Abstract
Erythrocytes have the same intracellular concentration of ascorbate as plasma, which is much lower than that of nucleated cells. To determine why erythrocytes are unable to concentrate ascorbate, we tested for the presence of ascorbate transporters in these cells. Human erythrocytes had very low rates of uptake of radiolabeled ascorbate, which was accounted for by lack of the ascorbate transporter SVCT2 in immunoblots. Using a cell culture model of Friend virus-infected mouse erythroblasts, immunoblots showed that the SVCT2 was present in the erythroblast stages, but was lost following extrusion of the nucleus in the formation of the reticulocyte stage. Rates of specific ascorbate transport correlated with the presence of the SVCT2. These results show that mature erythrocytes fail to concentrate ascorbate due to loss of the SVCT2 during maturation in the bone marrow.
Human erythrocytes have the same concentration of ascorbate as that in plasma [1;2]. This contrasts with nucleated blood cells and tissues, in which ascorbate concentrations are many-fold those in plasma [3;4]. For example, at plasma and erythrocyte ascorbate concentrations of 40–80 μM, lymphocyte ascorbate concentrations are about 4 mM [4]. The cause of this difference likely relates to the absence of a specific ascorbate transporter in erythrocytes, which have very low rates of ascorbate uptake [5–7]. In contrast, erythrocytes rapidly take up dehydroascorbate (DHA), the two-electron oxidized form of ascorbate, and reduce it to ascorbate [5–7]. Because of its negative charge at physiologic pH and hydrophilic nature, ascorbate crosses biological membranes only very slowly. Thus, ascorbate generated from DHA uptake is trapped in the erythrocyte and can temporarily reach concentrations to as high as 1–2 mM [2]. Nonetheless, the fact that ascorbate concentrations are the same in erythrocytes and plasma argues that DHA uptake and reduction does not generate the ascorbate concentration gradient seen in other cells.
The concentration gradient of ascorbate across the plasma membrane of nucleated cells is due to the presence of one or more specific ascorbate transporters, two of which have been cloned and termed “SVCT” (sodium-dependent vitamin C transporter) [8]. The SVCT1 and SVCT2 mediate sodium- and energy-depended ascorbate transport and generate a gradient supported by the plasma membrane sodium-potassium ATPase [8]. Xenopus oocytes also lack specific ascorbate uptake, and when injected with mRNA for the SVCT2, they acquire this transport and can concentrate ascorbate [8]. One would presume that erythrocytes lack both the SVCT1 and SVCT2, thus accounting for their inability to take up and accumulate ascorbate against a gradient. However, the absence of the SVCT protein(s) in erythrocytes has not been documented, nor has there been a mechanism proposed to account for this lack. In this work we confirm the absence of SVCT proteins in mature human and mouse erythrocytes and show in a mouse erythroblast culture system that the SVCT2 is lost with extrusion of the nucleus during reticulocyte formation.
Materials and methods
Materials
Analytical reagents, including, ascorbic acid, ascorbate oxidase, and dehydroascorbic acid, were supplied by Sigma/Aldrich Chemical Co. (St. Louis, MO).
Preparation and culture of mouse erythroblasts and reticulocytes
Developmentally synchronized proerythoblasts were purified from spleens of 8 to10 week-old, female CD2F1 mice in the acute erythroblastic stage of the disease caused by the “anemia-inducing” strain of Friend leukemia virus [9]. Erythroblasts were cultured for times indicated before removal of cells for experiments. At 44 h, some cultures were harvested, separated by unit gravity sedimentation to isolate fractions that were highly enriched in reticulocytes, extruded nuclei, or erythroblasts that had not under gone enucleation [10]. The purified reticulocytes were placed back in culture for another 3 days, and samples of reticulocytes were harvested at various times during their maturation in vitro.
Erythrocyte and erythrocyte membrane preparation
Human erythrocytes were obtained by venipuncture from normal human donors. Mouse erythrocytes were obtained by exsanguination following euthanization of 10–12 week old C57Bl/6 mice. Erythrocytes were rinsed three times by centrifugation in phosphate-buffered saline (PBS, consisting of 140 mM NaCl and 12.5 mM sodium phosphate, pH 7.4). With each rinse, the “buffy” coat of white cells was removed and discarded. White or “leaky” erythrocyte ghosts were prepared as described by Steck and Kant [11] in 5 mM phosphate buffer, pH 7.4 and stored at −70 °C for subsequent electrophoresis.
Preparation of mouse brain microvascular endothelial cells
Endothelial cells were prepared exactly as described by Song, et al. [12] and cultured for 7 days before they were scraped from the plate and prepared for gel electrophoresis.
Assay of erythrocyte ascorbate transport
Erythrocytes at a 6% packed cell volume or the indicated numbers of erythroid precursors were incubated at 37 °C in 0.5 ml of PBS that contained 5 mM D-glucose, 0.66 mM GSH, and 0.05 μCi of L-[1-14C]ascorbic acid. The total ascorbate concentration was 6–9 μM. At times indicated, cells were pelleted by centrifugation, and the supernatant was aspirated. The cells were rinsed three times in 2 ml of ice-cold “stop” solution that consisted of 25 μM cytochalasin B in PBS. The cells were lysed by vigorous mixing with 0.2 ml of 25% metaphosphoric acid and the lysate was partially neutralized with addition of 0.75 ml of phosphate-EDTA. The latter consisted of 100 mM sodium phosphate and 5 mM EDTA, pH 8.0. The lysate was centrifuged for 1 min at 13,600 × g and 0.5 ml of the supernatant were added with mixing to 4 ml of Ecolume liquid scintillation fluid (ICN, Costa Mesa, CA). The radioactivity of duplicate samples was measured in a Packard CA-2200 liquid scintillation counter, after allowing at least 1 h for decay of chemiluminescence. Results were normalized to the intracellular aqueous volume of the cells for erythrocytes, which was taken to be 70% of the packed cell volume [13] and are shown as mean ± standard error.
Electrophoresis and immunoblotting of SVCT proteins
Erythrocyte ghost membranes in 5 mM phosphate buffer were solubilized in a lysis buffer consisting of 150 mM NaCl, 1% Nonidet P40 (v/v), 0.5% sodium deoxycholate (w/v), 0.1% sodium lauryl sulfate (w/v), 0.1 mg/ml phenylmethylsulfonyl fluoride, and leupeptin, pepstatin, and aprotinin, each at 0.01 mg/ml. The lysate was mixed and stored on ice for 30 min. To this was added an equal volume of sample buffer, which consisted of 125 mM Tris-HCl, 20% (v/v) glycerol, 4% (w/v) sodium lauryl sulfate, 10% (v/v) mercaptoethanol, and 0.0025% bromphenol blue (w/v), pH 6.8. Samples were centrifuged for 10 s at 13,000 × g, and the solubilized material was subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis according to the method of Laemmli [14]. Electrophoresis and transfer to poly(vinylidine difluoride) membrane, was carried out as previously described [15]. The blot was probed with an affinity-purified rabbit polyclonal antibodies specific for the human or mouse SVCT2 or SVCT1 (#9926 and # 9921, respectively, Santa Cruz Biochemicals, Santa Cruz, CA) at 1:400 dilutions. The secondary antibody was anti-rabbit IgG conjugated to horseradish peroxidase (#A0545, Sigma-Aldrich, Inc., St. Louis, MO) and used at a 1:5000 dilution. Bands were stained using ECL Plus Western blotting reagents (RPN 2132 from Amersham Biosciences, Piscataway, NJ ). Locations of the bands were determined using pre-stained molecular weight markers.
Results
Human erythrocytes took up radiolabeled ascorbate very slowly (Fig. 1, triangles) compared to erythrocytes also incubated with ascorbate oxidase (Fig. 1, circles). This enzyme oxidizes ascorbate to its free radical, which then dismutates and generates radiolabeled DHA that is rapidly taken up by the cells on the glucose transporter. Uptake of L-[1-14C]DHA generated by ascorbate oxidase was so rapid that it was complete by about 10 min. When erythrocytes were treated just before the transport assay with 25 μM cytochalasin B, a specific glucose transport inhibitor, the apparent uptake of L-[1-14C]ascorbate was decreased to very low levels. This indicates that most of the observed uptake of radiolabeled ascorbate was actually due to uptake of L-[1-14C]DHA on the glucose transporter.
Fig. 1.
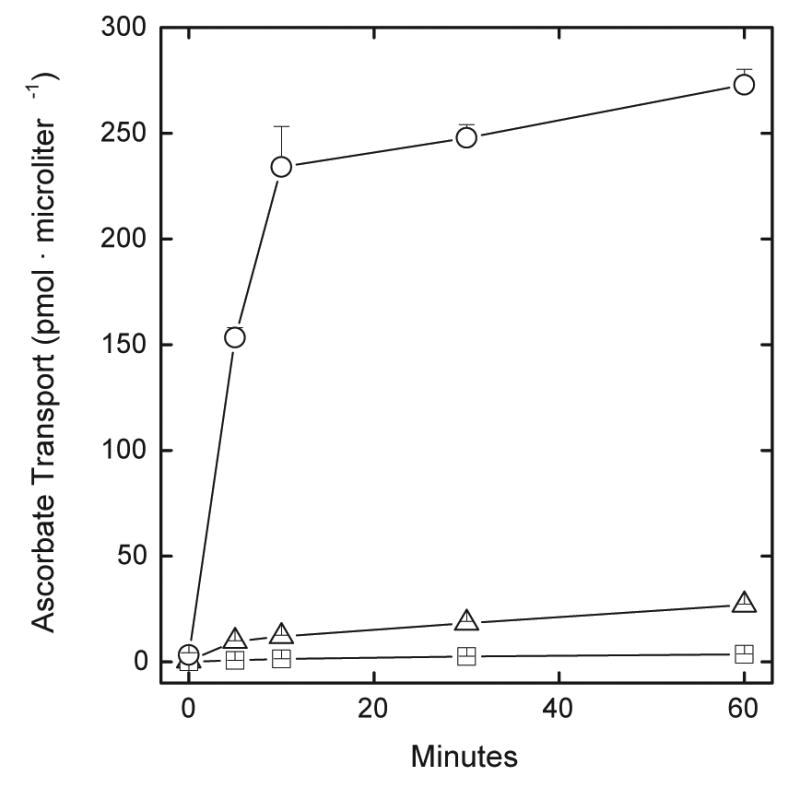
Ascorbate and DHA transport by human erythrocytes. Erythrocytes at a 4% packed cell volume were incubated at 37 °C in PBS that contained 5 mM D-glucose, 0.66 mM GSH, 0.05 μCi of L-[1-14C]ascorbate, and either no further additions (triangles), 0.1 unit/ml ascorbate oxidase (circles), or 25 μM cytochalasin B (squares). At the times indicated the cells were pelleted by centrifugation, rinsed three times in ice-cold “stop” solution, and taken for measurement of intracellular radioactivity as described under Methods. Results are shown from 4 experiments.
This lack of ascorbate transport in human erythrocytes was explained by the finding that the SVCT2 was not detected in immunoblots of human (Fig. 2, Lane A) and mouse (Fig. 2, Lane B) erythrocyte membranes. In contrast, there was strong immunostaining of a 74–76 kDa band in primary cultures of endothelial cells from mouse brain capillaries (Fig. 2, Lane C). The amount of protein loaded was comparable or slightly greater in the erythrocyte membranes, as indicated by actin immunostaining on the membrane prepared from the same gel.
Fig. 2.

Immunoblot of the SVCT2 in erythrocyte and endothelial cell membranes. Purified membranes from human (A) and mouse (B) erythrocytes as well as total endothelial cell membranes were loaded in each of 3 gel lanes and electrophoresis and immunoblotted for the SVCT2 and actin, as indicated in the figure. The migration of the 80 kDa molecular weight marker is indicated.
Membranes prepared from cultures of mouse erythroblasts, on the other hand, showed a doublet at the expected molecular weight range for the SVCT2 at 0 to 48 h of culture (Fig. 3). However, after the erythroblasts enucleated to form reticulocytes, they showed only very faint SVCT2 staining that gradually disappeared as culture was continued to 60 h. No band of a similar molecular weight was detected when an antibody against the SVCT1 was used (results not shown). These results show that the SVCT2 protein is present in the erythroblast stages, but is lost with extrusion of the nucleus.
Fig. 3.
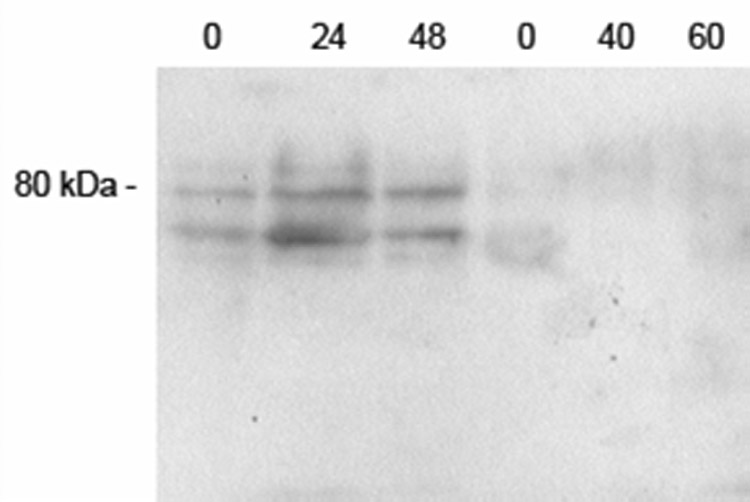
Immunoblotting of cultured erythroid cell membranes for the SVCT2. The cells were rinsed once in PBS and were extracted with lysis buffer. Protein from approximately 5 × 107 cells was applied to each lane of the gel. Electrophoresis and staining for SVCT2 was carried out as described under Methods. The location of the relevant molecular weight marker is shown to the left, and each lane is identified by the hours in culture before extrusion of the nucleus at 48 h and after extrusion to 60 h.
When cells in culture for 44 h were separated by sedimentation velocity, the fractions enriched for nuclei showed greater staining for SVCT2, although the bands were present in all fractions tested (Fig. 4A). In this separation, Fractions 1–7 and 8–10 contain largely nucleated erythroblasts, Fractions 11–12 and 13–18 contain mostly extruded nuclei, and Fractions 17–21 contain nuclei and reticulocytes [10]. Ascorbate transport was also measured in cells derived from the experiment shown in Fig. 4A, all in the presence of 25 μM cytochalasin B. As noted earlier, the latter will prevent uptake of any L-[1-14C]DHA on the glucose transporter. As can be seen in Fig. 4B, specific ascorbate transport remained high in the first 4 fractions, but was much lower in the last fraction containing mostly reticulocytes. Together, the results show that the SVCT2 protein and function is present in fractions containing erythroblasts, but not in fractions containing mostly reticulocytes.
Fig. 4.
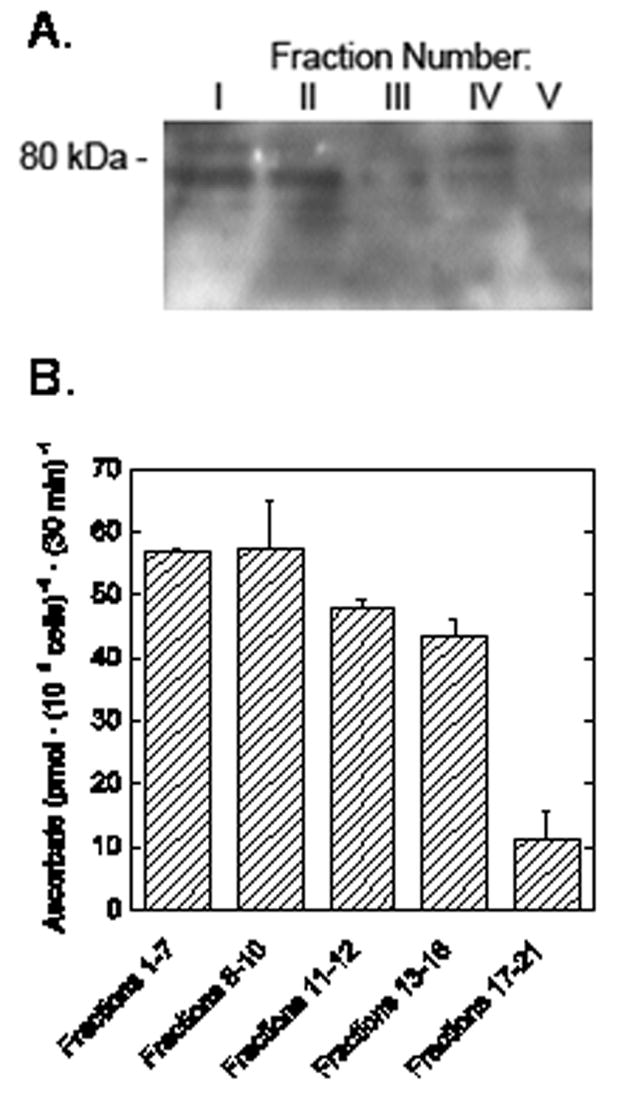
SVCT2 content and ascorbate transport in various erythroid fractions. Sedimentation velocity was used to separate cells cultured for 44 h into 5 fractions as described in the text. The cells in each fraction were rinsed in PBS and extracted with lysis buffer. Panel A. Membranes from approximately 5 × 107 cells were loaded into each gel lane. Electrophoresis and immunoblotting were carried out as described under Methods. The location of the relevant molecular weight marker is noted on the left. Panel B. Ascorbate transport was measured in duplicate in cells after fractionation. Results are shown from one of two such experiments performed.
Discussion
The study shows that mature erythrocytes lack the SVCT2 and that this accounts for their inability to take up ascorbate against a concentration gradient. Although the SVCT2 is present in erythroblasts derived from the bone marrow in mice, it is lost at the time the nucleus is extruded from the cells, which is about 42–48 h in culture and during maturation in the bone marrow in vivo. The SVCT2 is an intrinsic membrane protein that spans the plasma membrane 12 times, according to hydropathy analysis of its amino acid sequence [8]. Enucleating erythroblasts are known to shed specific plasma membrane proteins along with residual portions of organelles at this time. In addition to the SVCT2, shed proteins segregated to the plasma membrane surrounding the extruded nucleus include the receptors for concanavalin A [16] and wheat germ agglutinin [17].
Why some membrane proteins are lost during erythrocyte maturation and others, such as the anion and glucose transporters are not, remains unknown. Loss of the SVCT2 results in intracellular ascorbate concentrations in erythrocytes that are much lower than those in nucleated cells that have either isoform of the SVCT2. Whether erythrocytes might benefit from higher intracellular ascorbate is uncertain, since they do not synthesize collagen or have dioxygenase enzymes for which ascorbate serves as a co-factor [18]. Ascorbate is an excellent antioxidant and it can protect erythrocytes from extracellular oxidant stresses [19;20]. However, in a cell that contains high concentrations of iron, ascorbate could act as a pro-oxidant due to redox cycling of any free Fe3+ to Fe2+, which then can react with molecular oxygen to generate superoxide and eventually the highly reactive hydroxyl radical [21]. Nitrite that is generated from oxidation of nitric oxide is taken up by erythrocytes and can form methemoglobin [22]. Ascorbate can reduce this nitrite back to nitric oxide and thus recycle it [23]. We have shown that ascorbate modestly protects erythrocytes from methemoglobin formation due to nitrite, but only at non-physiologic nitrite concentrations above 0.5 mM [22]. Although ascorbate in erythrocytes will certainly act as a primary antioxidant, it does not appear that high intracellular erythrocyte concentrations are necessary for cell function or protection.
On the other hand, erythrocytes have a very high capacity to reduce DHA to ascorbate, which then will be sequestered inside the cells. Since erythrocytes are likely to encounter extremes of oxidant stress at sites of vascular inflammation in diseases such as atherosclerosis, substantial local amounts of DHA are likely to be generated from plasma ascorbate. Rapid uptake of DHA on the GLUT1 glucose transporter and reduction to ascorbate within erythrocytes will preserve the vitamin, which can then slowly leak out of erythrocytes back into plasma. Erythrocytes have also been shown to preserve and probably recycle ascorbate through reduction of extracellular free radical [24]. Erythrocytes thus may have a unique function to preserve ascorbate in blood: rather than simply sequestering ascorbate via the SVCT, they recycle it from its oxidized forms and slowly release it with time.
Acknowledgments
The authors thank Prapaporn Kopsombut and Melissa M. Rhodes for technical assistance. This work was supported by NIH grant DK050435 (JMM) and a Merit Review Award from the Dept. of Veterans Affairs (MJK).
Abbreviations used
- DHA
dehydroascorbic acid
- PBS
phosphate-buffered saline
- SVCT
sodium-dependent vitamin C transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Evans RM, Currie L, Campbell A. The distribution of ascorbic acid between various cellular components of blood, in normal individuals, and its relation to the plasma concentration. Br J Nutr. 1982;47:473–482. doi: 10.1079/bjn19820059. [DOI] [PubMed] [Google Scholar]
- 2.Mendiratta S, Qu ZC, May JM. Erythrocyte ascorbate recycling: Antioxidant effects in blood. Free Radic Biol Med. 1998;24:789–797. doi: 10.1016/s0891-5849(97)00351-1. [DOI] [PubMed] [Google Scholar]
- 3.Rose RC. Transport of ascorbic acid and other water-soluble vitamins. Biochim Biophys Acta. 1988;947:335–366. doi: 10.1016/0304-4157(88)90014-7. [DOI] [PubMed] [Google Scholar]
- 4.Levine M, Wang YH, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci USA. 2001;98:9842–9846. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hornig D, Weber F, Wiss O. Uptake and release of [1-14C]ascorbic acid and [1-14C]dehydroascorbic acid by erythrocytes of guinea pigs. Clin Chim Acta. 1971;31:25–35. doi: 10.1016/0009-8981(71)90358-5. [DOI] [PubMed] [Google Scholar]
- 6.Hughes RE, Maton SC. The passage of vitamin C across the erythrocyte membrane. Br J Haematol. 1968;14:247–253. doi: 10.1111/j.1365-2141.1968.tb01494.x. [DOI] [PubMed] [Google Scholar]
- 7.Wagner ES, White W, Jennings M, Bennett K. The entrapment of [14C]ascorbic acid in human erythrocytes. Biochim Biophys Acta. 1987;902:133–136. doi: 10.1016/0005-2736(87)90143-x. [DOI] [PubMed] [Google Scholar]
- 8.Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang YX, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 9.Koury MJ, Sawyer ST, Bondurant MC. Splenic erythroblasts in anemia-inducing Friend disease: a source of cells for studies of erythropoietin-mediated differentiation. J Cell Physiol. 1984;121:526–532. doi: 10.1002/jcp.1041210311. [DOI] [PubMed] [Google Scholar]
- 10.Koury MJ, Koury ST, Kopsombut P, Bondurant MC. In vitro maturation of nascent reticulocytes to erythrocytes. Blood. 2005;105:2168–2174. doi: 10.1182/blood-2004-02-0616. [DOI] [PubMed] [Google Scholar]
- 11.Steck TL, Kant JA. Preparation of impermeable ghosts and inside-out vesicles from human erythrocyte membranes. Methods Enzymol. 1974;31:172–180. doi: 10.1016/0076-6879(74)31019-1. [DOI] [PubMed] [Google Scholar]
- 12.Song L, Pachter JS. Culture of murine brain microvascular endothelial cells that maintain expression and cytoskeletal association of tight junction-associated proteins. In Vitro Cell Dev Biol Anim. 2003;39:313–320. doi: 10.1290/1543-706X(2003)039<0313:COMBME>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Orringer EP, Roer ME. An ascorbate-mediated transmembrane-reducing system of the human erythrocyte. J Clin Invest. 1979;63:53–58. doi: 10.1172/JCI109277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Due AD, Zhi-Chao Q, Thomas JM, Buchs A, Powers AC, May JM. Role of the C-terminal tail of the GLUT1 glucose transporter in its expression and function in Xenopus laevis oocytes. Biochemistry. 1995;34:5462–5471. doi: 10.1021/bi00016a017. [DOI] [PubMed] [Google Scholar]
- 16.Skutelsky E, Farquhar MG. Variations in distribution of con A receptor sites and anionic groups during red blood cell differentiation in the rat. J Cell Biol. 1976;71:218–231. doi: 10.1083/jcb.71.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geiduschek JB, Singer SJ. Molecular changes in the membranes of mouse erythroid cells accompanying differentiation. Cell. 1979;16:149–163. doi: 10.1016/0092-8674(79)90196-x. [DOI] [PubMed] [Google Scholar]
- 18.Arrigoni O, De Tullio MC. Ascorbic acid: much more than just an antioxidant. Biochim Biophys Acta Gen Subj. 2002;1569:1–9. doi: 10.1016/s0304-4165(01)00235-5. [DOI] [PubMed] [Google Scholar]
- 19.May JM, Qu ZC, Whitesell RR. Ascorbic acid recycling enhances the antioxidant reserve of human erythrocytes. Biochemistry. 1995;34:12721–12728. doi: 10.1021/bi00039a031. [DOI] [PubMed] [Google Scholar]
- 20.May JM, Qu ZC, Morrow JD, Cobb CE. Ascorbate-dependent protection of human erythrocytes against oxidant stress generated by extracellular diazobenzene sulfonate. Biochem Pharmacol. 2000;60:47–53. doi: 10.1016/s0006-2952(00)00312-9. [DOI] [PubMed] [Google Scholar]
- 21.Winterbourn CC. Hydroxyl radical production in body fluids. Roles of metal ions, ascorbate and superoxide. Biochem J. 1981;198:125–131. doi: 10.1042/bj1980125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.May JM, Qu ZC, Xia L, Cobb CE. Nitrite uptake and metabolism and oxidant stress in human erythrocytes. Am J Physiol Cell Physiol. 2000;279:C1946–C1954. doi: 10.1152/ajpcell.2000.279.6.C1946. [DOI] [PubMed] [Google Scholar]
- 23.Mirvish SS, Wallcave L, Eagen M, Shubik P. Ascorbate-nitrite reaction: possible means of blocking the formation of carcinogenic N-nitroso compounds. Science. 1972;177:65–68. doi: 10.1126/science.177.4043.65. [DOI] [PubMed] [Google Scholar]
- 24.May JM, Qu ZC, Cobb CE. Extracellular reduction of the ascorbate free radical by human erythrocytes. Biochem Biophys Res Commun. 2000;267:118–123. doi: 10.1006/bbrc.1999.1906. [DOI] [PubMed] [Google Scholar]


