Abstract
INTRODUCTION
Many surgeons will have encountered the scissors that would not cut, and the artery clip that comes off in a deep difficult location, but it would be reasonable to assume that new instruments should be of assured quality. This study reports the surprising findings of a local quality control exercise for new instruments supplied to a single trust.
MATERIALS AND METHODS
Between January 2004 and June 2004, all batches of new surgical instruments ordered by the Central Sterile Supplies Department of St Bartholomew's and the Royal London Hospitals were assessed by three clinical engineers, with reference to British Standards (BS) requirements.
RESULTS
Of 4800 instruments examined, 15% had potential problems. These included 116 with machining burrs and debris in the teeth of the tissue-holding regions, 71 defects of ratcheted instruments, 34 scissors with deficient cutting action, and 35 tissue forceps protruding guide pins. In addition, 254 instruments did not have a visible manufacturer's mark.
CONCLUSIONS
This study demonstrates the value of local quality control for surgical instruments. This is of importance in an increasingly hazard-conscious environment, where there are concerns over instrument sterilisation, surgical glove puncture and the potential for transmission of blood-borne and prion diseases.
Keywords: Surgical instruments, Quality assurance
A surgeon performing a surgical procedure should be able to assume that the instruments used are safe and reliable – particularly if they are new. To ensure the quality of these instruments, the Health Care Standards Policy Committee directed the British Standards Institution to produce requirements for the materials, design, dimensions and other features of surgical instruments. As a result, British Standards (BS), incorporating International Organisation of Standardisation (ISO) standards, were published.1 Each year, large numbers of new instruments are ordered by healthcare facilities across the UK, and those ordering them should be able to rely on these standards. This study reports the results of local quality control by the clinical engineering department of all new instruments supplied to a single NHS trust.
Materials and Methods
Over a 6-month period between January 2004 and June 2004, all new batches of surgical instruments delivered to the Barts and the London NHS Trust, from a variety of manufacturers, were assessed by three clinical engineers. The suppliers and manufacturers were informed beforehand. Where large numbers of identical instruments were delivered in a single batch, samples of these were examined as follows: 25–49 instruments 50%, 50–74 instruments 30%, 75–99 instruments 20%, and 100+ instruments 15%. In total, 4800 instruments were inspected, where necessary under magnification, for flaws as defined under BS quality assurance requirements.
Results
In total, 730 (15%) instruments failed the inspection. Table 1 shows the flaws that were identified. Figure 1 shows 3 views of the jaws of vascular clamps: a well-finished instrument on the left, an instrument with machining burrs in the teeth in the middle view and right views. Figure 2 shows a crack in the securing screw of scissors on the left, a crack though the end of the jaws of a needle holder in the middle view, and a major soldering fault in the surface of a wire bending forcep on the right. Figure 3 demonstrates protrusion of a sharp guide pin on gentle closure of tissue forceps.
Table 1.
Identified instrument flaws
| Principal flaw | Number of instruments |
|---|---|
| Machining burrs in teeth | 116 |
| Sharp burrs on handle grips | 8 |
| Soldering faults | 47 |
| Cracks | 91 |
| Failure of cutting action | 34 |
| Failure of correct meshing of ratchets | 71 |
| Failure of jaws of needle holders | 36 |
| Protruding tissue forceps guide pins | 35 |
| Corrosion | 28 |
| Deficient electrical insulation | 10 |
| Absent manufacturer's mark | 254 |
Figure 1.
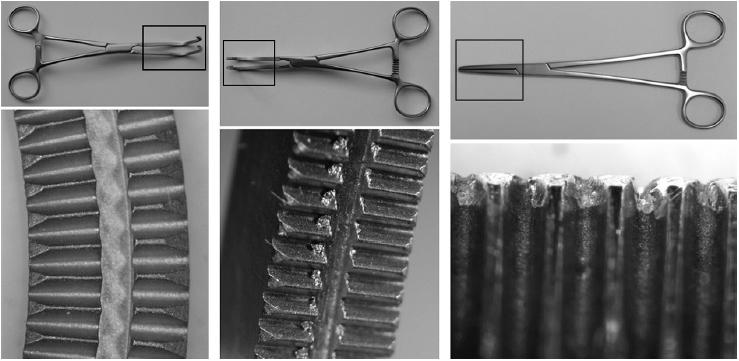
Magnified views of the jaws of vascular clamps – a well-finished example on the left, poorly finished examples in the middle and right views.
Figure 3.
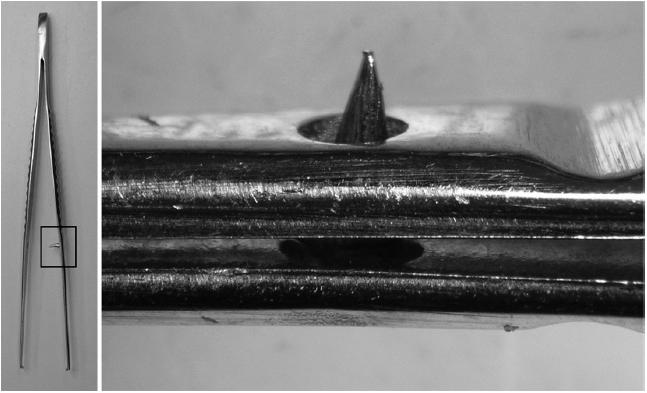
Protrusion of a forceps' guide pin.
Figure 2.
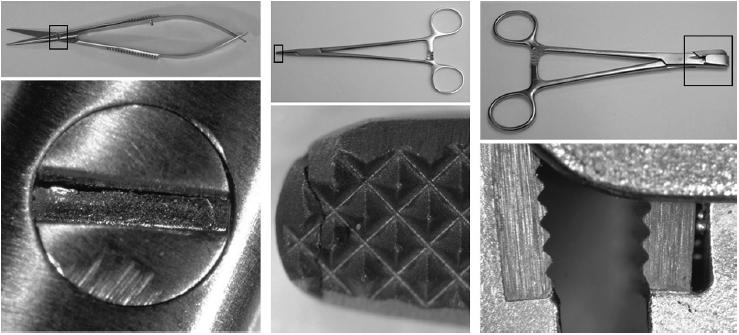
Magnified views showing a crack though the screw-head of fine scissors on the left, a cracked needle holder tip in the middle, and absence of solder to secure the jaw surface insert of a wire holder on the right.
Discussion
The commonest fault identified was lack of a maker's mark. BS states that ‘the instrument shall be marked with the name or registered trade mark of the manufacturer or supplier’.1 This may seem like a minor infringement, but in fact it is highly important. If an instrument fails in service, it is essential that the supplier and manufacturer can be notified, so that any potential problem can be rectified, to ensure the safety of the patient and theatre staff. In addition, there is the question of liability and insurance.
The commonest mechanical and structural fault was machining burr debris. BS states that ‘all surfaces must be free from pores, crevices and grinding marks’.1 The fine metallic surfaces of surgical instruments are the product of a number of engineering processes. The shapes and details are initially created by casting and pressing the metal into the required shape, but then finer detail is ground in. In modern, computer-controlled, laser-guided engineering, this should be a straightforward and reliable process, producing an extremely accurate surface, as is shown in the upper view of Figure 1. Sometimes, older methods are used but a fine finish should still be possible, as long as the surface is inspected and machine brush-polished. If this process is incomplete, metallic debris and surface imperfections will remain as shown in the middle view of Figure 1. This may be a problem in a number of ways. First, blood and tissue debris may collect in the imperfect surface. We have traditionally relied on sterilisation procedures to render such debris inert, but there are now concerns that prion disease may survive such processes.2 The metallic fragments may also wear off these surfaces, and remain as microscopic debris in the wound. Sharp burrs on instrument handles may contribute to previously unexplained surgical glove punctures. Although we cannot reference any reported instance of this, BS states that ‘there shall be no sharp edges other than those required by the pattern of the instrument’.1
Cracks and soldering faults may also provide niches for retention of blood and tissue, and serious defects may lead to instrument failure, such as the examples shown in Figure 2.
Every surgeon must have encountered the scissors that do not cut but, surprisingly, this can be a problem with new scissors. In this study, 34 scissors of various types did not meet the simple BS requirement, which describes how wet tissue paper (for fine dissecting scissors) and no. 18 gauze (for heavy tissue and suture scissors) must be cut cleanly and without tearing, for two-thirds of the length of the cutting blades.1
Most surgeons will also know the problem of the artery clip which comes off in a deep, difficult location. Contrary to popular myth, most surgeons do criticise their own technique for such problems, but may now be surprised to find that we identified 71 ratchet problems in new artery clips. BS describes in detail how the racks of these instruments should function so that they ‘mate accurately when engaged’,1 and should not spring open when left closed for 3 h on a test wire of specified diameter. The instruments that failed our assessments all had racks that did not engage correctly, and sprang open when tested in this way.
Many fine tissue forceps have guide pins to re-inforce the accuracy of the jaws mating. BS states that ‘if present, the guide pin shall be tapered to facilitate entry into the locating hole and shall not protrude from the hole when the jaws are closed’.1 We identified 35 guide pins which protruded on light, but complete, closure of the forceps’ jaws on naked eye inspection. This may be a source of glove puncture.
The stainless steel alloy from which modern instruments are made must also conform to BS. Procedures are described to test corrosion resistance, but it surprised us to find visible corrosion on new instruments.
Conclusions
This study demonstrates the value of local quality control for new surgical instruments. We have found a significant number of cases where new instruments did not appear to meet appropriate standards, and have discussed the potential problems that may result. It must be stressed that we have not shown any specific instance of harm to a patient or staff through these defects. Suppliers were informed, and remedial action taken. All defective instruments were replaced and reexamined prior to entering service.
References
- 1.British Standards Institution. The British Standard. BS 5194, parts 1–4. London: British Standards Institution; 1985. ISBN 0 580 14853 X. [Google Scholar]
- 2.Collinge J. Variant Creutzfeldt-Jakob disease. Lancet. 1999;354:317–23. doi: 10.1016/S0140-6736(99)05128-4. [DOI] [PubMed] [Google Scholar]
- 3.Lemmer K, Mielke M, Pauli G, Beekes M. Decontamination of surgical instruments from prion proteins in vitro studies on the detachment, destabilization and degradation of PrPSc bound to steel surfaces. J Gen Virol. 2004;85:3805–16. doi: 10.1099/vir.0.80346-0. [DOI] [PubMed] [Google Scholar]


