Abstract
INTRODUCTION
Xanthogranulomatous cholecystitis (XGC) is an uncommon variant of chronic cholecystitis characterised by marked thickening of the gallbladder wall and dense local adhesions. Pre-operative and intra-operative diagnosis is difficult and it often mimics a gallbladder carcinoma (GBC). Laparoscopic cholecystectomy (LC) is frequently unsuccessful with a high conversion rate. A series of patients with this condition led us to review our experience with XGC and to try to develop a care pathway for its management.
PATIENTS AND METHODS
A retrospective review of the medical records of 1296 consecutive patients who had undergone cholecystectomy between January 2000 and April 2005 at our hospital was performed. Twenty-nine cases of XGC were identified among these cholecystectomies. The clinical, radiological and operative details of these patients have been analysed.
RESULTS
The incidence of XGC was 2.2% in our study. The mean age at presentation was 60.3 years with a female:male ratio of 1.4:1. Twenty-three patients (79%) required an emergency surgical admission at first presentation. In three patients, a GBC was suspected both radiologically and at operation (10.3%), but was later disproved on histology. Seventeen patients (59%) had obstructive jaundice at first presentation and required an endoscopic retrograde cholangiopancreatography (ERCP) before LC. Of these, five had common bile duct stones. Abdominal ultrasound scan showed marked thickening of the gallbladder wall in 16 cases (55%). LC was attempted in 24 patients, but required conversion to an open procedure in 11 patients (46% conversion rate). A total cholecystectomy was possible in 18 patients and a partial cholecystectomy was the choice in 11 (38%). The average operative time was 96 min. Three patients developed a postoperative bile leak, one of whom required ERCP and placement of a biliary stent. The average length of stay in the hospital was 6.3 days.
CONCLUSIONS
Severe xanthogranulomatous cholecystitis often mimics a gallbladder carcinoma. Currently, a correct pre-operative diagnosis is rarely made. With increased awareness and a high index of suspicion, radiological diagnosis is possible. Preoperative counselling of these patients should include possible intra-operative difficulties and the differential diagnosis of gallbladder cancer. Laparoscopic cholecystectomy is frequently unsuccessful and a partial cholecystectomy is often the procedure of choice.
Keywords: Xanthogranulomatous cholecystitis, Gallbladder carcinoma, Laparoscopic cholecystectomy
Xanthogranulomatous cholecystitis (XGC) was first described as a distinct pathological condition in 1981 by Goodman and Ishak.1 It is a variant of cholecystitis characterised by intense acute or chronic inflammation, severe proliferative fibrosis with formation of multiple yellow-brown intramural nodules, and foamy histiocytes (see Fig. 1A,B). The inflammatory process often extends into neighbouring organs, such as liver, omentum, duodenum and colon. It may at times be confused with a carcinoma of the gallbladder.2 Clinically, it presents as either acute or chronic cholecystitis, and a correct pre-operative or intra-operative diagnosis is often difficult. Laparoscopic cholecystectomy (LC) is frequently unsuccessful due to the intense fibrosis, unclear anatomy and adhesions to the adjacent organs, and is associated with prolonged operating time and a higher complication rate.
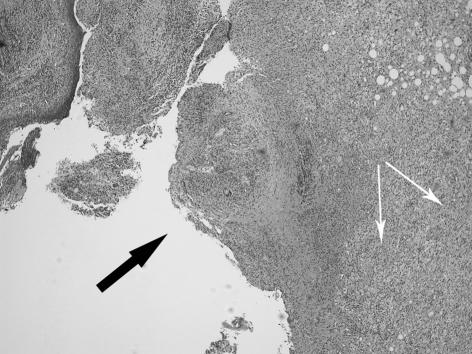
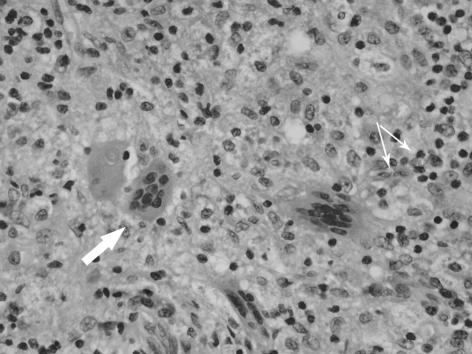
Figure 1.
(A) Xanthogranulomatous inflammation throughout the thickness of the gallbladder wall with mucosal ulceration (black arrow). The white arrows show the sheets of histiocytes in the stroma. Original magnification x2.5 objective, haematoxylin and eosin staining. (B) Xanthogranulomatous cholecystitis: close up of giant cells (big arrow) and histiocytes (small arrows), the latter forming the majority of the cells in the inflammatory infiltrate. Original magnification x40 objective; H&E staining).
Patients and Methods
A series of patients with this condition, led us to review the clinical files of all 1296 patients who had undergone cholecystectomy at our hospital between January 2000 and April 2005. Twenty-nine cases of XGC were identified. The histological diagnosis was based on the presence of diffuse or focal mural changes with the presence of xanthoma cells (foamy histiocytes containing lipids and bile pigment), giant multinucleate histiocytes, and acute or chronic inflammatory cells. We have analysed the clinical characteristics, preoperative imaging, intra-operative findings, postoperative course and histological features of these patients.
Results
Out of 1296 cholecystectomies reviewed, 29 had histologically proven XGC (2.2%). There were 17 women and 12 men with a female:male ratio of 1.4:1. The average age of the patients in this study was 60.3 years (range, 28–84 years). The clinical presentations are summarised in Table 1. Twenty-three patients (79%) required an emergency surgical admission at first presentation. A history of jaundice was obtained in 17 patients (59%). Three patients (10%) presented with a right upper quadrant mass, when a gallbladder carcinoma was suspected. The most common sonographic finding (Table 2) was a diffuse or focal thickening of the gallbladder wall (59%). Four patients had computerised tomography. A gallbladder carcinoma was suspected radiologically in three patients (10%), two of whom presented with a palpable mass. Seventeen patients (59%) presented with obstructive jaundice and required an endoscopic retrograde cholangiopancreatography (ERCP). Eight patients had normal findings, and a dilated common bile duct was found in nine patients (two with stones and seven without stones). An elective open cholecystectomy was performed in five patients (17%) in preference to LC, due to anticipation of intra-operative technical difficulty. Two of them had a Mirizzi's syndrome and the other three had a mass in the right hypochondrium from inflammatory adhesions and abscess formation. Laparoscopic cholecystectomy was attempted in 24 patients, but was unsuccessful in 11 cases (46% conversion rate). The most common reason for conversion was obscure anatomy of Calot's triangle (6/11 patients). Thickening of the gallbladder wall was the most consistent feature found at operation (82%), followed by adhesions to surrounding organs (79%; see Table 3). A partial cholecystectomy was carried out in 11 patients. The mean duration of operation was 59.6 min in open procedure (n = 5), 82.6 min in LC (n = 11), and 97.9 min in conversion (n = 13). Postoperative complications are shown in Table 4. The bile leaks were managed conservatively in two cases, and the other required ERCP and placement of a stent. There were no deaths in this series. The mean hospital stay was longer after open procedure when compared to either LC or conversion (Table 5).
Table 1.
Xanthogranulomatous cholecystitis: original clinical presentation
| Clinical presentation | Number |
|---|---|
| Acute cholecystitis | 14 |
| Cholangitis | 5 |
| Biliary colic | 4 |
| Chronic RUQ pain | 3 |
| RUQ mass | 3 |
RUQ, right upper quadrant.
Table 2.
Xanthogranulomatous cholecystitis: sonographic features
| Features | Number | |
|---|---|---|
| Wall thickness | ||
| Thick walled | 17 | |
| Thin walled | 5 | |
| Not reported | 7 | |
| Stones | ||
| Multiple | 18 | |
| Single | 9 | |
| Sludge | 1 | |
| No stones | 1 | |
| Other features | ||
| Pericystic collections | 5 | |
| Mass lesion (? carcinoma) | 3 | |
Table 3.
Xanthogranulomatous cholecystitis: operative findings
| Features | Number | ||
|---|---|---|---|
| Thick-walled gallbladder | 24 | ||
| Adhesions | 23 | ||
| Omental | (16) | ||
| Duodenal | (4) | ||
| Colonic | (3) | ||
| Obscure Calot's triangle anatomy | 12 | ||
| Empyaema of gallbladder | 9 | ||
| Features suggestive of gallbladder carcinoma | 3 | ||
| Gangrenous cholecystitis | 2 | ||
Table 4.
Xanthogranulomatous cholecystitis: postoperative complications
| Complication | Number |
|---|---|
| Bile leak | 3 |
| Biloma | 1 |
| Pneumonitis | 1 |
| Wound infection | 1 |
Table 5.
Xanthogranulomatous cholecystitis: in-patient length of stay
| Procedure | Number | Average (days) | Range (days) |
|---|---|---|---|
| LC | 13 | 3.4 | 2–6 |
| LC > Open | 11 | 6.8 | 4–12 |
| Open | 5 | 12.4 | 7–15 |
LC, laparoscopic cholecystectomy; LC > Open, conversion to open procedure.
Discussion
XGC is an uncommon entity, representing about 0.7–13.2% of all cholecystectomies.3,4 Guzman-Valdivia5 noted a male preponderance in a study of 182 cases of XGC. The disease is quoted to be common in the fifth and sixth decades of life.1,6 In our series, the incidence was 2.2% with slight female predominance, and the average age was 60.2 years.
XGC is characterised by a destructive inflammatory process of the gallbladder associated with deposition of bile pigments and cholesterol in the wall of the gallbladder. Macroscopically, the lesions vary from a small limited focus within a yellow-brown nodule in the gallbladder wall to diffuse involvement of the entire gallbladder with extension to the surrounding structures.1 Gallstones were present in 85–100% of patients in different series and obstruction to the cystic duct was noted in 80% of the cases.5,7 In our study, gallstones were present in all except one case (96%).
The pathogenesis of XGC is uncertain, but the current opinion favours a combination of acute inflammation of the gallbladder and its outflow obstruction due to gallstones.5,8 Bile enters into the stroma of the gallbladder wall through ruptured Rokitansky–Aschoff sinuses or mucosal ulcerations secondary to the presence of gallstones and/or acute inflammation (Fig. 1A). The extravasated bile in the stroma causes intense inflammatory reaction with accumulation of histiocytes which engulf the insoluble cholesterol and other bile lipids to form large round xanthoma cells (Fig. 1B). Micro-abscesses form in the gallbladder wall eventually resulting in xanthogranulomata. Finally, a fibrous reaction and scarring result from healing of the inflammatory reaction.
The clinical manifestations of XGC are those of acute or chronic cholecystitis. We noted that more than three-quarters of the patients with this condition required an acute surgical admission at first presentation. A right upper quadrant mass mimicking a gallbladder neoplasm was frequently noted. The intense inflammatory process often involves the surrounding organs and forms fistulous communications. In addition, XGC may manifest as Mirizzi's syndrome with obstructive jaundice.5,7
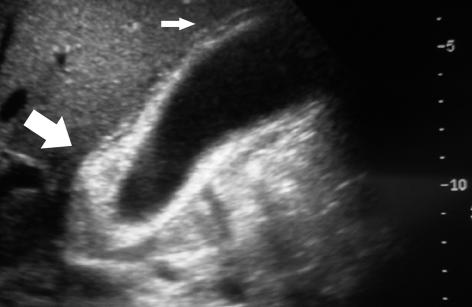
The sonographic findings in XGC (Fig. 2A) include the presence of gallstones (multiple, single or sludge), and moderate to marked thickening of the gallbladder wall (focal or diffuse). Three groups of researchers have reported a hypo-echoic nodule or band (sonolucent halo) in the gallbladder wall, to be the most characteristic finding in the disease.9–11 Occasionally, a complex, poorly defined mass mimicking malignancy is visualised on ultrasonography.12 Ultrasonography can also detect complications like gallbladder perforation with abscess formation and gas in the biliary tree due to fistula.6,13
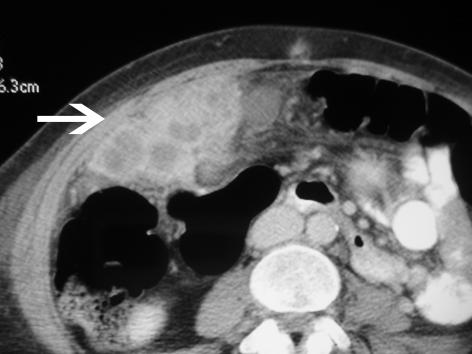
Figure 2.
(A) Ultrasonographic features of xanthogranulomatous cholecystitis, showing marked thickening of the gallbladder wall (big arrow) and ‘sonolucent halo’ (small arrow). (B) Computerised tomography of xanthogranulomatous cholecystitis showing a mixed density soft tissue mass (arrow) in the right upper quadrant in relation to gallbladder extending in to adjacent liver.
Computerised tomography (CT) is more helpful in severe XGC (Fig. 2B) and the findings are characterised by a hypodense band in the gallbladder wall with homogeneous contrast enhancement of the mucosa, thickening of the gallbladder wall.12,14 The hypodense band or low-attenuation areas in the wall of gallbladder correlate with foam and inflammatory cells or necrosis and/or abscess in XGC. Luminal surface enhancement of gallbladder wall represented preservation of the epithelial layer and might be helpful in differentiating XGC from gallbladder cancer.15 Kwon et al.7 reported non-visualisation of cystic duct on spiral computerised tomography after intravenous infusion cholangiography (IVC-SCT) as the most important factor in assessing the feasibility of successful LC in XGC. Pre-operative differentiation of XGC from gallbladder carcinoma is important for proper surgical management. However, overlaps of CT and sonographic features of XGC and GBC make it difficult. Hatakenaka et al.16 have demonstrated that chemicalshift gradient-echo MR imaging may play an important role in differentiating XGC from gallbladder carcinoma especially in cases in which a hypo-attenuated or hypo-echoic area in a thickened gall bladder wall is obscure.
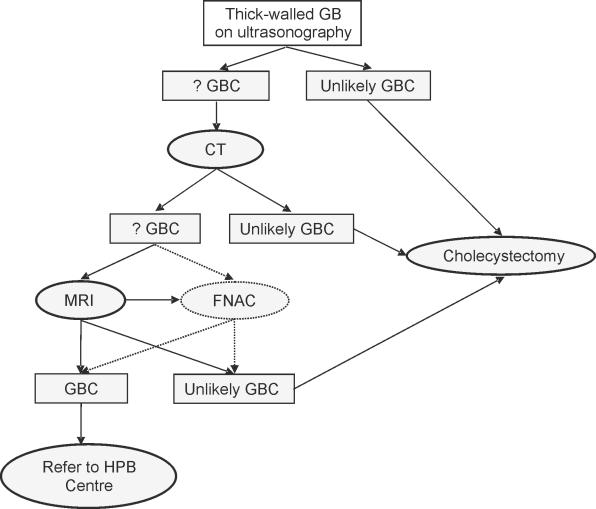
Fine needle aspiration cytology (FNAC) has been reported to play an important role in making the pre-operative diagnosis of adenocarcinoma and XGC. The overall sensitivity of detecting carcinoma was 90.6% and specificity 94.7%. The sensitivity of detecting malignancy was 80% when adenocarcinoma was associated with XGC.17 A proposed pre-operative investigative algorithm is depicted in Figure 3.
Figure 3.
Proposed algorithm for investigating a thick-walled gallbladder in a district general hospital. CT, computerised tomography; FNAC, fine needle aspiration cytology; GB, gallbladder; GBC, gallbladder carcinoma; HPB centre, hepatopancreaticobiliary centre; MRI, magnetic resonance imaging; US, ultrasonography.
We feel that in those cases where the pre-operative diagnosis is highly likely to be a gallbladder carcinoma, it is worth referring these patients to a hepatobiliary unit for further management which may involve a hepatic resection if warranted. Therefore, pre-operative counselling is important in these patients. Whenever a thick-walled gallbladder is encountered, it is important to consider XGC and gallbladder cancer in the differential diagnosis, and to counsel the patients of possible difficulties and management protocols including referral to specialist centres.
At operation, XGC may give the appearance of an advanced gallbladder carcinoma due to the marked thickening of the gallbladder wall and local destructive spread of the inflammation. Hence, an intra-operative frozen-section biopsy is often recommended.6,8,18 On the other hand, the severe inflammation may mask the presence of a carcinoma.19 In addition, a positive association between XGC and carcinoma of the gallbladder has been reported, with a 10% co-existence of these two lesions.6,20 There were no cases of co-existing carcinoma in our series. The incidence of carcinoma of the gallbladder in our hospital was 0.71% (n = 13 out of 1827 cholecystectomy specimens).
Operative findings commonly reveal the presence of prolific adhesions to surrounding tissues, thick-walled gallbladder often with fistulous communications, gallbladder perforations and abscess formation, resulting in technical difficulties and prolonged operating time.21 A complete resection of the gallbladder is not always possible especially due to poor visualisation of Calot's triangle.7 In our study, a total cholecystectomy was possible in only 18 out of 29 patients (62%). A high conversion rate up to 80% is reported in XGC.5 Our conversion rate in these cases was 46% (11 cases out of attempted 24).
The postoperative complication rate was noted to be higher in patients of XGC with 10.7% in partial cholecystectomy and 2.8% in total cholecystectomy.5,6 The over-all complication rate in this study was 20% (see Table 5). In addition, the length of stay in the hospital is generally longer in these patients. The average hospital stay was reported to be 21 days (range, 9–60 days) for open cholecystectomy as opposed to 5 days (range, 3–10 days) for LC.12 The average length of stay in our patients for open cholecystectomy was 12.4 days in comparison to 3.4 days for a successful LC.
Conclusions
Severe XGC can result in diagnostic dilemmas both preoperatively and intra-operatively due to close resemblance to gallbladder carcinoma. A correct pre-operative diagnosis requires awareness and a high index of suspicion. CT, MRI and FNAC are helpful tools in pre-operative diagnosis. Preoperative counselling should include possible differential diagnosis of gallbladder cancer. LC is frequently unsuccessful and often a partial cholecystectomy is the procedure of choice to avoid the complications of biliary injuries.
References
- 1.Goodman ZD, Ishak KG. Xanthogranulomatous cholecystitis. Am J Surg Pathol. 1981;5:653–9. doi: 10.1097/00000478-198110000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Duber C, Storkel S, Wagner PK, Muller J. Xanthogranulomatous cholecystitis mimicking carcinoma of the gallbladder: CT findings. J Comput Assist Tomogr. 1984;8:1195–8. doi: 10.1097/00004728-198412000-00034. [DOI] [PubMed] [Google Scholar]
- 3.Fligiel S, Lewin KJ. Xanthogranulomatous cholecystitis: case report and review of the literature. Arch Pathol Lab Med. 1982;106:302–4. [PubMed] [Google Scholar]
- 4.Solanki RL, Arora HL, Gaur SK, Anand VK, Gupta R. Xanthogranulomatous cholecystitis (XGC): a clinicopathological study of 21 cases. Indian J Pathol Microbiol. 1989;32:256–60. [PubMed] [Google Scholar]
- 5.Guzman-Valdivia G. Xanthogranulomatous cholecystitis: 15 years' experience. World J Surg. 2004;28:254–7. doi: 10.1007/s00268-003-7161-y. [DOI] [PubMed] [Google Scholar]
- 6.Houston JP, Collins MC, Cameron I, Reed MW, Parsons MA, Roberts KM. Xanthogranulomatous cholecystitis. Br J Surg. 1994;81:1030–2. doi: 10.1002/bjs.1800810735. [DOI] [PubMed] [Google Scholar]
- 7.Kwon AH, Matsui Y, Uemura Y. Surgical procedures and histopathologic findings for patients with xanthogranulomatous cholecystitis. J Am Coll Surg. 2004;199:204–10. doi: 10.1016/j.jamcollsurg.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Benbow EW. Xanthogranulomatous cholecystitis. Br J Surg. 1990;77:255–6. doi: 10.1002/bjs.1800770306. [DOI] [PubMed] [Google Scholar]
- 9.Lichtman JB, Varma VA. Ultrasound demonstration of xanthogranulomatous cholecystitis. J Clin Ultrasound. 1987;15:342–5. doi: 10.1002/jcu.1870150509. [DOI] [PubMed] [Google Scholar]
- 10.Casas D, Perez-Andres R, Jimenez JA, Mariscal A, Cuadras P, Salas M, et al. Xanthogranulomatous cholecystitis: a radiological study of 12 cases and a review of the literature. Abdom Imaging. 1996;21:456–60. doi: 10.1007/s002619900104. [DOI] [PubMed] [Google Scholar]
- 11.Kim PN, Ha HK, Kim YH, Lee MG, Kim MH, Auh YH. US findings of xanthogranulomatous cholecystitis. Clin Radiol. 1998;53:290–2. doi: 10.1016/s0009-9260(98)80129-3. [DOI] [PubMed] [Google Scholar]
- 12.Parra JA, Acinas O, Bueno J, Guezmes A, Fernandez MA, Farinas MC. Xanthogranulomatous cholecystitis: clinical, sonographic, and CT findings in 26 patients. AJR Am J Roentgenol. 2000;174:979–83. doi: 10.2214/ajr.174.4.1740979. [DOI] [PubMed] [Google Scholar]
- 13.Roberts KM, Parsons MA. Xanthogranulomatous cholecystitis: clinicopathological study of 13 cases. J Clin Pathol. 1987;40:412–7. doi: 10.1136/jcp.40.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu C, Hurwitz JL, Schuss A, Katz DS. Radiology-Pathology Conference: Xanthogranulomatous cholecystitis. Clin Imaging. 2003;27:421–5. doi: 10.1016/s0899-7071(02)00589-2. [DOI] [PubMed] [Google Scholar]
- 15.Shuto R, Kiyosue H, Komatsu E, Matsumoto S, Kawano K, Kondo Y, et al. CT and MR imaging findings of xanthogranulomatous cholecystitis: correlation with pathologic findings. Eur Radiol. 2004;14:440–6. doi: 10.1007/s00330-003-1931-7. [DOI] [PubMed] [Google Scholar]
- 16.Hatakenaka M, Adachi T, Matsuyama A, Mori M, Yoshikawa Y. Xanthogranulomatous cholecystitis: importance of chemical-shift gradient-echo MR imaging. Eur Radiol. 2003;13:2233–5. doi: 10.1007/s00330-002-1731-5. [DOI] [PubMed] [Google Scholar]
- 17.Krishnani N, Shukla S, Jain M, Pandey R, Gupta RK. Fine needle aspiration cytology in xanthogranulomatous cholecystitis, gallbladder adenocarcinoma and coexistent lesions. Acta Cytol. 2000;44:508–14. doi: 10.1159/000328522. [DOI] [PubMed] [Google Scholar]
- 18.Maeda T, Shimada M, Matsumata T, Adachi E, Taketomi A, Tashiro Y, et al. Xanthogranulomatous cholecystitis masquerading as gallbladder carcinoma. Am J Gastroenterol. 1994;89:628–30. [PubMed] [Google Scholar]
- 19.Benbow EW, Taylor PM. Simultaneous xanthogranulomatous cholecystitis and primary adenocarcinoma of gallbladder. Histopathology. 1988;12:672–5. doi: 10.1111/j.1365-2559.1988.tb01993.x. [DOI] [PubMed] [Google Scholar]
- 20.Benbow EW. Xanthogranulomatous cholecystitis associated with carcinoma of the gallbladder. Postgrad Med J. 1989;65:528–31. doi: 10.1136/pgmj.65.766.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixit VK, Prakash A, Gupta A, Pandey M, Gautam A, Kumar M, et al. Xanthogranulomatous cholecystitis. Dig Dis Sci. 1998;43:940–2. doi: 10.1023/a:1018802028193. [DOI] [PubMed] [Google Scholar]