastructural localization. FEMS Microbiol Lett. 2004;233:91–96. doi: 10.1016/j.femsle.2004.01.043. [DOI] [PubMed] [Google Scholar]
- [40].Zolnierowicz S. Type 2A protein phosphatase, the complex regulator of numerous signaling pathways. Biochem Pharmacol. 2000;60:1225–1235. doi: 10.1016/s0006-2952(00)00424-x. [DOI] [PubMed] [Google Scholar]
- [41].Lechward K, Awotunde OS, Swiatek W, Muszynska G. Protein phosphatase 2A: variety of forms and diversity of functions. Acta Biochim Pol. 2001;48:921–933. [PubMed] [Google Scholar]
- [42].Westphal RS, Anderson KA, Means AR, Wadzinski BE. A signaling complex of Ca2+‐calmodulin‐dependent protein kinase IV and protein phosphatase 2A. Science. 1998;280:1258–1261. doi: 10.1126/science.280.5367.1258. [DOI] [PubMed] [Google Scholar]
- [43].Anderson KA, Noeldner PK, Reece K, et al. Regulation and function of the calcium/calmodulin‐dependent protein kinase IV/protein serine/threonine phosphatase 2A signaling complex. J Biol Chem. 2004;279:31708–31716. doi: 10.1074/jbc.M404523200. [DOI] [PubMed] [Google Scholar]
- [44].Adams DG, Coffee RL, Jr, Zhang H, et al. Positive regulation of Raf1‐MEK1/2‐ERK1/2 signaling by protein serine/threonine phosphatase 2A holoenzymes. J Biol Chem. 2005 doi: 10.1074/jbc.M502464200. [DOI] [PubMed] [Google Scholar]
- [45].Davids BJ, Mehta K, Fesus L, et al. Dependence of Giardia lamblia encystation on novel transglutaminase activity. Mol Biochem Parasitol. 2004;136:173–180. doi: 10.1016/j.molbiopara.2004.03.011. [DOI] [PubMed] [Google Scholar]
- [46].Stefanic S, Palm D, Svard SG, Hehl AB. Organelle proteomics reveals cargo maturation mechanisms associated with Golgi‐like encystation vesicles in the early‐diverged protozoan Giardia lamblia. J Biol Chem. 2006;281:7595–7604. doi: 10.1074/jbc.M510940200. [DOI] [PubMed] [Google Scholar]
- [47].Bingham AK, Jarroll EL, Jr, Meyer EA, Radulescu S. Giardia sp.: physical factors of excystation in vitro, and excystation vs eosin exclusion as determinants of viability. Exp Parasitol. 1979;47:284–291. doi: 10.1016/0014-4894(79)90080-8. [DOI] [PubMed] [Google Scholar]
- [48].Slavin I, Saura A, Carranza PG, et al. Dephosphorylation of cyst wall proteins by a secreted lysosomal acid phosphatase is essential for excystation of Giardia lamblia. Mol Biochem Parasitol. 2002;122:95–98. doi: 10.1016/s0166-6851(02)00065-8. [DOI] [PubMed] [Google Scholar]
- [49].Feely DE, Gardner MD, Hardin EL. Excystation of Giardia muris induced by a phosphate‐bicarbonate medium: localization of acid phosphatase. J Parasitol. 1991;77:441–448. [PubMed] [Google Scholar]
- [50].Smith EF. Regulation of flagellar dynein by the axonemal central apparatus. Cell Motil Cytoskeleton. 2002;52:33–42. doi: 10.1002/cm.10031. [DOI] [PubMed] [Google Scholar]
- [51].Denu JM, Stuckey JA, Saper MA, Dixon JE. Form and function in protein dephosphorylation. Cell. 1996;87:361–364. doi: 10.1016/s0092-8674(00)81356-2. [DOI] [PubMed] [Google Scholar]


